Abstract
Background
This study prospectively evaluated the yield of fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) in patients with clinical stages II and III breast cancer and the impact of PET-CT results on prognosis.
Methods
In the course of 71 months, 254 consecutive patients with clinical stages II and III breast cancer (based on clinical examination, mammography, breast magnetic resonance imaging, and locoregional ultrasonography) underwent 18FDG-PET-CT. The yield was assessed in the whole population and for each American Joint Committee on Cancer subgroup. The prognostic impact of PET-CT findings was analyzed. Tests of statistical significance were two-sided.
Results
18FDG-PET-CT changed the clinical stage in 77 of 254 patients (30.3%; 95% confidence interval [CI] = 25.0% to 36.2%). It showed unsuspected N3 disease (infraclavicular, supraclavicular, or internal mammary nodes) in 40 patients and distant metastases in 53. PET-CT revealed distant metastases in 2.3% (1 of 44) of clinical stage IIA, 10.7% (6 of 56) of stage IIB, 17.5% (11 of 63) of stage IIIA, 36.5% (27 of 74) of stage IIIB, and 47.1% (8 of 17) of stage IIIC patients. Among 189 patients with clinical stage IIB or higher disease and adequate follow-up, disease-specific survival was statistically significantly shorter in the 47 patients scored M1 on 18FDG-PET-CT in comparison with those scored M0, with a three-year disease-specific survival of 57% vs 88% (P < .001). In multivariable analysis, only distant disease on PET-CT and triple-negative phenotype were statistically significant prognostic factors. The relative risk of death was 26.60 (95% CI = 6.60 to 102.62) for M1 vs M0 patients.
Conclusions
The yield of 18FDG-PET-CT appeared substantial in patients with clinical stage IIB or higher breast cancer. In these patients, 18FDG-PET-CT provided powerful prognostic stratification.
Several studies have pointed out the limited value of fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) in staging breast cancer patients with tumors less than 2cm and no palpable nodes (clinical T1N0, stage I), which currently represents the majority of newly diagnosed cases (1,2). Low sensitivity of PET, compared with the sentinel node technique, in assessing axillary lymph node involvement is well known (3), and the risk of distant metastases is low in early stage disease. The case might be different for higher-risk categories. Many reports have documented the value of 18FDG-PET-CT in stage III patients with locally advanced or inflammatory breast cancer (4–6). In all these studies, the yield from 18FDG-PET-CT as a single procedure was higher than that offered by a conventional staging approach combining bone scan (BS), chest imaging (chest x-ray or CT), and abdominal imaging (liver ultrasound or contrast-enhanced CT). Some recent studies have suggested that PET-CT might also be valuable in stage II disease (7–12). However, the precise subgroups of patients in whom PET-CT could be useful are not clearly defined.
This prospective study examined the yield of 18FDG-PET-CT in a population of 254 patients with clinical stage II or III breast cancer, as well as in each specific substage (IIA, IIB, IIIA, IIIB, and IIIC). The impact of PET-CT findings on patient’s outcome was also examined.
Methods
Study Design
This single-institution prospective study (protocol “Asaint”) included consecutive patients with newly diagnosed, biopsy-proven breast cancer and clinical stage II or III disease seen at the Breast Diseases Unit at Saint-Louis Hospital, Paris, France, between January 2006 and November 2011. Clinical stage was based on clinical examination, mammography, breast magnetic resonance imaging, and ultrasound of breast and locoregional nodal basins.
18FDG-PET-CT in breast cancer greater than 2cm is optional in France (13) and part of routine clinical practice in our institution. Patients staged node positive after sentinel node biopsy were not included. Other exclusion criteria were previous history of breast or other cancer, uncontrolled diabetes mellitus, pregnancy, and age younger than 18 years.
The main objective was to determine the percentage of stage modification resulting from the use of 18FDG-PET-CT as a single procedure in the population with stages II and III disease and within each American Joint Committee on Cancer subgroup (IIA, IIB, IIIA, IIIB, and IIIC). Other objectives were the yield in distant metastases, the yield in N3 disease, and the prognostic impact of these findings on survival.
Sample size was calculated for the main objective (stage modification) assuming the following three hypotheses: 1) distant metastases or unsuspected N3 disease (infraclavicular, supraclavicular, or internal mammary nodes) are meaningful findings; 2) stage modification refers to clinical staging (in order to minimize radiation exposure and costs, 18FDG-PET-CT would be a substitute to other distant workup, not an additional procedure); and 3) in early studies, based on PET alone instruments, unsuspected lesions were uncovered in about 20% (10%–30%) of patients (14–17). With this projected yield, it was estimated that inclusion of 250 patients would allow measuring the true yield within a 95% confidence interval (CI) not wider than 10% (true yield ±5%). The population included five clinical substages, and equal partition was used for simplification. In a subgroup of 50 patients, the yield would be measured with a confidence interval of plus or minus 10%.
18FDG-PET-CT was performed before any treatment. Findings from 18FDG-PET-CT were used together with results from other staging procedures to adapt treatment as judged appropriate by the institutional multidisciplinary team.
Informed consent was obtained before 18FDG-PET-CT imaging, and the study followed the guidelines of the institutional ethical committee.
Histology and Immunohistochemistry Analysis
It was performed on the core needle biopsy before any neoadjuvant chemotherapy. Tumor grade used the modified Scarff-Bloom-Richardson system. Tumors were estrogen receptor positive if showing moderate or high positivity of at least 10% of cells. The same criteria were used for progesterone receptor. Tumors were human epidermal growth factor receptor 2 (HER2) positive if more than 30% of cells showed definite membrane staining. Control by fluorescence in situ hybridization (FISH) or silver-enhanced in situ hybridization (SISH) was done for ambiguous cases. Triple-negative breast cancer (TNBC) is negative for estrogen and progesterone receptors and does not overexpress HER2.
18FDG-PET-CT Imaging
Patients fasted 6 hours, and blood glucose level had to be less than 7 mmol/L. 18FDG (5 MBq/kg, not exceeding 500 MBq) was injected in the arm opposite to the tumor. Imaging was performed 60 minutes later, from midthigh level to the base of the skull with the arms raised, on a Gemini XL PET-CT (germanium oxyorthosilicate–based PET + 16-slice CT; Philips). CT data was acquired without contrast enhancement and using the following parameters: 120kV; 100 mAs; pitch, 0.81; and slice thickness, 2.5mm. PET data was collected in a three-dimensional mode, with 2 minutes per table position, and reconstructed.
PET-CT Interpretation and Modification in Staging
18FDG uptake, findings on the CT part of PET-CT, and fusion images were considered altogether. PET-CT was read by two nuclear medicine physicians (D. Groheux and E. Hindié). If interpretation differed, consensus was reached with the help of a third reader.
Lymph node interpretation considered any well-defined focus with 18FDG uptake clearly higher than surrounding background (18). Anatomic location on PET-CT was based on American Joint Committee on Cancer classification (1). For distant metastases, form and intensity of 18FDG uptake as well as CT findings were considered altogether. Bone evaluation was performed as described by Nakamoto (19). 18FDG uptake corresponding to degenerative findings on the underlying CT (eg, on facet articulation) and uptake in a rib fracture with a history of trauma were considered non-suspicious. However, high uptake on a classic area of metastasis (eg, body of a vertebra, pedicle, long bone) was considered malignant even if the CT part showed subtle or no changes, in agreement with the well-known high sensitivity of 18FDG-PET compared with CT for early bone marrow involvement (19). For lung evaluation, we considered pulmonary nodule(s) with high 18FDG uptake or the presence of multiple small angiocentric nodules on the CT part (even in the absence of high FDG uptake) to be suspicious.
Nuclear physicians were blind to any other systemic imaging that could have been ordered in parallel (bone scan, chest x-ray or CT, liver ultrasound, or abdominal-pelvic CT). The choice of conventional procedures was at the discretion of oncologists. Bone scan was routinely performed.
Infraclavicular and supraclavicular N3 disease received ultrasound-directed biopsy. Internal mammary nodes were not routinely biopsied because of the small risk of pneumothorax or bleeding. In early studies, 18FDG uptake in the internal mammary basin was predictive of tumor involvement (16).
Distant involvement was confirmed by biopsy, especially in the case of oligometastases. If biopsy proved difficult to perform, conventional imaging and additional directed radiological studies were helpful. 18FDG-PET-CT findings were compared with BS and magnetic resonance imaging for bone lesions; with dedicated CT for lung and pleura; and with contrast-enhanced CT, ultrasound, and/or magnetic resonance imaging for liver lesions.
Modification in staging resulting from 18FDG-PET-CT was compared with clinical stage.
Outcome Evaluation
Disease-specific survival (DSS) was determined as the percentage of patients who had not died from breast cancer as of April 2012. The start of treatment was used as the beginning of follow-up. Patients had follow-up visits every 4 months for 2 years, then twice yearly.
Statistical Analysis
All statistical tests were two-sided. P values less than .05 were considered statistically significant.
The Fisher exact test was used to evaluate odds ratios and 95% confidence intervals.
Log-rank test was used to compare survivals of patients with and without distant metastases on 18FDG-PET-CT. Impact of N3 status on DSS was also assessed.
Relative risk and statistical significance of prognostic factors in regards to DSS were first assessed by univariate analysis using the Cox proportional hazard model and log-rank test. Variables showing poor statistical significance were discarded from the multivariable model. Finally, subgroups of phenotypes showing statistically homogeneous survival were merged. The Cox proportional hazard assumption was checked by testing the hypothesis of non-correlation between survival times and the scaled Schoenfeld residuals. When needed, the Cox proportional hazard model was stratified in order to maintain the Cox proportionality hazard assumption. Analyses were performed using R 2.12.0 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
This study included 254 patients. The main characteristics of the patients are outlined in Table 1.
Table 1.
Characteristics of patients and tumors at diagnosis*
| Characteristic | Patients, No. (%) |
|---|---|
| AJCC clinical stage and TNM† | |
| IIA | 44 (17) |
| T1 N1 M0 | 2 |
| T2 N0 M0 | 42 |
| IIB | 56 (22) |
| T2 N1 M0 | 33 |
| T3 N0 M0 | 23 |
| IIIA | 63 (25) |
| T3 N1 M0 | 37 |
| T1 N2 M0 | 1 |
| T2 N2 M0 | 11 |
| T3 N2 M0 | 14 |
| IIIB | 74 (29) |
| T4 N0 M0 | 14 |
| T4 N1 M0 | 36 |
| T4 N2 M0 | 24 |
| IIIC | 17 (7) |
| T1 N3 M0 | 2 |
| T2 N3 M0 | 1 |
| T3 N3 M0 | 5 |
| T4 N3 M0 | 9 |
| Tumor type | |
| Invasive ductal carcinoma | 218 (86) |
| Invasive lobular carcinoma | 21 (8) |
| Others | 15 (6) |
| Grade | |
| 1 | 10 (4) |
| 2 | 116 (46) |
| 3 | 119 (47) |
| Unspecified | 9 (3) |
| Breast tumor phenotype ‡ | |
| ER positive / HER2 negative | 130 (51) |
| HER2 positive | 51 (20) |
| Triple negative | 69 (27) |
| Unspecified | 4 (2) |
* N = 254; AJCC = American Joint Committee on Cancer; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; TNM = tumor nodes metastases
† Clinical classification before fluorodeoxyglucose positron emission tomography/computed tomography according to the seventh edition of the AJCC Staging Manual (1). Clinical stage was based on clinical examination, mammography, breast magnetic resonance imaging, and ultrasound of breast and locoregional nodal basins.
‡ Tumors were ER positive if more than 10% of cells showed staining by immunohistochemistry. Tumors were considered to overexpress HER2 (HER2 positive) if more than 30% of invasive tumor cells showed definite membrane staining resulting in a so-called fishnet appearance.
PET-CT Findings in the Whole Population
18FDG-PET-CT was positive in all 17 patients with known N3 nodes (clinical stage IIIC) and revealed unsuspected N3 disease (infraclavicular, supraclavicular, or internal mammary nodes) in 40 additional patients (17%).
Distant metastases were uncovered by 18FDG-PET-CT in 53 patients (20.8%; 95% CI = 16.3% to 26.3%). Sites of involvement were bone (n = 35), distant lymph nodes (n = 20), liver (n = 13), lung (n = 9), and pleura (n = 2). Twenty-one women had distant lesions at more than one site.
Twenty-five patients with multiple metastases received confirmation by more than one image modality. Twenty-one patients, mostly with oligometastatic disease, received confirmation by biopsy or surgery. In seven cases, biopsy was not feasible, and these patients were considered M1 on the basis of 18FDG-PET-CT.
Altogether, 18FDG-PET-CT changed staging in 77 of 254 patients (30.3%; 95% CI = 25.0% to 36.2%). Figures 1 and 2 show two examples.
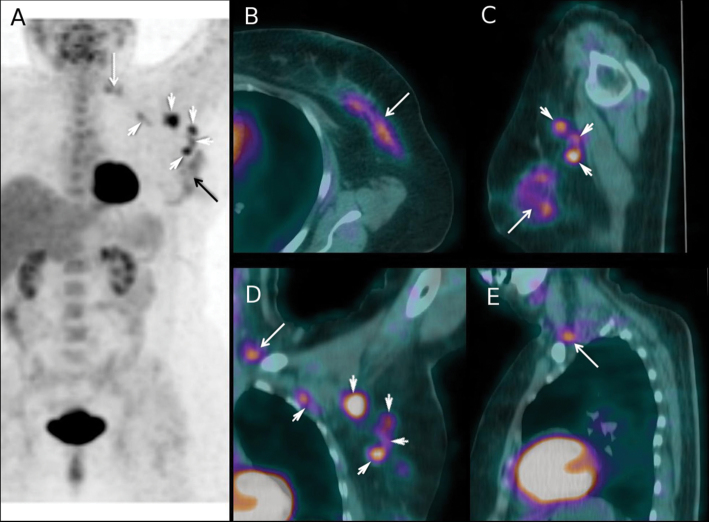
Figure 1.
Fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) findings in a 63-year-old woman with a 90-mm invasive mixed ductal/lobular carcinoma of the left breast (tumor phenotype = estrogen receptor positive, human epidermal growth factor receptor 2 negative, grade 2) and matted lymph nodes in axilla level 1. Based on clinical examination and loco-regional workup, the patient was clinically staged IIIA (cT3 cN2a cM0). A) Maximum intensity projection image PET showing 18FDG uptake in the primary tumor (black arrow), in lymph nodes at level 1 and 2 of axilla (white arrowheads), and in supraclavicular lymph nodes (white arrow). B) Axial projection PET-CT fusion images through the left breast showing the primary tumor (white arrow). C) PET-CT sagittal projection showing the primary tumor (white arrow) and three level 1 axillary lymph nodes (white arrowheads). D) PET-CT coronal section showing 18FDG uptake in level 1 and level 2 nodes (white arrowheads) and in a supraclavicular node (white arrow). E) PET-CT sagittal section showing 18FDG uptake in one supraclavicular node (white arrow). After PET-CT, cancer was classified cT3 cN3c cM0 (stage IIIC). The neoadjuvant chemotherapy regimen was performed as planned (4 cycles of epirubicin 75mg/m² plus cyclophosphamide 750mg/m² followed by 4 courses of docetaxel 100mg/m²). After completion of neoadjuvant chemotherapy, the patient underwent mastectomy, axillary lymph node dissection, and, because of data from the initial PET-CT scan, additional dissection of the supraclavicular area. Pathological examination showed residual tumor in breast with >50% pathological response, six axillary metastases (with evidence of therapeutic effect), and tumor-involved supraclavicular nodes (with evidence of therapeutic effect). Radiation therapy to the chest wall and locoregional basins was performed, and the patient received adjuvant hormone therapy. At 24 months of follow-up, no recurrence was observed.
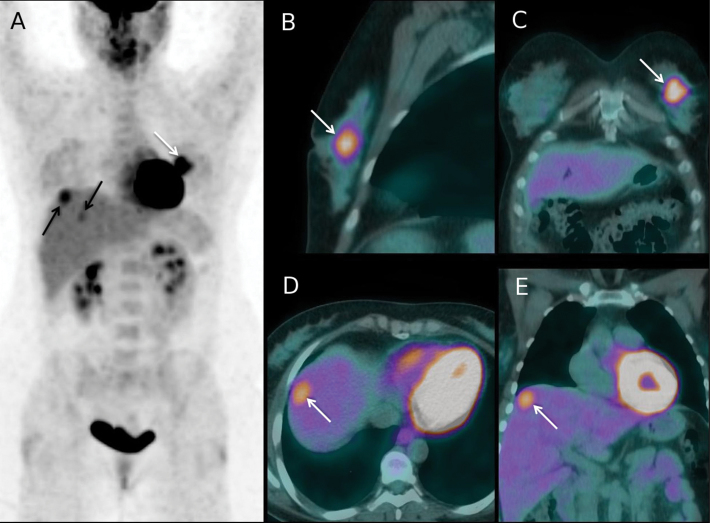
Figure 2.
Fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) findings at initial staging in a 45-year-old woman with a 60-mm invasive ductal carcinoma of the left breast (tumor phenotype = estrogen receptor positive, human epidermal growth factor receptor 2 negative, grade 2). The patient had no regional lymph nodes metastases at clinical and ultrasound examination (cT3 cN0 cM0; stage IIB). A) Maximum intensity projection PET image showing 18FDG uptake in the primary tumor (white arrow), and also FDG uptake foci in the liver (black arrows). B, C) Sagittal and coronal projections PET-CT fusion images through the left breast showing the primary tumor (white arrows). D) Axial projection PET-CT fusion image through the liver dome showing a 18FDG-avid liver lesion (white arrow). E) Coronal projection image showing the same metastasis (white arrow). No metastases had been depicted on initial liver ultrasound but abdominal contrast-enhanced CT performed after PET-CT confirmed liver metastases. The patient was upstaged to cT3 cN0 cM1 (stage IV). Chemotherapy regimen was adapted to metastatic disease. Following primary chemotherapy, mastectomy and axillary clearance showed a residual breast primary with pathological response >50% and no metastases in the 14 removed nodes. Then, right liver surgery was performed and showed residual metastases. Radiation therapy to the chest wall only (without regional nodal basins irradiation) was performed, and the patient received adjuvant hormone therapy. In the 7 months since liver surgery, no recurrence was documented.
Considering ancillary findings, 18FDG-PET-CT depicted a second primary cancer in three patients (lung, n = 1; thyroid, n = 1; colon, n = 1).
Comparison With Conventional Workup
Out of the 53 patients with distant metastases uncovered by 18FDG-PET-CT, 23 (43%) were reported negative on unguided conventional workup. However, conventional imaging workup was not homogeneous. Nevertheless, all patients received BS in our department so that a direct comparison could be performed. 18FDG-PET-CT was positive in 35 of 36 patients with bone involvement, whereas planar BS was positive in 26. False-positive findings occurred in three patients with 18FDG-PET-CT and in seven patients with BS because of benign osteo-articular lesions. 18FDG-PET-CT outperformed BS, with four misclassifications vs 17 for BS (Fisher exact test, P =.006).
Findings According to Tumor Phenotype and Grade
N3 disease (clinical + PET-detected) was more frequent in patients with grade 3 tumors in comparison with lower grades (29% vs 13%; odds ratio [OR] = 2.6; 95% CI = 1.31 to 5.32; P =.004). The rate of distant metastases did not differ (17% vs 21%; OR = 1.35; 95% CI = 0.68 to 2.72; P =.42) (Table 2).
Table 2.
Fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) findings according to tumor phenotype and Scarff-Bloom-Richardson (SBR) grade*
| PET-CT findings | ER positive†/ HER2 negative‡ | HER2 positive‡ | TNBC | Grade 3 | Grades 1 and 2 | Total |
|---|---|---|---|---|---|---|
| Total patients | 130§ | 51§ | 69§ | 119|| | 126|| | 254 |
| Patients with N3 lymph nodes | 21 (16%) | 16 (31%) | 19 (28%) | 34 (29%) | 17 (13%) | 57 (22%) |
| Total patients with distant metastases | 28 (22%) | 13 (26%) | 11 (16%) | 20 (17%) | 27 (21%) | 53 (21%) |
| Only bone metastases | 13 (10%) | 5 (10%) | 2 (3%) | 7 (6%) | 11 (9%) | 21 (8%) |
| Extra-skeletal metastases only | 5 (4%) | 6 (12%) | 7 (10%) | 9 (8%) | 8 (6%) | 18 (7%) |
| Skeletal and extra-skeletal metastases | 10 (8%) | 2 (4%) | 2 (3%) | 4 (3%) | 8 (6%) | 14 (6%) |
* N = 254; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer.
† Tumors were considered ER positive if more than 10% of cells showed staining by immunohistochemistry.
‡ Tumors were considered to overexpress HER2 (HER2 positive) if more than 30% of invasive tumor cells showed definite membrane staining resulting in a so-called fishnet appearance.
§ Phenotype was unspecified in four cases.
|| Grade was unspecified in nine tumors.
N3 disease was more frequent in TNBC (28%) and HER2-positive (31%) patients combined than in estrogen receptor–positive and HER2-negative patients (16%) (OR = 2.13; 95% CI = 1.11 to 4.15; P =.01). The rates of distant involvement did not differ: TNBCs (16%), HER2 positive (26%), estrogen receptor positive and HER2 negative (22%) (P =.42). However, the sites of involvement differed. TNBC patients and HER2-positive patients had a high proportion of extra-skeletal metastases (Table 2).
Yield of PET-CT According to Clinical Stage
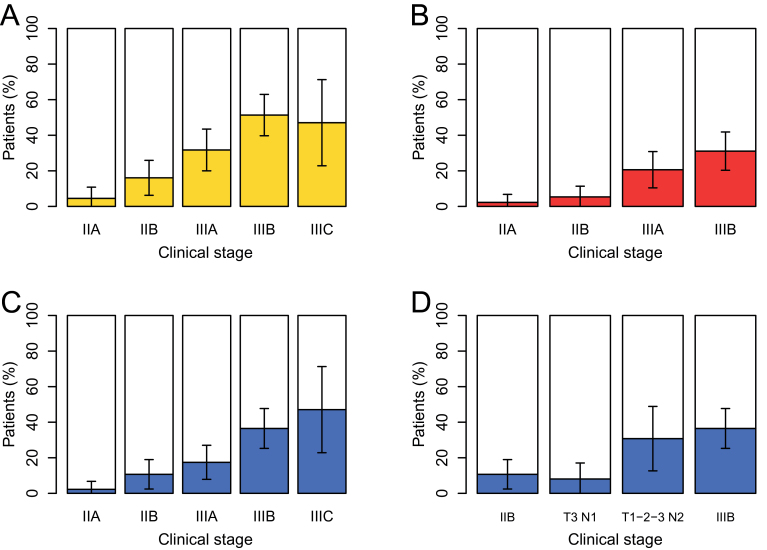
Following 18FDG-PET-CT imaging, the rates of stage modification in the different clinical American Joint Committee on Cancer subgroups were 4.5% (95% CI = 0% to 10.8%) for stage IIA; 16.1% (95% CI = 6.3% to 25.9%) for stage IIB; 31.7% (95% CI = 20.0% to 43.5%) for stage IIIA; 51.4% (95% CI = 39.7% to 62.9%) for stage IIIB; and 47.1% (95% CI = 22.8% to 71.3%) for stage IIIC (Figure 3, A).
Figure 3.
Yield of fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) according to initial clinical American Joint Committee on Cancer subgroup and tumor nodes metastases subset. A) Percentage of stage modification. B) Percentage of N3 disease uncovered by PET-CT. All patients with clinical N3 (stage IIIC) were also confirmed on PET-CT and are not represented here. C) Percentage of patients with distant metastases uncovered by PET-CT. D) Percentage of patients with distant metastases uncovered by PET-CT for specific tumor node metastases subsets of stage IIIA. The yield in T3 N1 patients is similar to that of stage IIB, whereas the yield in patients with clinical N2 disease is similar to that in stage IIIB patients (D). Error bars on each graph show the 95% confidence interval.
Figure 3, B shows the yield in N3 disease, and Figure 3, C shows the yield in distant metastases. The yield in distant metastases increased progressively along subgroups (P <.001; χ2 test for trend in proportions). PET-CT revealed distant metastases in 1 of 44 (2.3%; CI = 0 to 6.8%) stage IIA patients, 6 of 56 (10.7%; CI = 2.4% to 18.9%) stage IIB patients, 11 of 63 (17.5%; CI = 7.9% to 27.0%) stage IIIA patients, 27 of 74 (36.5%; CI = 25.3% to 47.7%) stage IIIB patients, and 8 of 17 (47.1%; CI = 22.8% to 71.3%) stage IIIC patients (Figure 3, C).
Stage IIIA is heterogeneous. The rate of distant metastases in T3N1 patients (primary operable) was similar to that in stage IIB (T2N1/T3N0) patients. It was much higher in patients with N2 disease, close to that found in stage IIIB patients (Figure 3, D).
Thus, the yield of 18FDG-PET-CT was limited in patients with stage IIA disease. When considering all other categories combined (stage IIB disease and higher), distant metastases were found in 24.9% of patients (95% CI = 19.4% to 31.0%).
Distant Metastases Detected by PET-CT and Prognosis
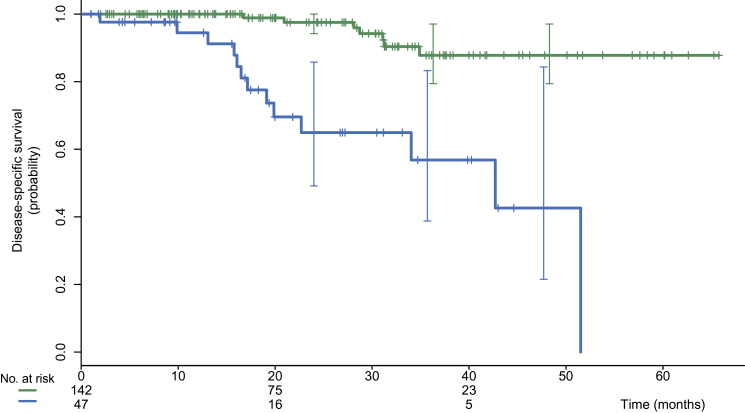
The impact on prognosis was examined in patients with clinical stage IIB disease and higher. Full information was available for 189 patients, with a mean follow-up of 23 months. Twenty patients died.
Among these 189 patients with stage IIB and higher, 18FDG-PET-CT showed distant metastases in 47 patients (24.9%). DSS was shorter in the 47 patients scored M1 on 18FDG-PET-CT in comparison with those scored M0 (log-rank P <.001), with a 3-year DSS of 57% vs 88% (Figure 4). The impact on survival was present in the locally advanced group as well as in stage IIB and primary operable IIIA (T2N1/T3N0/T3N1) disease (log-rank P <. 001).
Figure 4.
Kaplan–Meier disease-specific survival for 189 patients with clinical stages IIB, IIIA, IIIB, and IIIC disease and adequate follow-up. Comparison of patients with and without distant metastases on fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT). The upper curve (in green color online) shows patients without distant metastases (n = 142; events = 7). The lower curve (in blue color online) shows patients with distance metastases detected by PET-CT (n = 47; events = 13). Log-rank P less than .001.
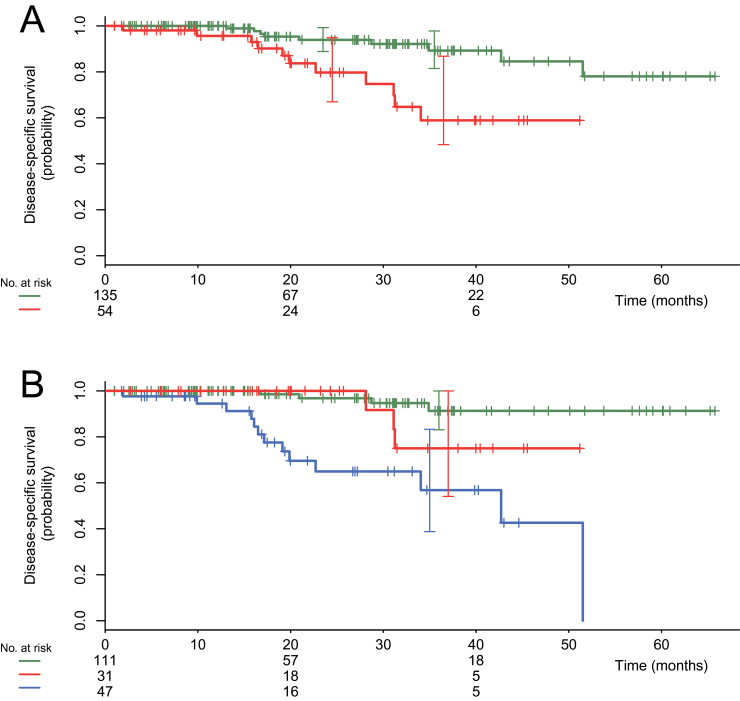
N3 Status and Prognosis
Figure 5, A shows that DSS was shorter in women with N3 status (clinical or PET-detected) (log-rank P =.001). However, distant metastases were more frequent in this group (43% vs 18%; OR = 3.41, 95% CI = 1.61 to 7.28; P <.001). When considering patients without distant metastases, DSS was not statistically significantly different between the 31 patients with N3 disease and the 111 patients without, although the curves show a trend for poorer survival (log-rank P = .16) (Figure 5, B).
Figure 5.
Kaplan–Meier disease-specific survivals for the 189 patients with clinical stages IIB, IIIA, IIIB, and IIIC disease. Comparison of patients with and without N3 disease (infraclavicular, supraclavicular, or internal mammary lymph nodes) shown by fluorodeoxyglucose positron emission tomography/computed tomography. A) Comparison of patients with and without N3 disease. The upper curve (in green color online) shows patients without N3 disease (n =135; events = 9). The lower curve (in red color online) shows patients with N3 disease (n = 54; events = 11). Log-rank P equals .001. B) Women with M1 disease separated from others. The upper curve (in green color online) shows patients without N3 disease and without distant metastases (n = 111; events = 4). The intermediate curve (in red color online) shows patients with N3 disease and without distant metastases (n = 31; events = 3). The lower curve (in blue color online) shows patients with distant metastases (n = 47; events = 13). Log-rank P equals .16 between the two upper curves.
Univariate and Multivariable Analysis
In univariate analysis, three variables had a negative prognostic impact on DSS: inflammatory breast cancer, triple-negative status, and M1 disease on PET-CT (Table 3). Multivariable analysis showed that two independent variables were statistically significant: TNBC and M1 disease on PET-CT (Table 3). The relative risk of death was 26.60 (95% CI = 6.60 to 102.62) for patients scored M1 on PET-CT vs patients scored M0 and 18.58 (95% CI = 5.24 to 65.85) for patients with TNBC vs patients with other phenotypes combined.
Table 3.
Univariate and multivariable analysis: impact of various clinical and biological factors and of fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET-CT) findings on disease-specific survival*
| Variable | No. (%) | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Reference category | Log rank P | RR(95% CI) | Cox P | Reference category | RR(95% CI) | Cox P | ||
| Clinical N+ disease | 156 (82.5%) | Clinical N0 disease | .71 | 1.32 (0.30 to 5.72) | .71 | † | † | † |
| Inflammatory cancer | 31 (16.5%) | Noninflammatory breast cancer | <.001 | 4.85 (2.00 to 11.73) | <.001 | Noninflammatory breast cancer | 2.03 (0.75 to 5.52) | .17 |
| Grade 3 breast cancer | 92 (48.5%) | Grade 1 or 2 breast cancer | .28 | 1.61 (0.67 to 3.90) | .29 | † | † | † |
| Phenotype‡ER-positive/HER2-negative tumors§HER2-positive tumors§ TNBC§ | 98|| (52.0%)37|| (19.5%)52|| (27.5%) | ER-positive/HER2-negative tumors§ | <.001 | 10.42 (0.09 to 1.80)4.48 (1.82 to 11.00) | -.24.001 | ††Non-TNBC§ | ††18.58 (5.24 to 65.85) | ††<.001 |
| Distant metastases on PET-CT | 47 (25.0%) | No distant metastases on PET-CT | <.001 | 8.41 (3.33 to 21.27) | <.001 | No distant metastases on PET-CT | 26.60 (6.60 to 102.62) | <.001 |
* The analysis was performed in patients with clinical stage IIB and higher breast cancer and full information (N = 189). All statistical tests were two-sided. CI, confidence interval; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; RR = relative risk; TNBC = triple-negative breast cancer.
† Corresponding variables were discarded from the multivariable Cox proportional hazard model because they were not significant in the univariate analysis.
‡ Stratified univariate Cox proportional hazard model was considered for the corresponding variables.
§ Tumors were considered ER positive if more than 10% of cells showed staining by immunohistochemistry. The same criterion was used for progesterone receptor. Tumors were considered to overexpress HER2 (HER2-positive) if more than 30% of invasive tumor cells showed definite membrane staining resulting in a so-called fishnet appearance. TNBC is negative for estrogen and progesterone receptors and is without HER2 overexpression.
|| Phenotype was undetermined in 2 of the 189 patients.
The 52 patients with TNBC showed poorer prognosis, both within the M0 group and the M1 group. Seven of the 142 M0 patients died; five had a triple-negative phenotype. Thirteen of the 47 M1 patients died; seven of these had a triple-negative phenotype.
Discussion
Although the degree of uptake varies depending on histological type, tumor grade, and phenotype, the overwhelming majority of breast tumors are 18FDG-avid (20).
The aim of this prospective study was to examine the yield of 18FDG-PET-CT as a single examination in a large population of patients with clinical stage II and III breast cancer and in each specific substage. Therefore, nuclear physicians were blind to all conventional imaging studies that could have been ordered in parallel (eg, BS, chest x-ray, CT, liver ultrasound).
Stage modification due to detection of N3 disease (infraclavicular, supraclavicular, or internal mammary nodes) and/or distant metastases occurred in 4.5% of stage IIA patients, 16.1% of stage IIB patients, 31.7% of stage IIIA patients, 51.4% of stage IIIB patients, and 47.1% of stage IIIC patients. We suggested that PET-CT might be justified starting with stage IIB.
The overall low yield in stage IIA (4.5%) might challenge the use in this group if considering cost of 18FDG-PET-CT imaging, increased patient anxiety, potential for delaying care, risk of invasive procedures stimulated by false-positive results, and exposure to ionizing radiation (21). Stage IIA was mainly represented by T2N0 disease; only two patients had T1N1 disease. Sentinel node-positive patients were not included.
The rate of distant metastases uncovered with 18FDG-PET-CT showed a steady increase across subgroups: 2.3% in stage IIA patients, 10.7% in stage IIB patients, 17.5% in stage IIIA patients, 36.5% in stage IIIB patients, and 47.1% in stage IIIC patients. Within stage IIIA, the risk differed between patients with T3N1 disease and those with N2 disease (Figure 3, D). Interestingly, management recommendations would usually distinguish between these entities (22).
One advantage of 18FDG-PET-CT is its ability to examine extra-axillary nodes, chest, abdomen, and bone in a single session (4–12). We observed a higher accuracy for 18FDG-PET-CT compared with planar BS, which is in agreement with reports from Fuster et al. (8) and Morris et al. (23). Not all patients in the present study received chest diagnostic CT or abdominal-pelvic diagnostic CT (or magnetic resonance imaging), making a comparison for other sites of involvement difficult.
The impact of 18FDG-PET-CT findings on prognosis was examined in patients with clinical stage IIB or higher disease. The 3-year DSS was statistically significantly shorter in women who were scored M1 on PET-CT in comparison with those scored M0 (57% vs 88%; P < .001). This impact was also apparent in the subset of patients with stage IIB and primary operable IIIA (T2N1/T3N0/T3N1).
Besides prognostic value, the information from 18FDG-PET-CT can be helpful in adapting treatment. Early detection of distant metastases might lead to refinement in the type of systemic treatment. It can also lead to local treatments in some patients (eg, liver surgery, radiofrequency ablation, radiation therapy to bone metastases). Detection of N3 disease might influence the extent of surgery and the design of radiation fields (24,25).
In the absence of distant metastases, the prognostic impact of N3 disease did not reach statistical significance (P =.16). A possible explanation could be that patients with N3-detected nodes received a more aggressive treatment with curative intent (Figure 1). Patients with supraclavicular metastases were previously categorized as stage IV. These patients are potentially curable in the era of multimodality imaging and therapy (24–26).
In a multivariable analysis, two independent prognostic variables were highly statistically significant: M1-disease on 18FDG-PET-CT and a triple-negative phenotype. Findings that patients with TNBC are at high risk, even in the absence of distant metastases on initial PET-CT staging, reinforces the feeling that monitoring tumor response during neoadjuvant chemotherapy is needed (27).
The proportion of extra-skeletal metastases in patients with TNBC was high (Table 2). TNBC is more likely to metastasize to viscera, particularly lungs, brain, and liver (28). In that regard, it is important to note that some patients with isolated brain metastases might have escaped detection.
Our study has some limitations, mainly resulting from difficulties in performing confirmatory biopsies of distant metastases seen on 18FDG-PET-CT in every case. The remarkable progression in the yield of 18FDG-PET-CT with advancing clinical stage and the correlation between PET-CT findings and prognosis are strong arguments that the overwhelming majority of patients classified as M+ on the basis of 18FDG-PET-CT have true-positive metastases.
Another limit is the single-institution design. These results thus need to be validated in independent settings. We checked that our series was representative. Phenotype distribution (TNBC, 27%; HER2-enriched, 20%; estrogen receptor–positive and HER2-negative, 51%) is roughly similar to other reports (29,30).
In conclusion, 18FDG-PET-CT has substantial yield in breast cancer patients with clinical stage IIB or higher breast cancer, and findings from this examination have prognostic value. It is hoped that the information provided at initial staging might lead to better management of these patients who account for the largest mortality rate from breast cancer.
The authors are solely responsible for the study design, data collection, analysis and interpretation of the data, writing the manuscript, and decision to submit the manuscript for publication. While this study was under way, we lost our colleague, Professor Jean-luc Moretti (1946–2010), head of the Nuclear Medicine Department at Saint-Louis Hospital, Paris, France.
References
- 1. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7thed. New York: Springer; 2010;. [Google Scholar]
- 2. Groheux D, Hindié E, Rubello D, et al. Should FDG PET/CT be used for the initial staging of breast cancer?. Eur J Nucl Med Mol Imaging. 2009; 36(10):1539–1542 [DOI] [PubMed] [Google Scholar]
- 3. Hindié E, Groheux D, Brenot-Rossi I, Rubello D, Moretti JL, Espié M. The sentinel node procedure in breast cancer: nuclear medicine as the starting point. J Nucl Med. 2011; 52(3):405–414 [DOI] [PubMed] [Google Scholar]
- 4. Yang WT, Le-Petross HT, Macapinlac H, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008; 109(3):417–426 [DOI] [PubMed] [Google Scholar]
- 5. Carkaci S, Macapinlac HA, Cristofanilli M, et al. Retrospective study of 18F-FDG PET/CT in the diagnosis of inflammatory breast cancer: preliminary data. J Nucl Med. 2009; 50(2):231–238 [DOI] [PubMed] [Google Scholar]
- 6. Alberini J-L, Lerebours F, Wartski M, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer. 2009; 115(21):5038–5047 [DOI] [PubMed] [Google Scholar]
- 7. Groheux D, Moretti J-L, Baillet G, et al. Effect of 18F-FDG PET/CT imaging in patients with clinical stage II and III breast cancer. Int J Radiat Oncol Biol Phys. 2008; 71(3):695–704 [DOI] [PubMed] [Google Scholar]
- 8. Fuster D, Duch J, Paredes P, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol. 2008; 26(29):4746–4751 [DOI] [PubMed] [Google Scholar]
- 9. Segaert I, Mottaghy F, Ceyssens S, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010; 16(6):617–624 [DOI] [PubMed] [Google Scholar]
- 10. Aukema TS, Straver ME, Peeters MJ, et al. Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II–III breast cancer. Eur J Cancer. 2010; 46(18):3205–3210 [DOI] [PubMed] [Google Scholar]
- 11. Groheux D, Giacchetti S, Espié M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011; 52(10):1526–3154 [DOI] [PubMed] [Google Scholar]
- 12. Koolen BB, Vrancken Peeters MJ, Aukema TS, et al. 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: comparison with conventional imaging techniques. Breast Cancer Res Treat. 2012; 131(1):117–126 [DOI] [PubMed] [Google Scholar]
- 13. Bourguet P, Hitzel A, Houvenaeghel G, et al. Synthesis bulletin of 2005 surveillance. Clinical practice recommendations: the use of PET-FDG in cancers of the breast, ovary and uterus. Bull Cancer. 2006; 93(4):385–390 [PubMed] [Google Scholar]
- 14. Yap CS, Seltzer MA, Schiepers C, et al. Impact of whole-body 18F-FDG PET on staging and managing patients with breast cancer: the referring physician’s perspective. J Nucl Med. 2001; 42(9):1334–1337 [PubMed] [Google Scholar]
- 15. Schirrmeister H, Kühn T, Guhlmann A, et al. Fluorine-18 2-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med. 2001; 28(3):351–358 [DOI] [PubMed] [Google Scholar]
- 16. Bellon JR, Livingston RB, Eubank WB, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC). Am J Clin Oncol. 2004; 27(4):407–410 [DOI] [PubMed] [Google Scholar]
- 17. Van der hoeven JJ, Krak NC, Hoekstra OS, et al. 18F-2-fluoro-2-deoxy-d-glucose positron emission tomography in staging of locally advanced breast cancer. J Clin Oncol. 2004; 22(7):1253–1259 [DOI] [PubMed] [Google Scholar]
- 18. Heusner TA, Kuemmel S, Hahn S, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009; 36(10):1543–1550 [DOI] [PubMed] [Google Scholar]
- 19. Nakamoto Y, Cohade C, Tatsumi M, Hammoud D, Wahl RL. CT appearance of bone metastases detected with FDG PET as part of the same PET/CT examination. Radiology. 2005; 237(2):627–634 [DOI] [PubMed] [Google Scholar]
- 20. Groheux D, Giacchetti S, Moretti J-L, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011; 38(3):426–435 [DOI] [PubMed] [Google Scholar]
- 21. Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012; 30(14):1715–1724 [DOI] [PubMed] [Google Scholar]
- 22. National Comprehensive Cancer Network Clinical practice guidelines in oncology: breast cancer. Version 2, 2012. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed July 11, 2012
- 23. Morris PG, Lynch C, Feeney JN, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010; 28(19):3154–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park HJ, Shin KH, Cho KH, et al. Outcomes of positron emission tomography–staged clinical N3 breast cancer treated with neoadjuvant chemotherapy, surgery, and radiotherapy. Int J Radiat Oncol Biol Phys. 2011; 81(5):689–695 [DOI] [PubMed] [Google Scholar]
- 25. Walker GV, Niikura N, Yang W, et al. Pretreatment staging positron emission tomography/computed tomography in patients with inflammatory breast cancer influences radiation treatment field designs. Int J Radiat Oncol Biol Phys. 2012; 83(5):1381–1386 [DOI] [PubMed] [Google Scholar]
- 26. Brito RA, Valero V, Buzdar AU, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol. 2001; 19(3):628–633 [DOI] [PubMed] [Google Scholar]
- 27. Groheux D, Hindié E, Giacchetti S, et al. Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med. 2012; 53(2):249–254 [DOI] [PubMed] [Google Scholar]
- 28. Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009; 115(2):423–428 [DOI] [PubMed] [Google Scholar]
- 29. Straver ME, Rutgers EJ, Rodenhuis S, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010; 17(9):2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Esserman LJ, Berry DA, Demichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 trial—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012; 30(26)3242–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]