Critical involvement of the leukocyte and 5-lipoxygenase metabolites in retinal capillary degeneration using a chimeric mouse model of diabetic retinopathy.
Keywords: 5-lipoxygenase, inflammation, transcellular synthesis
Abstract
Understanding the early pathogenesis of DR may uncover new therapeutic targets to prevent or slow the progression of this sight-threatening disorder. We investigated the role of leukocyte-mediated generation of LTs in regulation of retinal capillary degeneration and inflammation in the diabetic mouse. We generated (1) chimeric mice that lacked the ability to generate LTs by transplanting 5LO−/− bone marrow cells into ND.WT mice and into SD.WT mice and (2) “control” chimeric mice by transplanting WT bone marrow cells into 5LO−/− mice or into WT mice. Retinas from diabetic chimeric mice with WT marrow demonstrated capillary degeneration to the same extent as retinas from diabetic, nonchimeric WT mice. In contrast, retinas from diabetic chimeric mice with 5LO−/− marrow developed significantly less capillary degeneration and pericyte loss (P<0.05). In the retinas from chimeric mice with WT marrow, diabetes induced a rise in leukocyte adherence to the microvasculature, expression of the NF-κB p65 subunit, and ICAM1, superoxide generation, and retinal microvascular permeability, yet these characteristic responses were blunted by >50% in diabetic chimeras containing 5LO−/− leukocytes (P<0.05). Our data suggest the critical involvement of leukocytes and LTs in the regulation of inflammation and capillary degeneration in DR.
Introduction
DR is a slowly progressive disorder that threatens the vision of the millions of individuals living with diabetes mellitus and millions more at risk of developing diabetes mellitus. Whereas treatment to keep blood glucose levels and blood pressure near normal are the best therapies to date, this does not completely reduce the risk of developing the retinopathy [1–3]. Furthermore, long-term normoglycemia is not accomplished easily by the majority of individuals. Prevention and early intervention in the disease process are hindered by the clinically “silent” phase of the disease, with little clinically detectable lesions until a decade or more following diagnosis. During this early, nonproliferative phase, a histological exam reveals focal areas of capillary degeneration [4, 5]. This leads to areas of retinal ischemia and triggers the later vasoproliferative phase. Most therapies for DR target the proliferative phase of the disease [6–10]. Earlier intervention requires identifying critical cellular pathways leading to the silent initiation of capillary degeneration, and recent findings suggest that the leukocyte may play a role [11, 12].
Leukocytes participate in acute and chronic inflammatory responses throughout the body [13–19]. This is facilitated uniquely by their abilities to specifically target “injured” tissues, adhere to the vasculature, and locally secrete proinflammatory mediators. Animal studies of DR have presented strong evidence that a chronic inflammatory process within the retina results in the degeneration of retinal capillaries, a hallmark feature of early DR [12, 20–22]. The proinflammatory phenotype of the diabetic retina is characterized by increased leukocyte adherence to the retinal microvasculature and activation of inflammatory steps, such as NF-κB and ICAM1, in various retinal cells [12, 21, 22]. Increased permeability of the retinal microvasculature has also been reported [23, 24]. Several questions still remain regarding the role of the leukocyte response in mediating these alterations in inflammation and permeability within the diabetic retina, including what are the potential paracrine mediators of “cross-talk” between the intravascular leukocyte and the retinal microvascular endothelial cells, and what is the relative contribution of the leukocyte to this local inflammatory response and the development of histopathology?
LTs are potent mediators of inflammatory processes with autocrine and paracrine effects [25–27]. This diverse family of arachidonic acid metabolites includes: LTB4, a key chemokine involved in leukocyte recruitment to areas of tissue or vascular damage, and LTC4, a classic regulator of vascular permeability [27–30]. 5LO catalyzes the initial step in the synthesis of LTs and is principally expressed in leukocytes [26, 31, 32]. In the synthetic cascade, 5LO generates the short-lived intermediate LTA4, which can be metabolized further by the leukocyte or can be transferred to other neighboring cells, where the downstream metabolites LTB4 and LTC4 are generated [26]. Under various disease states, leukocytes can be activated to locally produce LTs that initiate and amplify the inflammatory process [14, 17, 19]. For example, LTB4 binds to BLT1, leading to activation of NF-κB inflammatory cascades, generation of cytokines, and production of ROS [33–36]. Our previous studies have suggested a role for LTs in the development of early stages of DR [20, 37]. Diabetic 5LO knockout mice develop less capillary degeneration than do WT diabetic mice [20]. More recently, we demonstrated that retinal glial cells robustly produce LTB4 and that microvascular endothelial cells can synthesize LTB4 and LTC4, but both require the transcellular transfer of the precursor LTA4 from another cellular source, as described above [37]. In addition, BLT1 expression is increased in the retina of diabetic mice, and retinal glial cells also demonstrate enhanced expression of BLT1 when exposed to high-glucose conditions [20, 37]. Clinically, vitreous samples from humans confirm the selective presence of elevated levels of 5LO metabolites in patients with early DR [38]. As 5LO is expressed predominantly in the leukocyte, these results collectively suggest that leukocytes are critical to the diabetes-induced, LT-mediated inflammation in the retina.
Our current experiments were designed to investigate the role of the leukocyte in the inflammatory response in the diabetic retina and the role of LTs as potential mediators of cross-talk between leukocytes and the retinal microvasculature. We generated chimeric mice that selectively lack the 5LO gene in cells (notably, leukocytes) derived from bone marrow. We now report that marrow-derived cells regulate the diabetes-induced inflammatory response in the mouse retina and that LT production may be an early and major contributor to the inflammatory response leading to DR.
MATERIALS AND METHODS
Animals
WT C57BL/6 mice and a breeding pair of B6.129S2-Alox5tm1Fun/J were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). When the mice, all males, were 20–25 g body weight (∼2 months of age), they were randomly assigned to become diabetic or remain as ND [20, 39, 40]. Diabetes was induced by sequential daily i.p. injections of a freshly prepared solution of streptozotocin in citrate buffer (pH 4.5) at 45 mg/kg body weight. Insulin was given as needed to achieve slow weight gain without preventing hyperglycemia and glucosuria (typically 0–0.2 U neutral protamine Hagedorn insulin s.c., zero to three times/week) [20, 39, 40]. The animals remained insulin-deficient but not grossly catabolic. The animals had free access to food and water and were maintained under a 14-h on/10-h off light cycle. Food consumption and body weight were measured weekly. Glycosylated hemoglobin was measured every 2–3 months to estimate the average level of hyperglycemia (Variant kit, Bio-Rad Laboratories, Hercules, CA, USA). Retinas were harvested at 3 months of diabetes duration for leukostasis and superoxide measurement and at 9 months of diabetes duration for retinal histopathology.
Generation of chimeric animals
The recipient mice were treated with two doses of 600 rads ionizing radiation at an interval of 3 h to kill endogenous bone marrow cells. Generation of chimeras took place following confirmation of diabetes induction (∼2 weeks after injection of streptozotocin) in mice to be recipients. Immediately after the final irradiation, bone marrow cells were harvested from femur and tibia of donor mice and suspended in PBS to give ∼20 million cells/mL PBS. Donor mice included WT mice and 5LO−/−. Each recipient mouse received 4 million cells by i.v. tail-vein injection within 2 h of irradiation. Recipient mice included: ND.WT, SD.WT, and SD.5LO−/− mice. The following chimeras were generated: 5LO−/− bone marrow injected into ND.WT and SD.WT mice, denoted as 5LO−/− → ND.WT and 5LO−/− → SD.WT; WT marrow injected into ND.5LO−/− and SD.5LO−/− mice, referred to as WT → ND.5LO−/− and WT → SD.5LO−/−; and WT marrow injected into ND.WT and SD.WT mice, labeled as WT → ND.WT and WT → SD.WT. At the time of death, the recipients' peripheral blood leukocytes were isolated as described below and analyzed for the presence or absence of 5LO mRNA by RT-PCR using the following primers: upstream (5-ATGGATGGAGTGGAACCCCGG-3) and downstream (5-CTGTACTTCCTGTTCTAAACT-3) of the neo insertion site in the mouse 5LO gene; β-actin primers were: upstream (5-CAGAAGGAGATTACTGCTCTGGCT-3) and downstream (5 GTGAGGGACTTCCTGTAACCACTT-3). With the use of a Peltier thermal cycler (PTC-200, MJ Research, Waltham, MA, USA), 35 PCR cycles were carried out at 95°C for 5 min, 94°C for 15 s, 57°C for 1 min, 72°C for 1 min, and a final extension of 72°C for 10 min. Reaction products were separated on a 1% agarose gel, and bands were visualized using ethidium bromide (0.1%). The gel was scanned using VersaDoc (Model 3000) imaging system from Bio-Rad Laboratories. Actin amplification was used as a positive control, and PCR was carried out in the absence of cDNA for each set of primers as a negative control.
Isolation of peripheral blood leukocytes
Mouse blood (500 μl) was collected by cardiac puncture in a vacutainer tube containing 3.6 mg K2 EDTA and mixed with 4 mL 1× RBC lysis buffer (eBioscience, San Diego, CA, USA). It was gently rocked for 5 min to facilitate RBC lysis and then centrifuged at 300 g for 7 min to sediment WBC in the bottom. The RBC lysis step was repeated. After carefully removing the supernatant, the WBCs were washed with PBS and used for PCR, Western blot, or for mass spectrometry of LT generation.
Western blot analysis
Leukocytes were homogenized in buffer containing protease inhibitors (leupeptin 1 μg/mL, aprotinin 1 μg/mL, 1 mM PMSF, and 0.2 mM Na3VO4) and protein concentration determined (Bio-Rad Laboratories). Equivalent amounts of sample proteins were loaded, separated by SDS-PAGE, and transferred to nitrocellulose membranes. The blots were blocked in 5% nonfat dry milk overnight at 4°C and then hybridized for 3 h with appropriate dilution of the primary antibody against BLT1. Blots were washed and incubated with suitable secondary antibody coupled to HRP (Bio-Rad Laboratories) at a dilution of 1:3000 for 1 h. After extensive washing, protein bands were visualized by ECL (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and evaluated by densitometry. Membranes then were stripped and reprobed with antibody against tubulin (Sigma Chemical, St. Louis, MO, USA) to confirm equal protein loading. Densitometric analysis was performed using the public domain NIH Image program developed at the U.S. National Institute of Health (Bethesda, MD, USA) with the Scion Image 1.63 program.
Mass spectrometry of LT generation
Cells were treated with the calcium ionophore A23187 to mobilize calcium for activation of cytosolic PLA2α, which cleaves arachidonic acid from membrane phospholipids and makes it available for LT synthesis [37, 41]. Cells were resuspended in HBSS (1 ml) with CaCl2 (2 mmol/L) and MgCl2 (0.5 mmol/L) and then treated with calcium ionophore A23187 dissolved in DMSO (0.5 μmol/L final concentration in buffer) for 10 min. The media were collected and the reaction terminated by addition of 1 mL ice-cold methanol containing labeled internal standards ([d4]LTB4 and [d8]5-HETE, 2 ng each; and [d5]LTC5, [d4]PGE2, and [d4]TXB2, 5 ng each). Samples were diluted with water to a final methanol concentration <15% and then extracted using a solid-phase extraction cartridge (Strata C18-E, 100 mg/1 ml; Phenomenex, Torrance, CA, USA). The eluate (1 ml methanol) was dried down and reconstituted in 40 μl HPLC solvent A (8.3 mM acetic acid buffered to pH 5.7 with NH4OH) plus 20 μl solvent B (acetonitrile/methanol, 65/35, v/v) [37, 41]. An aliquot of each sample (25 μl) was injected into a HPLC system, and separation of the different metabolites was conducted using a C18 column (Gemini, 150×2 mm 5 μM; Phenomenex) eluted at a flow rate of 200 μl/min with a linear gradient from 45% to 98% of mobile phase B. Solvent B was increased from 45% to 75% in 12 min, increased to 98% in 2 min, and held at 98% for a further 11 min before re-equilibration at 45%. The HPLC system was interfaced directly into the electrospray source of a triple-quadrupole mass spectrometer (Sciex API 3000, PE Sciex, Thornhill, ON, Canada), where mass spectrometric analyses were performed in a negative ion mode using multiple reaction monitoring of the specific mass-to-charge transitions: 335 → 195 for LTB4 and Δ6-trans-LTB4 isomers; 335 → 115 for 5,6-diHETE isomers; 624 → 272 for LTC4; 495 → 177 for LTD4; 438 → 333 for LTE4; 319 → 115 for 5-HETE; 351 → 271 for PGE2 and PGD2; 369 → 169 for TXB2; 629 → 72 for [d5]LTC4; 327 → 116 for [d8]5-HETE; 339 → 197 for [d4]LTB4; 373 → 173 for [d4]TXB2; and 355 → 275 for [d4]PGE2. Quantitation was performed using a standard isotope dilution curve, as described previously [37, 41].
Isolation of retinal blood vessels
Retinal vasculatures were isolated as described previously [5, 38–40, 42, 43]. Briefly, freshly isolated eyes were fixed with 10% neutral-buffered formalin. Following dissection, retinas were rinsed in water overnight and then incubated with 1 ml elastase solution (40 U/ml; Calbiochem, La Jolla, CA, USA) containing 0.1 M NaF, pH 6.5, 150 mM NaCl, and 5 mM EDTA at 37°C for 1 h. After elastase digestion, nonvascular cells were removed by gentle brushing, and the isolated vasculature was dried to a microscope slide, stained with hematoxylin and periodic acid-schiff, and analyzed for pathology.
Quantitation of acellular capillaries and pericyte ghosts
Acellular capillaries and pericyte ghosts were quantitated in eight fields in the midretina (40× magnifications) in a masked manner [20, 40]. Acellular capillaries were identified as capillary-sized vessel tubes having no nuclei anywhere along their length and were reported/mm2 of retinal area. Tubes with a diameter <20% of the diameter of adjacent capillaries or a length <40 μm were identified as strands and not counted as acellular capillaries. Pericyte ghosts were identified as spaces in the capillary basement membranes from which pericytes had disappeared. Approximately 1000 capillary cells in eight field areas in the midretina were evaluated in a masked manner, and the number of pericyte ghosts was reported/1000 capillary cells. Ghosts on any acellular capillary were excluded.
Quantitative measurement of leukostasis
The number of leukocytes adherent to the microvasculature was determined at 3 months of diabetes [11, 20]. Following cardiac catheterization, anesthetized mice (100 mg/ml Ketaset:100 mg/ml Xylazine=5:1) were exanguinated by perfusion with PBS. Fluorescein-coupled Con A lectin (20 μg/ml in PBS; Vector Laboratories, Burlingame, CA, USA) was then infused, as described previously. Flat-mounted retinas were viewed via fluorescence microscopy, and brightly fluorescent leukocytes were counted in the entire retina.
Immunostaining of retinal slices
Formalin-fixed, paraffin-embedded sections were deparaffinized using three changes of xylene. The tissue sections were then subjected to an antigen-retrieving protocol in sodium citrate buffer (10 mmol/l sodium citrate, 0.05% Tween 20, pH 6.0) by microwaving for 15 min (three times, 5 min each). The tissue endogenous peroxides were quenched using 3.0% hydrogen peroxide for 10 min, and nonspecific binding sites were blocked using 1.5% normal goat serum for 20 min (Vector Laboratories). The tissue sections were then incubated overnight with rabbit polyclonal antibody (1:100 in PBS) against NF-κB p65 (Santa Cruz Biotechnology) or ICAM-1 (rabbit polyclonal from Proteintech Group; Chicago, IL, USA). Unbound primary antibody was washed off using PBS containing 0.05% Tween 20. Biotinylated secondary anti-rabbit antibody (1:200 dilutions for 30 min; Vector Laboratories) and ABC reagent (30 min at room temperature; Vector Laboratories) were applied to the sections. DAB substrates with nickel enhancer were used to stain the sections. Processing time was identical among experimental groups. Sections were washed, counterstained with nuclear Fast Red, dehydrated, and permanently mounted using Permount solution. As performed previously for the staining intensity of the p65 subunit of NF-κB, sections were scored based on the intensity of the nuclear staining on a scale of one to four, with one (lightest gray), two (light gray), three (medium gray), and four (black) [20]. Sections stained for ICAM1 were similarly scored on a scale of one to four based on the intensity of staining of cell membranes [44]. All grading of slides was completed in a masked manner.
For retinal slices immunostained with antibody against albumin, the sections were incubated with 0.5% rabbit serum followed by sheep polyclonal to HSA (1:1000 in PBS; Abcam, Cambridge MA, USA) and then FITC-conjugated anti-sheep antibody (1:2000 in PBS; Abcam) [45]. The sections were washed extensively with PBS containing 0.05% Tween 20 and were mounted with mounting medium (Vectashield, Vector Laboratories) for fluorescence microscopy. Images were taken throughout the retinal layers using a Nikon microscope and fluorescence intensity averaged over 0.1 mm2 for each retinal slice using NIS-Elements software (Eclipse 80i and NIS-Elements AR 3.0, Tokyo, Japan).
Superoxide measurement
Fresh retinas from animals were analyzed for superoxide production as described previously [46]. Briefly, retinas were placed in 0.2 ml Krebs/HEPES buffer and allowed to equilibrate in the dark at 37°C under 95% O2/5% CO2 conditions for 30 min. To each tube, 25 μl lucigenin (0.5 mM; Sigma Chemical) was added, and the photon emission was detected for 10 s by a luminometer (Analytical Luminescence Laboratory, San Diego, CA, USA). Retinal protein was quantified/sample (Bio-Rad Laboratories), and the luminescence was expressed as RLU/mg protein.
Statistical analysis
All results are expressed as means ± sd. The data were analyzed by ANOVA, followed by Tukey's multiple comparison test. The only exception was analysis of immunohistochemistry data, which compared pairs of columns based on rank using Mann-Whitney. Differences were considered statistically significant when the P values were <0.05.
Assurances
All animal experiments were in accordance with the guidelines for the Treatment of Animals in Research outlined by the Association for Research in Vision and Ophthalmology.
RESULTS
Characterization of diabetic chimeric mice
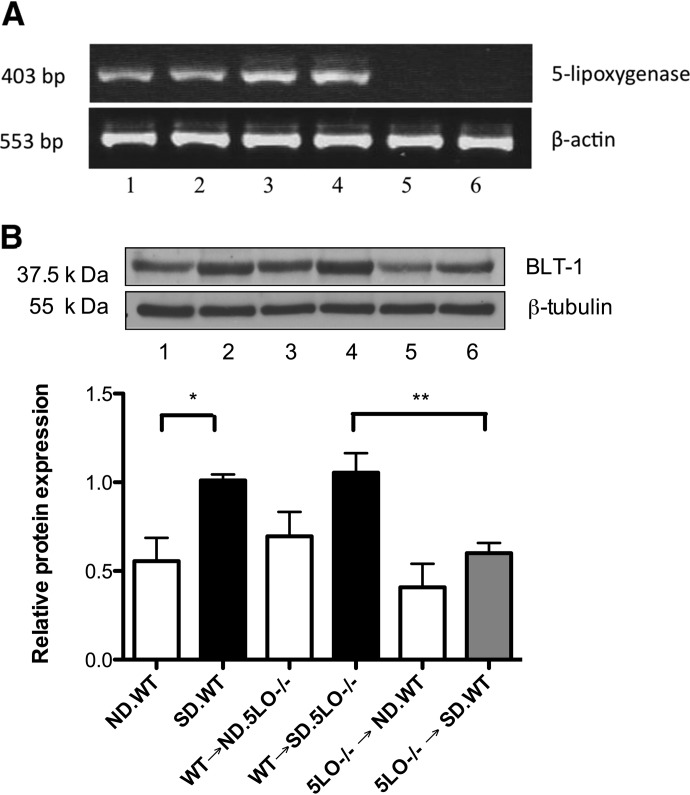
Generation of chimeras took place following confirmation of diabetes induction in mice to be recipients. Donor mice included WT mice and 5LO−/− mice. Recipient mice included: ND.WT, SD.WT, and SD.5LO−/− mice. The following chimeras were generated: 5LO−/− bone marrow injected into ND.WT and SD.WT mice, denoted as 5LO−/− → ND.WT and 5LO−/− → SD.WT; WT marrow injected into ND.5LO−/− and SD.5LO−/− mice, referred to as WT → ND.5LO−/− and WT → SD.5LO−/−; and WT marrow injected into ND.WT and SD.WT mice, labeled as WT → ND.WT and WT → SD.WT. Glycemia, as measured by glycohemoglobin levels, was unaffected by gene deletions or chimera formation (Table 1). As a confirmation of chimera formation, RT-PCR of peripheral blood leukocytes from these mice verified the absence of 5LO gene expression (Fig. 1A). As anticipated, WT and control chimeric mice—the latter generated using bone marrow cells from a WT mouse, specifically WT → ND.5LO−/− and WT → SD.5LO−/−—robustly expressed 5LO in their peripheral blood leukocytes (Fig. 1A). Previously, we demonstrated that hyperglycemia leads to enhancement of LT generation, especially LTB4. Accordingly, WT → ND.5LO−/− generated 3.45 ± 1.3 ng LTB4/million cells, whereas WT → SD.5LO−/− produced 6.57 ± 0.4 ng LTB4/million cells. In contrast to these results and in agreement with the lack of 5LO mRNA detection, stimulated bone marrow-derived cells from 5LO−/− → ND.WT and 5LO−/− → SD.WT were deficient in LT production and generated only 0.08 ± 0.01 ng LTB4/million cells and 0.08 ± 0.04 ng LTB4/million cells, respectively. Leukocytes from 5LO−/− → SD.WT chimeric mice developed less hyperglycemia-enhanced BLT1 expression compared with control chimeric mice, consistent with a decrease in LT-regulated inflammatory signaling in 5LO−/− → SD.WT chimeric mice (Fig. 1B).
Table 1. Glycohemoglobin Levels.
| Experimental group | GHba (average+sd%) |
|---|---|
| ND.WT | 3.32 ± 0.16% |
| 5LO−/− → ND.WT | 3.3 ± 0.16% |
| WT → ND.5LO−/− | 3.12 ± 0.16% |
| WT → ND.WT | 2.9 ± 0.2% |
| SD.WT | 11.42 ± 1.69% |
| 5LO−/− → SD.WT | 11.62 ± 1.31% |
| WT → SD.5LO−/− | 11.05 ± 1.26% |
| WT → SD.WT | 11.4 ± 0.7% |
GHb, Glycohemoglobin.
Figure 1. Analysis of 5LO synthesis and signaling cascades in the leukocytes of chimeric mice.
(A) RT-PCR showing the expression of 5LO mRNA (band separated on a 1% agarose gel and visualized using 0.1% ethidium bromide) in circulating leukocytes. Lane 1: ND.WT; lane 2, SD.WT; lane 3, WT → ND.5LO−/−; lane 4, WT → SD.5LO−/−; lane 5, 5LO−/− → ND.WT; and lane 6, 5LO−/− → SD.WT. As anticipated, there is a lack of 5LO mRNA in mice transplanted with 5LO−/− bone marrow. (B) Western blot analysis of BLT1 expression in leukocytes from chimeric mice and control mice demonstrated that mice carrying 5LO−/− leukocytes lack a diabetes-induced rise in BLT1 expression. Data are representative of four different mice from each group. *, **P<0.05.
Inhibition of diabetes-induced capillary degeneration in retinas from 5LO−/− → SD.WT chimeric mice
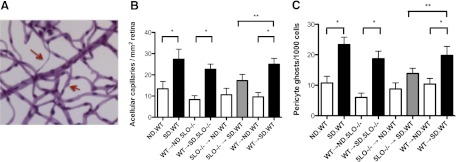
At a diabetes duration of 9 months, WT → SD.WT chimeric mice (designed to control for the process of chimera formation) demonstrated the expected diabetes-induced increase in capillary degeneration to the same extent as standard nonchimeric diabetic WT mice (Fig. 2A; P<0.05). Similarly, diabetic mice whose marrow-derived cells (but no other cells) contained 5LO (WT→SD.5LO−/− chimeric mice) demonstrated the diabetes-induced increase in the number of degenerate acellular capillaries compared with ND.WT → ND.5LO−/− mice (Fig. 2A; P<0.05). Notably, capillary degeneration and pericyte loss were inhibited by >25% in diabetic 5LO−/− mice in their marrow cells only (5LO−/−→SD.WT) compared with diabetic chimeric mice with circulating WT leukocytes (Fig. 2B and C; P<0.05). These data demonstrate that 5LO in marrow-derived cells, but not other cells in the body, played a critical role in the diabetes-induced degeneration of retinal capillaries.
Figure 2. Inhibition of diabetes-induced acellular capillary formation and pericyte loss in chimeric mice with circulating 5LO−/− leukocytes.
(A) Acellular capillaries (upper red arrow) and pericyte ghosts (lower red arrow) were quantitated in eight fields in the mid-retina (400× original magnification) in a masked manner. (B and C) Diabetic mice with WT circulating leukocytes (black bars) demonstrated a significant increase in the number of acellular capillaries (B) and pericyte loss (C) compared with matched ND control mice (white bars; *P<0.005), whereas diabetic chimeric mice with circulating 5LO−/− leukocytes were protected from the diabetes-induced increase in acellular capillary formation and pericyte loss despite a similar degree of hyperglycemia over the 9-month diabetes duration (gray bars; **P<0.05). Data represent eight to 12 mice/group.
Inhibition of retinal leukostasis in 5LO−/− → SD.WT chimeric mice
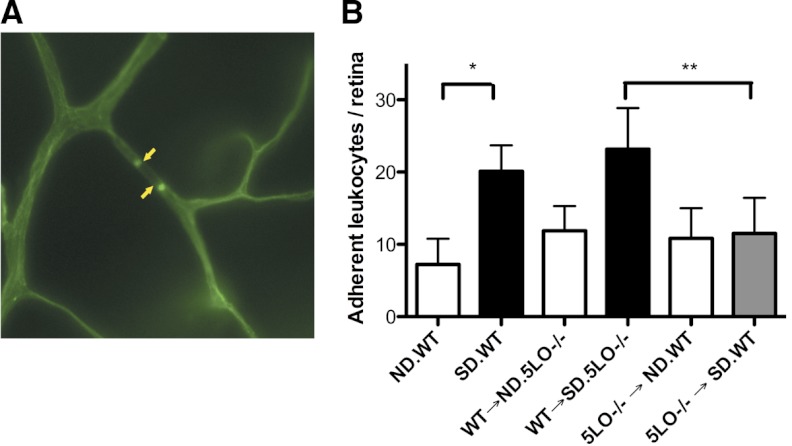
Retinas from diabetic mice compared with ND mice demonstrate an increase in the number of leukocytes adherent to the microvasculature. The enhanced adherence of leukocytes was reproduced well in the diabetic chimeric mice carrying WT leukocytes (Fig. 3; P<0.005). In contrast, retinas from 5LO−/− → SD.WT were protected from the diabetes-induced increase in leukocyte adherence (Fig. 3; P<0.005).
Figure 3. Leukostasis is diminished in chimeric mice with circulating 5LO−/− leukocytes.
The number of leukocytes adherent to the microvasculature or leukostasis was determined at 3 months of diabetes after perfusion with fluorescently labeled Con A. (A) Flat-mounted retinas were viewed via fluorescence microscopy, and brightly fluorescent leukocytes (yellow arrows) were counted in the entire retina. (B) The number of leukocytes adherent to the retinal microvasculature of the SD.WT and WT → SD.5LO−/− mice (black bars) was increased significantly compared with the retinas from ND control mice (white bars; *P<0.005) and 5LO−/− → SD.WT mice (gray bar; **P<0.05). Data represent six to eight mice.
Suppression of retinal markers of inflammation in 5LO−/− → SD.WT chimeric mice
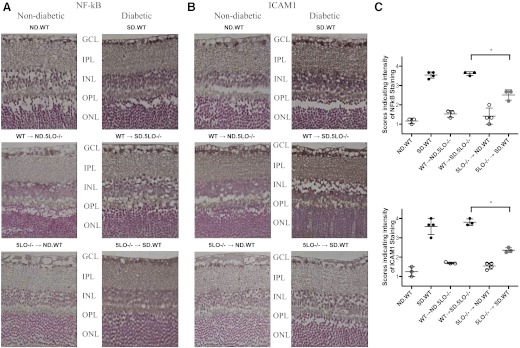
We examined the nuclear expression of the p65 subunit of NF-κB and one downstream target of NF-κB, ICAM1, by immunohistochemical analysis of paraffin-embedded sections of mouse retina. Retinas from diabetic WT mice and WT → SD.5LO−/− mice demonstrated at least a threefold increase in expression of the NF-κB p65 subunit and ICAM1 in the ganglion cell layer, inner nuclear layer, and outer nuclear layer when compared with retinas from ND WT mice (Fig. 4). Retinas from 5LO−/− → SD.WT mice uniformly expressed less of each of these markers (Fig. 4; P<0.05).
Figure 4. Inhibition of inflammatory markers in the retina of mice with circulating 5LO−/− leukocytes.
Sections of mouse retina were analyzed for expression of the p65 subunit of NF-κB (A) and ICAM1 (B) using immunohistochemistry and the intensity of the staining scored as described in Materials and Methods. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. (C) Increased expression of NF-κB and ICAM1 was detected in the retinas from diabetic mice with circulating WT leukocytes (black circles) that was not observed in ND mice (white circles) or diabetic mice with circulating 5LO−/− leukocytes (gray circles). Data represent three to four mice/group. *P < 0.05.
Decrease of retinal microvascular permeability in 5LO−/− → SD.WT chimeric mice
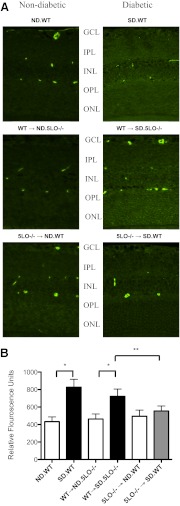
Vascular permeability was determined in retinal slices by immunofluorescent detection of extravasated endogenous albumin, a previously described marker of permeability in diabetic rodent models. Retinas from diabetic 5LO−/− → SD.WT chimeras demonstrated markedly less extravasation of albumin compared with WT and WT → SD.5LO−/− mice (Fig. 5; P<0.05).
Figure 5. Inhibition of retinal vascular permeability as measured by albumin extravasation.
Retinal sections were stained for albumin as mentioned in Materials and Methods, and the intensity of fluorescence for a 0.1-mm2 area of retina was measured using Eclipse 80i and NIS-Elements AR 3.0 software. (A) A representative immunofluorescent-stained retinal slice is shown from each group of mice. Diabetic mice with circulating WT leukocytes exhibited increased microvascular vascular leakage compared with the matched ND control mice, as indicated by intense staining for albumin throughout the retinal layers, whereas the ND and diabetic mice with circulating 5LO−/− leukocytes showed diminished microvascular leakage, as indicated by very faint staining for albumin. Punctate, intense staining corresponds with cross-sections of vessels with remaining intravascular albumin. (B) The average fluorescence for each group by selection of nonvessel-containing areas of each retinal layer is summarized in the accompanying graph. Data represent three to four mice/group. *, **P<0.05.
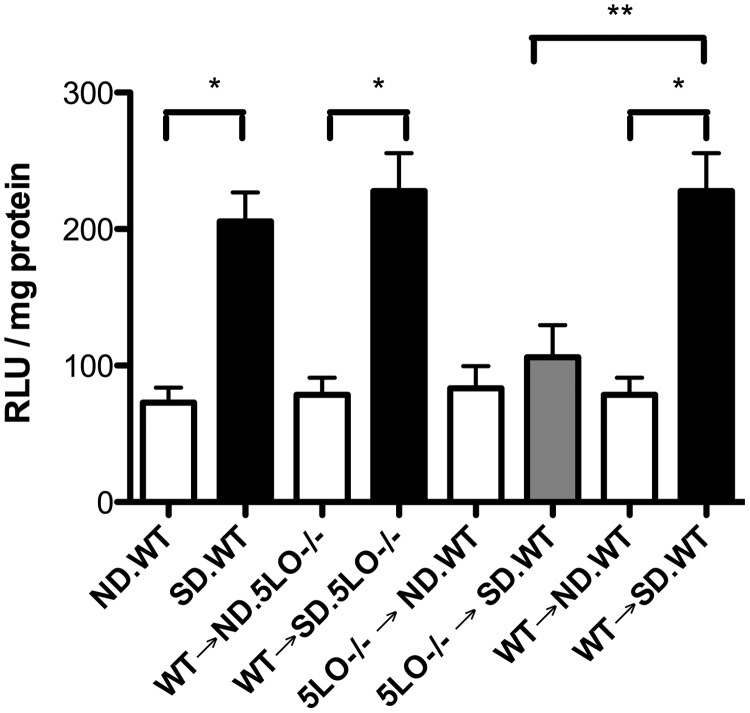
Superoxide generation is reduced in 5LO−/− → SD.WT chimeric mice
Retinas from diabetic mice containing 5LO in their marrow demonstrated a nearly 2.8-fold increase in superoxide production compared with ND mice with circulating WT leukocytes (Fig. 6; P<0.006). Retinas from 5LO−/− diabetic mice in marrow-derived cells (5LO−/−→SD.WT) did not show the diabetes-induced increase in superoxide generation (Fig. 6; P<0.005).
Figure 6. Inhibition of superoxide production by the retina from mice with circulating 5LO−/− leukocytes.
Freshly isolated retinas were incubated in Krebs-HEPES buffer as described in Materials and Methods, and the superoxide generation was measured by chemiluminescence using lucigenin. Superoxide production by the retina in ND (white bars) and 5LO−/− → SD.WT mice (gray bar) was diminished significantly compared with diabetic WT and WT → SD.5LO−/− (black bars; *,**P<0.05). Data represent six to eight samples/group.
DISCUSSION
Leukocytes are essential in development of the inflammatory response in many tissues, and this depends largely on their synthesis of the potent proinflammatory LTs [16, 47–52]. With the use of the chimeric mouse strategy and the SD mouse model, we have demonstrated that reduction of LT synthesis in chimeric mice, which lacked 5LO only in their marrow-derived cells, resulted in a decrease in the development of retinal microvascular capillary degeneration and pericyte loss, hallmark features of early DR. In addition to reduced retinal histopathology in diabetic 5LO−/− mice in their marrow-derived cells, multiple intermediary steps in the pathogenic cascade of capillary degeneration were affected, including: (1) less leukostasis in the retinal microvasculature; (2) less production of the proinflammatory mediators, specifically NF-κB, which promotes transcription of the cellular adhesion molecule ICAM1; (3) less retinal vascular permeability; and (4) less generation of superoxide production by the retinal cells. As leukocytes are the major cell type released by bone marrow, and prior studies have implicated inflammatory processes within leukocytes in diabetes-induced capillary cell death in diabetes, the data suggest that leukocytes and their unique production of LT precursors are critical initiators of the inflammatory response and histopathology of early DR [11, 12, 53].
It is important to emphasize that the experimental process leading to chimera formation did not alter the course of the diabetes-induced changes in the retinal microvasculature. Notably, the chimeric controls in which leukocytes from a WT “donor” were transplanted into the 5LO−/− “host” developed the characteristic capillary degeneration and inflammatory responses to the same extent as the diabetic, nonchimeric WT mice. The choice of the 5LO−/− mice as the host for these chimeric control mice excludes the possibility that any resident retinal cells were participating in the initiation of the LT-driven inflammatory response. This experimental design underscores the dependence of the retinal inflammatory response and capillary degeneration on a “signal” largely being driven by nonvascular cells traveling in the blood that is subsequently “transmitted” to the vascular and extravascular cells.
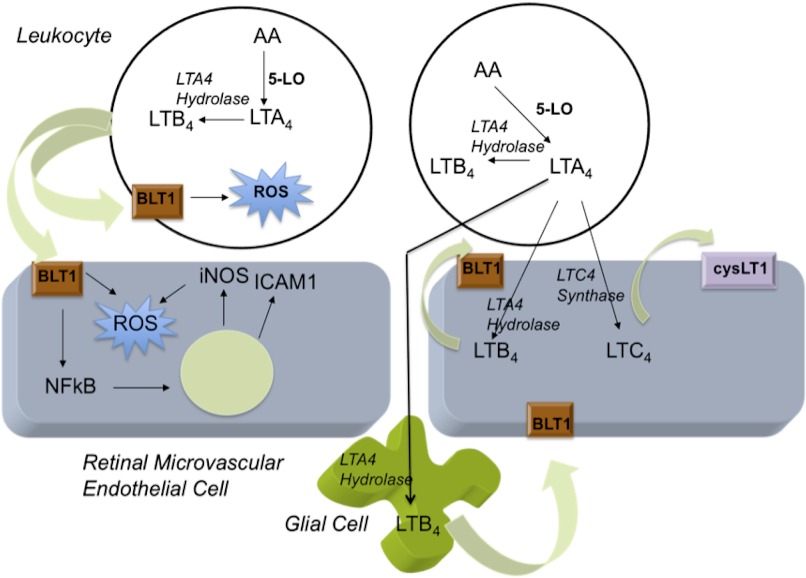
Transmission and amplification of this inflammatory signal apparently rely on paracrine mediators, such as the LTs. Our chimeric results confirm in vivo our prior in vitro investigations on transcellular biosythesis of LTs by retinal cells. Collectively, these studies support the following pathogenic model of LT synthesis and action in the retina of diabetic mice: (1) hyperglycemia primes leukocytes to generate 5LO metabolites including LTB4 (which promotes leukostasis and likely regulates the leukocyte's generation and release of superoxide) and LTA4 (which passes transcellulary to retinal endothelial cells, where it is converted to LTC4); (2) LTC4 binds to the cysLT1 receptor on the retinal microvascular endothelial cells, inducing vascular permeability; and (3) intraretinal LTB4 synthesis regulates proinflammatory cascades and superoxide generation in the retinal microvascular endothelial cell, apparently contributing to the sequelae of endothelial cell death and capillary degeneration (Fig. 7). The change in permeability likely permits leukocyte-pericyte and leukocyte-glial cell interaction to occur, facilitating LTA4 passage and further retinal LTB4 and LTC4 synthesis. Inherent to this model, the leukocyte may initiate the inflammatory response in the diabetic retina, but the development of chronic inflammation would rely on the resident retinal cells participating in the subsequent inflammatory response.
Figure 7. Proposed model of LT generation and action in the retina during diabetes.
Leukocytes initiate the inflammatory LT cascade. In diabetes, resident retinal cells amplify the generation of LTs. These mediators induce the generation of other proinflammatory molecules and superoxide via paracrine and autocrine actions. AA, arachidonic acid.
Our results replicate well what others have shown in relation to the inflammatory responses generated by the diabetic retina with increases in the expression of NF-κB and ICAM1 [21, 22, 34, 43]. BLT1 activation has been linked to activation of NF-κB and ICAM1 and generation of multiple proinflammatory cytokines [33–36, 54]. Our prior work demonstrated a diabetes-induced increase in synthesis of the LTB4 receptor BLT1 by the retina during diabetes, but we now extend that observation to include increased BLT1 expression in the leukocytes from diabetic mice. This suggests that leukocytes may serve as a peripheral “marker” of early retinal disease. Future studies will investigate whether a leukocyte-based assay of LT generation and/or BLT1 expression may serve as an effective biomarker of retinal disease in patients living with diabetes.
As in other inflammatory conditions, the leukocyte is a critical regulator of the inflammatory response in the retina during diabetes. LTs act as mediators of the inflammatory message, providing the necessary cross-talk from leukocyte to the retinal cells. The subsequent activation of proinflammatory cascades in retinal cells likely contributes to the chronic inflammation and oxidative stress that may ultimately damage the retinal capillaries. Inhibition of LT synthesis and signaling is a novel target for drug therapy to prevent DR.
ACKNOWLEDGMENTS
This work was supported by grants to R.A.G-K. from the National Eye Institute (K08EY16833, R01EY021535), to T.S.K. from the Medical Research Service of the U.S. Department of Veterans Affairs and U.S. National Institutes of Health (R01EY00300), and to R.M. from the U.S. National Institutes of Health (HL34303). Histology assistance was provided by Case Western Reserve University Visual Science Research Center Core Facilities (P30EY11373).
Footnotes
- 5LO
- 5-lipoxygenase
- 5LO−/−
- 5-lipoxygenase-deficient
- BLT1
- high-affinity leukotriene B4 receptor
- cysLT1
- cysteinyl leukotriene 1
- DR
- diabetic retinopathy
- LT
- leukotriene
- ND
- nondiabetic
- RBC
- red blood cell
- RLU
- relative light unit
- SD
- streptozotocin diabetic
- TXB2
- thromboxane B2
- WBC
- white blood cell
AUTHORSHIP
R.T. participated in the conception and design of the experiments and conducted most of the experiments. S.Z. participated in the analysis of leukotrienes. J.T. participated in preparation of elastase digests. G.L. performed the leukostasis assay. S.Z., R.M., and T.S.K. participated in the design of experiments. R.A.G-K. supervised the entire study, participated in the conception and design of the project, and wrote the manuscript.
REFERENCES
- 1. (1995) The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44, 968–983 [PubMed] [Google Scholar]
- 2. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2002) Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287, 2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. (2010) Preventing blindness due to diabetic retinopathy. Control glycaemia and blood pressure, and monitor the eyes. Prescrire Int. 19, 35–38 [PubMed] [Google Scholar]
- 4. Engerman R. L. (1989) Pathogenesis of diabetic retinopathy. Diabetes 38, 1203–1206 [DOI] [PubMed] [Google Scholar]
- 5. Tang J., Mohr S., Du Y. D., Kern T. S. (2003) Non-uniform distribution of lesions and biochemical abnormalities within the retina of diabetic humans. Curr. Eye Res. 27, 7–13 [DOI] [PubMed] [Google Scholar]
- 6. Abu El-Asrar A. M., Al-Mezaine H. S. (2010) Advances in the treatment of diabetic retinopathy. Discov. Med. 9, 363–373 [PubMed] [Google Scholar]
- 7. Schwartz S. G., Flynn H. W., Jr., Scott I. U. (2009) Pharmacotherapy for diabetic retinopathy. Expert Opin. Pharmacother. 10, 1123–1131 [DOI] [PubMed] [Google Scholar]
- 8. Scott I. U., Danis R. P., Bressler S. B., Bressler N. M., Browning D. J., Qin H., Diabetic Retinopathy Clinical Research Network (2009) Effect of focal/grid photocoagulation on visual acuity and retinal thickening in eyes with non-center-involved diabetic macular edema. Retina 29, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva P. S., Sun J. K., Aiello L. P. (2009) Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin. Ophthalmol. 24, 93–99 [DOI] [PubMed] [Google Scholar]
- 10. Subramanian M.L., Ness S., Abedi G., Ahmed E., Daly M., Feinberg E., Bhatia S., Patel P., Nguyen M., Houranieh A. (2009) Bevacizumab vs ranibizumab for age-related macular degeneration: early results of a prospective double-masked, randomized clinical trial. Am. J. Ophthalmol. 148, 875–882 [DOI] [PubMed] [Google Scholar]
- 11. Joussen A. M., Murata T., Tsujikawa A., Kirchhof B., Bursell S. E., Adamis A. P. (2001) Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol. 158, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joussen A. M., Poulaki V., Le M. L., Koizumi K., Esser C., Janicki H., Schraermeyer U., Kociok N., Fauser S., Kirchhof B., Kern T. S., Adamis A. P. (2004) A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 18, 1450–1452 [DOI] [PubMed] [Google Scholar]
- 13. Chew E.Y. (2011) Fatty acids and retinopathy. N. Engl. J. Med. 364, 1970–1971 [DOI] [PubMed] [Google Scholar]
- 14. Cuzzocrea S., Rossi A., Mazzon E., Di Paola R., Genovese T., Muià C., Caputi A. P., Sautebin L. (2005) 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab. Invest. 85, 808–822 [DOI] [PubMed] [Google Scholar]
- 15. Dahlen S. E. (2006) Treatment of asthma with antileukotrienes: first line or last resort therapy? Eur. J. Pharmacol. 533, 40–56 [DOI] [PubMed] [Google Scholar]
- 16. Letts L. G., (1987) Leukotrienes: role in cardiovascular physiology. Cardiovasc. Clin. 18, 101–113 [PubMed] [Google Scholar]
- 17. Mathis S., Jala V. R., Haribabu B. (2007) Role of leukotriene B4 receptors in rheumatoid arthritis. Autoimmun. Rev. 7, 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osher E., Weisinger G., Limor R., Tordjman K., Stern N. (2006) The 5 lipoxygenase system in the vasculature: emerging role in health and disease. Mol. Cell. Endocrinol. 252, 201–206 [DOI] [PubMed] [Google Scholar]
- 19. Peters-Golden M. (2008) Expanding roles for leukotrienes in airway inflammation. Curr. Allergy Asthma Rep. 8, 367–373 [DOI] [PubMed] [Google Scholar]
- 20. Gubitosi-Klug R. A., Talahalli R., Du Y., Nadler J. L., Kern T. S. (2008) 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 57, 1387–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kern T.S., (2007) Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007, 95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang W., Liu H., Al-Shabrawey M., Caldwell R. W., Caldwell R. B. (2011) Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res. 2, 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frey T., Antonetti D. A. (2011) Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid. Redox Signal. 15, 1271–1284 [DOI] [PubMed] [Google Scholar]
- 24. Chronopoulos A., Tang A., Beglova E., Trackman P. C., Roy S. (2010) High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes 59, 3159–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gijon M. A., Zarini S., Murphy R. C. (2007) Biosynthesis of eicosanoids and transcellular metabolism of leukotrienes in murine bone marrow cells. J. Lipid Res. 48, 716–725 [DOI] [PubMed] [Google Scholar]
- 26. Murphy R. C., Gijon M. A. (2007) Biosynthesis and metabolism of leukotrienes. Biochem. J. 405, 379–395 [DOI] [PubMed] [Google Scholar]
- 27. Samuelsson B. (2000) The discovery of the leukotrienes. Am. J. Respir. Crit. Care Med. 161, S2–S6 [DOI] [PubMed] [Google Scholar]
- 28. Samuelsson B. (1991) Arachidonic acid metabolism: role in inflammation. Z. Rheumatol. 50 (Suppl. 1), 3–6 [PubMed] [Google Scholar]
- 29. Samuelsson B. (1997) Some recent advances in leukotriene research. Adv. Exp. Med. Biol. 433, 1–7 [DOI] [PubMed] [Google Scholar]
- 30. Samuelsson B., Claesson H. E. (1990) Leukotriene B4: biosynthesis and role in lymphocytes. Adv. Prostaglandin Thromboxane Leukot. Res. 20, 1–13 [PubMed] [Google Scholar]
- 31. Samuelsson B., Funk C. D. (1989) Enzymes involved in the biosynthesis of leukotriene B4. J. Biol. Chem. 264, 19469–19472 [PubMed] [Google Scholar]
- 32. Samuelsson B., Haeggstrom J. Z., Wetterholm A. (1991) Leukotriene biosynthesis. Ann. N. Y. Acad. Sci. 629, 89–99 [DOI] [PubMed] [Google Scholar]
- 33. Bäck M., Bu D. X., Bränström R., Sheikine Y., Yan Z. Q., Hansson G. K. (2005) Leukotriene B4 signaling through NF-κB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc. Natl. Acad. Sci. USA 102, 17501–17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiu H., Johansson A. S., Sjöström M., Wan M., Schröder O., Palmblad J., Haeggström J. Z. (2006) Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc. Natl. Acad. Sci. USA 103, 6913–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sánchez-Galán E., Gómez-Hernández A., Vidal C., Martín-Ventura J. L., Blanco-Colio L. M., Muñoz-García B., Ortega L., Egido J., Tuñón J. (2009) Leukotriene B4 enhances the activity of nuclear factor-κB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc. Res. 81, 216–225 [DOI] [PubMed] [Google Scholar]
- 36. Xu S., Lu H., Lin J., Chen Z., Jiang D. (2010) Regulation of TNFα and IL1β in rheumatoid arthritis synovial fibroblasts by leukotriene B4. Rheumatol. Int. 30, 1183–1189 [DOI] [PubMed] [Google Scholar]
- 37. Talahalli R., Zarini S., Sheibani N., Murphy R. C., Gubitosi-Klug R. A. (2010) Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 51, 1699–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwartzman M. L., Iserovich P., Gotlinger K., Bellner L., Dunn M. W., Sartore M., Grazia Pertile M., Leonardi A., Sathe S., Beaton A., Trieu L., Sack R. (2010) Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes 59, 1780–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du Y., Miller C. M., Kern T. S. (2003) Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic. Biol. Med. 35, 1491–1499 [DOI] [PubMed] [Google Scholar]
- 40. Zheng L., Du Y., Miller C., Gubitosi-Klug R. A., Ball S., Berkowitz B. A., Kern T. S. (2007) Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 50, 1987–1996 [DOI] [PubMed] [Google Scholar]
- 41. Murphy R. C., Barkley R. M., Zemski Berry K., Hankin J., Harrison K., Johnson C., Krank J., McAnoy A., Uhlson C., Zarini S. (2005) Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal. Biochem. 346, 1–42 [DOI] [PubMed] [Google Scholar]
- 42. Kern T. S., Engerman R. L. (2001) Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes 50, 1636–1642 [DOI] [PubMed] [Google Scholar]
- 43. Zheng L., Szabo C., Kern T. S. (2004) Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κB. Diabetes 53, 2960–2967 [DOI] [PubMed] [Google Scholar]
- 44. Hattori T., Matsubara A., Taniguchi K., Ogura Y. (2010) Aldose reductase inhibitor fidarestat attenuates leukocyte-endothelial interactions in experimental diabetic rat retina in vivo. Curr. Eye Res. 35, 146–154 [DOI] [PubMed] [Google Scholar]
- 45. Ellis E. A., Grant M. B., Murray F. T., Wachowski M. B., Guberski D. L., Kubilis P. S., Lutty G. A. (1998) Increased NADH oxidase activity in the retina of the BBZ/Wor diabetic rat. Free Radic. Biol. Med. 24, 111–120 [DOI] [PubMed] [Google Scholar]
- 46. Kowluru R. A., Kowluru V., Xiong Y., Ho Y. S. (2006) Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic. Biol. Med. 41, 1191–1196 [DOI] [PubMed] [Google Scholar]
- 47. Banasiak N. C., Meadows-Oliver M. (2005) Leukotrienes: their role in the treatment of asthma and seasonal allergic rhinitis. Pediatr. Nurs. 31, 35–38 [PubMed] [Google Scholar]
- 48. Chen M., Lam B. K., Kanaoka Y., Nigrovic P. A., Audoly L. P., Austen K. F., Lee D. M. (2006) Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 203, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dahlen S.E., Haeggstrom J. Z., Samuelsson B., Rabe K. F., Leff A. R. (2000) Leukotrienes as targets for treatment of asthma and other diseases. Current basic and clinical research. Am. J. Respir. Crit. Care Med. 161, S1. [DOI] [PubMed] [Google Scholar]
- 50. del Giudice M. M., Pezzulo A., Capristo C., Alterio E., Caggiano S., de Benedictis D., Capristo A. F. (2009) Leukotriene modifiers in the treatment of asthma in children. Ther. Adv. Respir. Dis. 3, 245–251 [DOI] [PubMed] [Google Scholar]
- 51. Di Lorenzo G., Pacor M. L., Mansueto P., Esposito-Pellitteri M., Ditta V., Lo Bianco C., Leto-Barone M. S., Di Fede G., Rini G. B. (2006) Is there a role for antileukotrienes in urticaria? Clin. Exp. Dermatol. 31, 327–334 [DOI] [PubMed] [Google Scholar]
- 52. Sharma J. N., Mohammed L. A. (2006) The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology 14, 10–16 [DOI] [PubMed] [Google Scholar]
- 53. Li G., Wang X., Talahalli R., Gubitosi-Klug R. A., Kern T. S. (2012) Bone marrow-derived cells play a critical role in the development of early stages of diabetic retinopathy and tactile allodynia in mice. Diabetes, doi: 10.2337/db11-1249 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patarroyo M., Prieto J., Rincon J., Timonen T., Lundberg C., Lindbom L., Asjö B., Gahmberg C. G. (1990) Leukocyte-cell adhesion: a molecular process fundamental in leukocyte physiology. Immunol. Rev. 114, 67–108 [DOI] [PubMed] [Google Scholar]