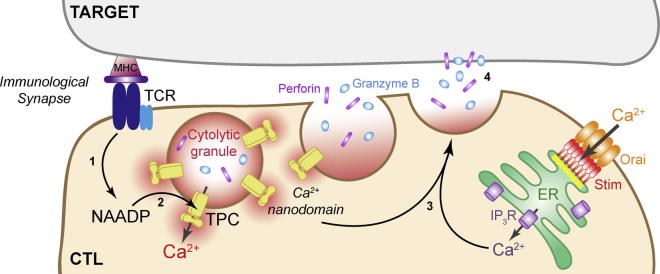
Summary
A cytotoxic T lymphocyte (CTL) kills an infected or tumorigenic cell by Ca2+-dependent exocytosis of cytolytic granules at the immunological synapse formed between the two cells. Although inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release from the endoplasmic reticulum activates the store-operated Ca2+-influx pathway that is necessary for exocytosis, it is not a sufficient stimulus [1–4]. Here we identify the Ca2+-mobilizing messenger nicotinic acid adenine dinucleotide phosphate (NAADP) and its recently identified molecular target, two-pore channels (TPCs) [5–7], as being important for T cell receptor signaling in CTLs. We demonstrate that cytolytic granules are not only reservoirs of cytolytic proteins but are also the acidic Ca2+ stores mobilized by NAADP via TPC channels on the granules themselves, so that TPCs migrate to the immunological synapse upon CTL activation. Moreover, NAADP activates TPCs to drive exocytosis in a way that is not mimicked by global Ca2+ signals induced by IP3 or ionomycin, suggesting that critical, local Ca2+ nanodomains around TPCs stimulate granule exocytosis. Hence, by virtue of the NAADP/TPC pathway, cytolytic granules generate Ca2+ signals that lead to their own exocytosis and to cell killing. This study highlights a selective role for NAADP in stimulating exocytosis crucial for immune cell function and may impact on stimulus-secretion coupling in wider cellular contexts.
Graphical Abstract
Highlights
► The T cell receptor mobilizes acidic Ca2+ stores via NAADP/two-pore channels (TPCs) ► Exocytotic granules are themselves acidic Ca2+ stores decorated with TPCs ► TPCs move to the immunological synapse upon TCR activation ► NAADP stimulates exocytosis more efficiently than does IP3/Ca2+ influx alone
Results and Discussion
The compartmentation of cell signaling pathways is important for maintaining the fidelity between extracellular stimuli and appropriate downstream responses. For Ca2+ signaling, not all sources (or patterns) of intracellular Ca2+ are equivalent, and Ca2+ channels can differentially couple to cellular processes [1, 8]. In T cells, the exocytosis of cytolytic factors is clearly Ca2+ dependent but, although Ca2+ influx via STIM/Orai is a necessary pathway, it is not a sufficient stimulus for exocytosis because it requires the additional activation of protein kinases [2, 9]. We investigated whether other Ca2+ channels couple more directly to exocytosis. All three major Ca2+-mobilizing second messengers—inositol 1,4,5-trisphosphate (IP3), cyclic ADP-ribose (cADPR), and nicotinic acid adenine dinucleotide phosphate (NAADP)—increase in concentration following T cell receptor activation [10, 11]. However, the molecular and organellar targets of NAADP are controversial in T cells [12], and the relationship between NAADP and exocytosis is unknown. To that end, we have investigated whether NAADP-dependent Ca2+ signaling via a recently identified molecular target, the two-pore channel (TPC) [5–7, 13], is an overlooked molecular component of T cell cytolytic granule exocytosis.
NAADP Mobilizes Acidic Ca2+ Stores in Cytotoxic T Lymphocytes
First, using a pharmacological approach, we characterized the intracellular Ca2+ stores in a primary human cytotoxic T lymphocyte (CTL) clone and found pH-neutral as well as acidic Ca2+ stores. Neutral stores were revealed by ionomycin, a Ca2+ ionophore that can only act at neutral organelles [14], and cyclopiazonic acid (CPA), a sarcoendoplasmic reticulum Ca2+-ATPase inhibitor [15] (see Figures S1B–S1D available online). Similarly, agents that release Ca2+ from acidic organelles also produced Ca2+ responses, i.e., bafilomycin A1 (a V-type H+-ATPase inhibitor), nigericin and monensin (protonophores), and glycyl-phenylalanine 2-naphthylamide (GPN, which osmotically lyses lysosomes [16]) (Figures S1E–S1I). The presence of acidic Ca2+ stores is crucial because NAADP is predominantly (though not universally [12]) accepted to release Ca2+ from acidic (endolysosomal) Ca2+ stores [13, 17], thereby distinguishing it from IP3 and cADPR that target the neutral endoplasmic reticulum (ER).
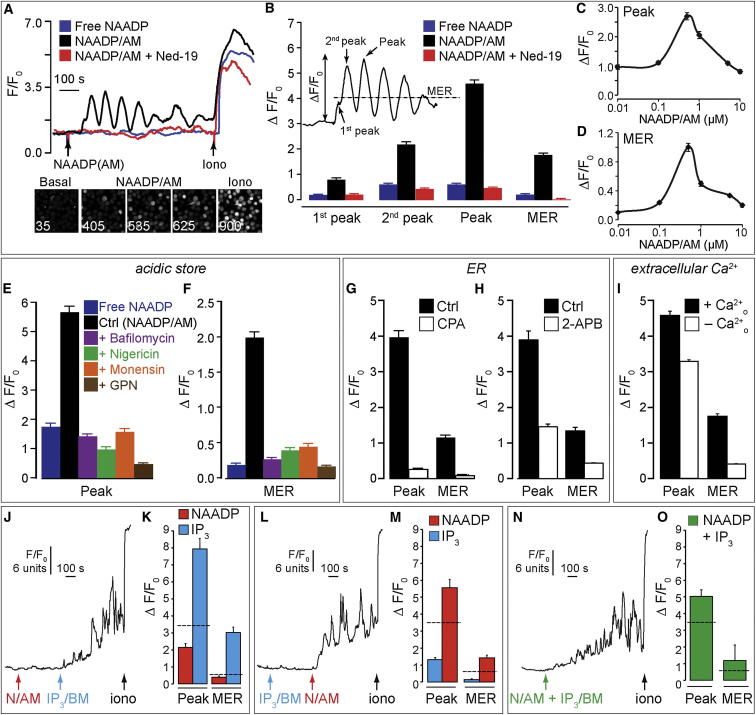
To test whether NAADP could evoke Ca2+ signals in CTLs, we indirectly introduced NAADP into the cytosol by bath application of a membrane-permeant ester precursor (NAADP/AM) that is converted to NAADP by intracellular esterases. NAADP/AM elicited Ca2+ oscillations, with the initial spike commonly comprising distinct first and second phases (Figures 1A and 1B); oscillations were blocked by the NAADP antagonist trans-Ned-19 (Figures 1A and 1B) [18]. The response to NAADP/AM was not an artifact of contaminating nonesterified (free) NAADP acting at plasma membrane receptors because equimolar extracellular NAADP was without effect (Figures 1A and 1B). Strikingly, NAADP/AM-induced Ca2+ release displayed a “bell-shaped” concentration-response curve (Figures 1C and 1D), which is a hallmark of this second messenger [19]. Repeating experiments in Ca2+-free medium (to eliminate Ca2+ influx), NAADP/AM still caused Ca2+ oscillations, thus confirming intracellular stores as the primary Ca2+ source (Figures S1J–S1N). That NAADP required acidic Ca2+ stores was confirmed by inhibition of oscillations by bafilomycin A1, nigericin, monensin, or GPN (Figures 1E and 1F). Given that Ned-19 acts upon non-ER Ca2+ channels [18], this provides compelling evidence that NAADP targets acidic organelles in primary CTLs, contrasting with results in a Jurkat cell line [20].
Figure 1.
NAADP-Induced Ca2+ Release from Acidic Intracellular Stores in Cytotoxic T Lymphocytes
(A) Single-cell Ca2+ traces normalized to initial fluorescence (F/F0). Bottom panels show fluorescence confocal images of fluo-3-loaded cytotoxic T lymphocytes (CTLs) taken at the time indicated in seconds at basal, during NAADP/AM application and upon addition of 1 μM ionomycin. NAADP/AM (2.5–10 μM) elicited Ca2+ oscillations, which were blocked by the NAADP antagonist trans-Ned-19 (10 μM). No Ca2+ responses were observed in response to 2.5–10 μM nonesterified (free) NAADP/DMSO.
(B) Fluorescence changes (ΔF/F0) were calculated as shown in the inset. MER (mean elevated ratio) was defined as the mean fluorescence divided by F0 for the period after the addition of NAADP/AM.
(C and D) NAADP/AM-induced Ca2+ release displayed a “bell-shaped” concentration-response curve, characteristic of this second messenger.
(E and F) Ca2+ signals to 2.5–10 μM NAADP/AM were inhibited by preincubation with 1 μM bafilomycin A1, 10 μM nigericin, 1 μM monensin, or 50 μM GPN. n = 383−1,030 cells.
(G and H) CTLs were treated with 10 μM CPA in Ca2+-free buffer (G) or 2 μM 2-APB (H) prior to application of 10 μM NAADP/AM. n = 81–178 cells.
(I) Ca2+ signals with 2.5–10 μM NAADP/AM in 0.5 mM extracellular Ca2+ (+Ca2+o) or Ca2+-free medium containing 100 μM EGTA (–Ca2+o). n = 730–1,030 cells.
(J–O) Traces from single cells (J, L, and N) and Ca2+ changes (K, M, and O) (n = 66–99 cells) upon addition of 1 μM NAADP/AM followed by 5 μM IP3/BM (J and K) or vice versa (L and M). Cells responded to ionomycin (1 μM) at the end of the recording. The dotted line represents the sum of NAADP/AM alone and IP3/BM alone responses. In (N) and (O), 5 μM IP3/BM and 1 μM NAADP/AM were added simultaneously. ∗∗∗p < 0.001 versus Ctrl. All phases of the Ca2+ signal for free NAADP versus NAADP/AM + inhibitor are not significantly different.
Error bars are mean ± SEM. See also Figure S1.
The long-standing “trigger hypothesis” or two-pool model [13, 21] describes NAADP as a provider of an initial “trigger” bolus of Ca2+ that is subsequently amplified by Ca2+ release from the ER by virtue of the Ca2+ sensitivity of the IP3 receptor (IP3R) or ryanodine receptor (RyR), so-called Ca2+-induced Ca2+ release (CICR). We tested for the coinvolvement of the ER, first by depleting the ER with the Ca2+-ATPase inhibitor CPA, which did indeed abrogate NAADP/AM responses (Figures 1G, S2C, and S1D). Also consistent with ER involvement is the fact that NAADP/AM stimulated Ca2+ influx (Figure 1I), a natural consequence of ER depletion and recruitment of the dominant store-regulated Ca2+ entry pathway in T cells.
Given that IP3R1–3 and RyR1 were all detected by PCR in these primary T cells (Figures S3A and S3B), we tested which ER channel family was functionally important. We found no evidence of functional RyRs, either with or without NAADP/AM (Figures S1O–S1Q), conceivably due to a low RyR abundance [22]. In contrast, using a cell-permeant analog of the major Ca2+-mobilizing second messenger IP3 (IP3/BM), IP3Rs were demonstrably active (Figures 1J–1O, 2G, 2H, S2A, and S2B), and complementary pieces of evidence suggested that they contribute to NAADP-evoked responses: first, IP3R blockade with 2-APB (2-aminoxydiphenylborate) (Figures S2A and S2B) [23] profoundly reduced NAADP/AM-stimulated Ca2+ oscillations (Figure 1H). Conversely, costimulating IP3Rs enhanced NAADP/AM-induced Ca2+ release (Figures 1J–1O), and in a synergistic (greater than additive) manner that depended upon the order of addition, with the greatest effect occurring when NAADP was added first. Together, these data are consistent with the trigger hypothesis whereby NAADP provides the trigger Ca2+ from acidic stores that is subsequently amplified by IP3Rs on the ER [13, 19].
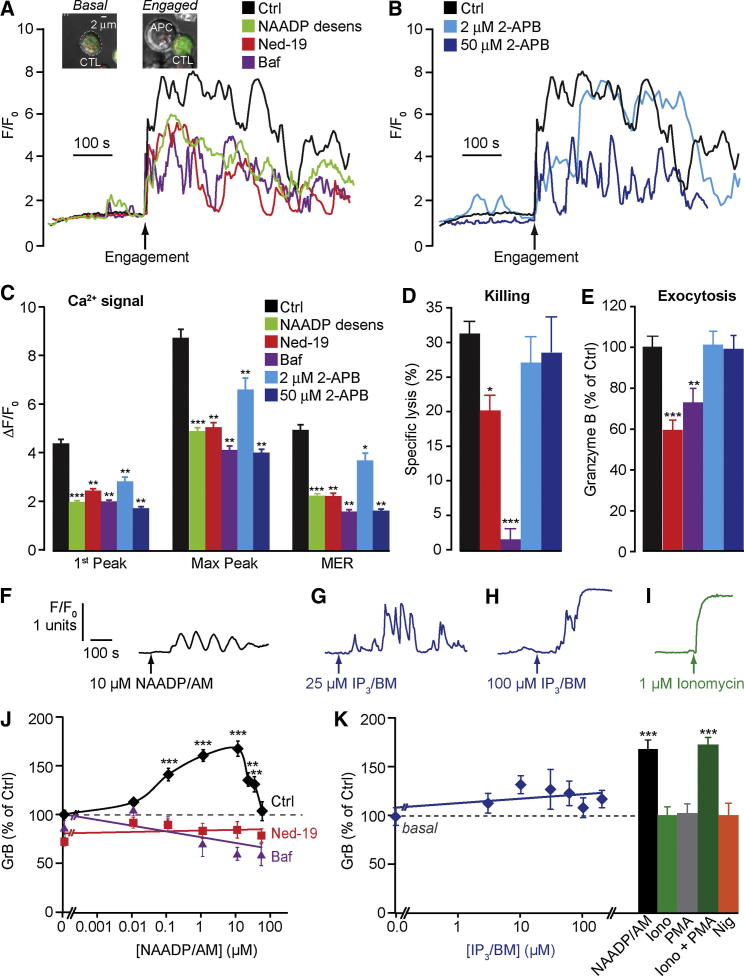
Figure 2.
NAADP Is Involved in Physiological T Cell Receptor Activation
(A–C) Ca2+ signals in CTLs upon engagement with antigen-presenting cells (APCs) loaded with 100 nM ESO 9C peptide. CTLs were incubated with 0.1% DMSO (Ctrl), 10 μM trans-Ned-19, 1 μM bafilomycin A1, or 2 μM or 50 μM 2-APB prior to engagement and throughout the experiment.
(A and B) Single-cell Ca2+ traces. Inset: confocal images of CTL loaded with fluo-3 and Lysotracker Red to label acidic organelles before (basal) and after engagement with APCs.
(C) Peak Ca2+ changes (ΔF/F0) and MER (mean value of fluorescence throughout the postengagement period) as normalized to F0. n = 130–239 cells.
(D) Lysis of target cells pulsed with ESO 9C peptide by CTL (measured by release of 51Cr after 2 hr) under conditions as in (A)–(C). n = 6.
(E) Granzyme B secretion by CTLs 15 min after antigen presentation, under conditions as in (A)–(C) and measured by ELISA. n = 6–15.
(F–I) Ca2+ signal upon addition of NAADP/AM (F), IP3/BM (G and H), and ionomycin (I) to fluo-3-loaded CTL.
(J and K) In the absence of antigen presentation, CTLs were stimulated with NAADP/AM in the presence of 0.1% DMSO (Ctrl), 10 μM trans-Ned-19, or 1 μM bafilomycin A1 (J); CTLs were treated with IP3/BM, 10 μM NAADP/AM, 10 μM nigericin, 1 μM ionomycin, 50 nM PMA, or a combination of 1 μM ionomycin and 50 nM PMA (K). After 30 min, supernatants were assayed for granzyme B by ELISA (n = 3).
∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05. Error bars are mean ± SEM. See also Figure S2.
The T Cell Receptor Recruits the NAADP Pathway
Having defined NAADP signaling in CTLs, we determined whether it was involved during physiological T cell receptor (TCR) activation. CTLs were stimulated upon engagement with peptide-loaded antigen-presenting cells (APCs), which resulted in the expected two phases of Ca2+ increase: an immediate spike (Ca2+ release from intracellular stores), followed by a slower, sustained increase or Ca2+ oscillations (dependent on Ca2+ influx; Figures 2A and 2B [24]). Acidic Ca2+ stores and NAADP did indeed contribute to TCR-induced Ca2+ signals as judged by the effect of inhibitors of the NAADP pathway: Ned-19 or bafilomycin A1 and NAADP self-desensitization profoundly reduced the amplitudes of the Ca2+ peaks as well as the mean fluorescence (an index of how sustained the response is; Figures 2A and 2C). In addition to NAADP, IP3Rs and Ca2+ influx were also recruited as expected (and required; Figure 2B): 2-APB attenuated Ca2+ oscillations at low (2 μM) and high (50 μM) concentrations, respectively (Figure 2C). Together, these results highlight a physiological role for NAADP-induced Ca2+ release that interleaves with Ca2+-induced Ca2+ release (IP3Rs) and Ca2+ influx.
NAADP Stimulates Cytolytic Granule Exocytosis
We next asked whether NAADP/acidic stores were important for exocytosis and target cell killing. CTL-dependent killing of target cells can be effected by two processes: (1) the Ca2+-dependent vectorial exocytosis of cytolytic proteins granzyme B and perforin from CTL cytolytic granules (a type of secretory lysosome [25]), and (2) the cell-surface expression of the death receptor Fas ligand [26]. A link between Ca2+ influx and cytolytic granule exocytosis is well established [3], but a role for acidic Ca2+ stores in driving secretion is less understood. Therefore, we measured granzyme B secretion at early times (15–30 min) after APC engagement. Testing for NAADP and acidic Ca2+ stores, we found that Ned-19 or bafilomycin A1 substantially inhibited granzyme B exocytosis (Figure 2E) and this translated into an inhibition of cell killing (Figure 2D). Importantly, this was not merely a consequence of a reduced global Ca2+ signal, because 2 μM and 50 μM 2-APB also lowered the TCR Ca2+ signal to the same extent (Figure 2C) and yet secretion and cell killing were unaffected (Figures 2D and 2E). Bafilomycin A1 had a disproportionate effect upon killing compared to exocytosis, which is probably due to an additional effect upon the target cells themselves: bafilomycin A1 (and other V-ATPase inhibitors) have been shown to profoundly block the perforin-dependent cytotoxicity mediated by CTLs [27]. In keeping with the Ca2+ data, 10 μM ryanodine did not affect TCR-stimulated granzyme B release (93% ± 8% of control, n = 8), excluding a role for RyRs in exocytosis. In summary, these data implicate NAADP in TCR-stimulated exocytosis.
We directly tested whether NAADP could stimulate exocytosis, even in the absence of TCR activation. Remarkably, application of NAADP/AM per se was sufficient to evoke granzyme B secretion in a Ned-19- and bafilomycin A1-sensitive manner (Figure 2J). Moreover, the hallmark bell-shaped NAADP/AM concentration-response curve previously observed with Ca2+ signals (Figures 1C and 1D) was preserved with granzyme B exocytosis (Figure 2J). Together, the data indicate that NAADP can and does drive exocytosis.
If NAADP simply acts by elevating global Ca2+, then any experimental manipulation that mimics this should stimulate exocytosis. Therefore, we raised Ca2+ in other ways and assessed secretion. IP3/BM evoked robust Ca2+ signals in CTLs, and in a classic pattern dependent on the stimulus intensity: lower concentrations of IP3/BM stimulated oscillations, whereas higher concentrations gave a strong peak-and-plateau Ca2+ pattern (indicative of Ca2+ release and influx through Orai/STIM activation; Figures 2G and 2H). Although IP3-induced Ca2+ signals mimic those seen following stimulation by physiological TCR engagement [24] or NAADP/AM (Figures 2A and 2F), granzyme B secretion was not observed (Figure 2K). Likewise, the Ca2+ ionophore ionomycin produced a strong Ca2+ signal (Figure 2I) but failed to evoke exocytosis (unless combined with phorbol esters to activate PKC/ERK [4, 26]; Figure 2K). Interestingly, releasing Ca2+ from the acidic Ca2+ store using nigericin (Figure S1E) was not sufficient to evoke exocytosis, reinforcing the unique properties of NAADP (Figure 2K). That is, in the absence of TCR activation, only NAADP-induced Ca2+ release coupled efficiently to exocytosis, thereby reinforcing the different roles of second messengers, i.e., NAADP and IP3 [13, 19].
What underlies this differential coupling? A major difference between NAADP and the other agents is that NAADP ultimately recruits three Ca2+ sources: acidic Ca2+ stores (Figures 1E and 1F), the ER (Figures 1G and 1H), and, consequently, extracellular Ca2+ (Figure 1I). In contrast, IP3 and ionomycin only share the latter two (neither mobilizes acidic Ca2+ stores [14]). This suggests that Ca2+ release from acidic stores by NAADP is a crucial permissive factor for exocytosis. Indeed, although Ca2+ influx via Orai/STIM is crucial for lymphocyte function, it cannot drive exocytosis without the pharmacological activation of protein kinases [2, 9]. Therefore, NAADP is the first Ca2+ pathway demonstrated to drive exocytosis without additional stimuli. Given that the global Ca2+ signal is a poor predictor of exocytosis, we hypothesize that local Ca2+ nanodomains around the acidic Ca2+ stores are what distinguishes NAADP from the other stimuli. This underscores the importance of where the Ca2+ is released, i.e., local versus global Ca2+ responses, and so we turned to identifying the molecular target of NAADP, and thereby its subcellular locale.
NAADP-Dependent Ca2+ Signals during TCR Activation Require Two-Pore Channels
Recently, the major ion channel target for NAADP has been identified as members of the TPC family that appropriately reside on acidic compartments of the endolysosomal system in various mammalian cell types [5–7]. To address the importance of TPCs in TCR activation, we used Jurkat cells transduced with the NY-ESO-1157–165-HLA A2-restricted TCR 1G4 [25], hereafter referred to as Jurkat 1G4 cells, because they are amenable to genetic manipulation and express the 1G4 TCR, which has the same specificity as the TCR expressed by the 4D8 CD8+ T cell clone used above. We established that Jurkat 1G4 cells mirrored the 4D8 T cell clone in several key aspects: first, each cell type expresses both TPC1 and TPC2 isoforms as judged by RT-PCR (Figures S3C and S3F), and second, NAADP/AM elicited Ca2+ responses in Jurkat 1G4 cells that were sensitive to Ned-19 and bafilomycin A1 (Figures 3A–3C), consistent with acidic Ca2+ store involvement [22, 28].
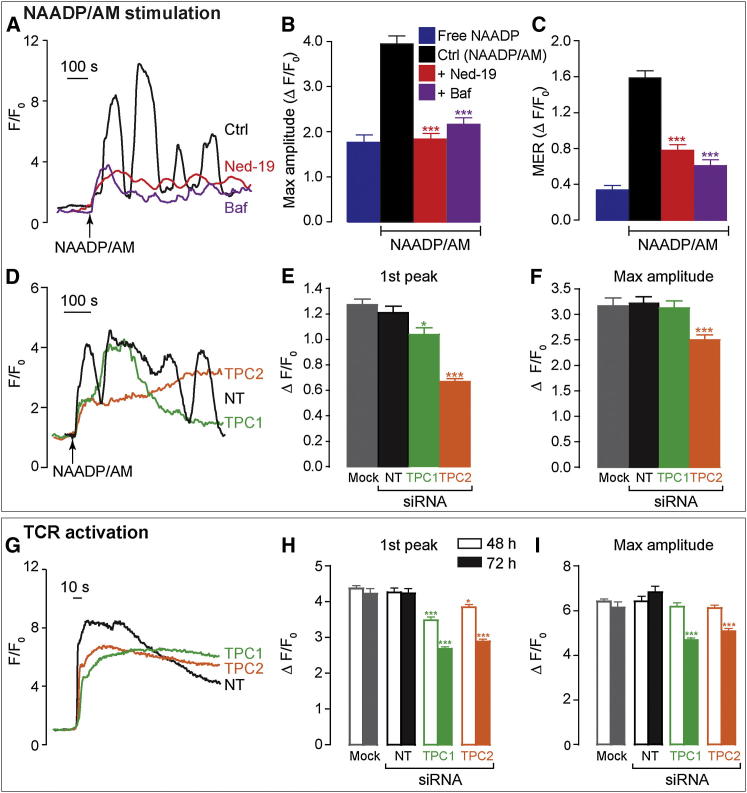
Figure 3.
TPCs Are Necessary for TCR-Triggered Ca2+ Release via NAADP
(A–C) Ca2+ oscillations in Jurkat 1G4 cells induced by 2.5–10 μM NAADP/AM were inhibited by 10 μM trans-Ned-19 or 1 μM bafilomycin A1. Single-cell Ca2+ traces (A) and summary of maximum peak Ca2+ changes and MER (B and C) are shown. n = 88–103 cells.
(D–I) Jurkat 1G4 cells were nucleofected with 100 pmol siRNA (nontargeting [NT], TPC1 and TPC2) or without siRNA (Mock) and incubated for 72 hr (D–G) or 48–72 hr (H and I). siRNA-treated cells were stimulated with 10 μM NAADP/AM (D–F) or by contact with 10 μg/ml anti-human CD3-coated coverslips (G–I). Single-cell Ca2+ traces (D and G) and summary of peak Ca2+ changes (E–F and H–I) are shown for n = 213–383 cells from six independent siRNA experiments.
Error bars are mean ± SEM. See also Figure S3.
The functional role of TPCs in NAADP-induced Ca2+ release was tested by suppressing TPC1 and TPC2 expression with small interfering RNA (siRNA) knockdown (assessed by RT-qPCR and western blot; Figures S3G–S3I). Consequently, NAADP/AM-evoked Ca2+ release was reduced by TPC siRNAs, with a more pronounced inhibition of Ca2+ signals seen with TPC2 than with TPC1 siRNA (Figures 3D–3F and S3J). Statistical analysis suggested that only a subpopulation of cells were affected by siRNA (Figure S3J), presumably a function of the transfection efficiency (although we could not empirically identify which cells were transfected). The Ca2+ signal was significantly reduced in amplitude (by 30%–54%)—a partial effect consistent with incomplete TPC knockdown (Figures S3G–S3I)—and underscored the importance of TPCs for NAADP action. Because NAADP is important for TCR signaling (Figure 2), it follows that TPCs should likewise be required, and indeed, TCR-induced Ca2+ signals were reduced with either TPC1 or TPC2 siRNA (maximal inhibition 44% for each isoform; Figures 3G–3I and S3K). Incidentally, RyRs were absent in Jurkat 1G4 cells (Figure S3E, as with other Jurkat T cell clones [22, 28]), confirming that RyRs are not obligatory for NAADP action, though they have been proposed as NAADP receptors by others [12, 13]. The data affirm both TPC isoforms as necessary components of NAADP-dependent Ca2+ signals during TCR activation, and so CTLs join the emerging list of cells that utilize the NAADP/TPC couple [13, 16].
TPCs on Exocytotic Granules Translocate to the Immunological Synapse
Thus far, we had not determined precisely which acidic vesicle class (or classes) the NAADP/TPC axis was associated with. To that end, we tagged TPC1 and TPC2 with fluorescent proteins and compared their distribution in Jurkat 1G4 cells with other acidic vesicle markers. Both TPC1 (Figures S4A–S4C) and TPC2 (Figures S4D–S4F) colocalized with two markers of the cytolytic granules themselves (granzyme B and LAMP-1), although the TPC1 overlap was slightly lower than that for TPC2 (distribution differences between the two isoforms are a recurring theme [13]). This overlap was not peculiar to Jurkat 1G4 cells, because we observed a similar pattern in primary CTLs: using immunofluorescence, endogenous TPC2 extensively colocalized with granzyme B and LAMP-1 (Figures S4G–S4I). The specificity of TPC2 antibody labeling was verified by preblocking with the immunogenic peptide, which reduced labeling by 87.4% ± 0.4%. Unfortunately, we were unable to obtain specific staining with anti-TPC1 antibodies. NAADP therefore targets the exocytotic vesicles themselves.
That TPCs are found on cytolytic granules agrees with the NAADP acidic store pharmacology (Figures 1E and 1F) because cytolytic granules are the acidic, secretory lysosomes of CTL and natural killer (NK) cells [25, 29]. Indeed, TPC2 was identified in the proteome of NK cell secretory lysosome membranes [30], and such a localization provides the appropriate architecture for NAADP-evoked local Ca2+ domains that selectively couple to exocytosis. That secretory vesicles act as Ca2+ stores has some precedents [13, 31, 32].
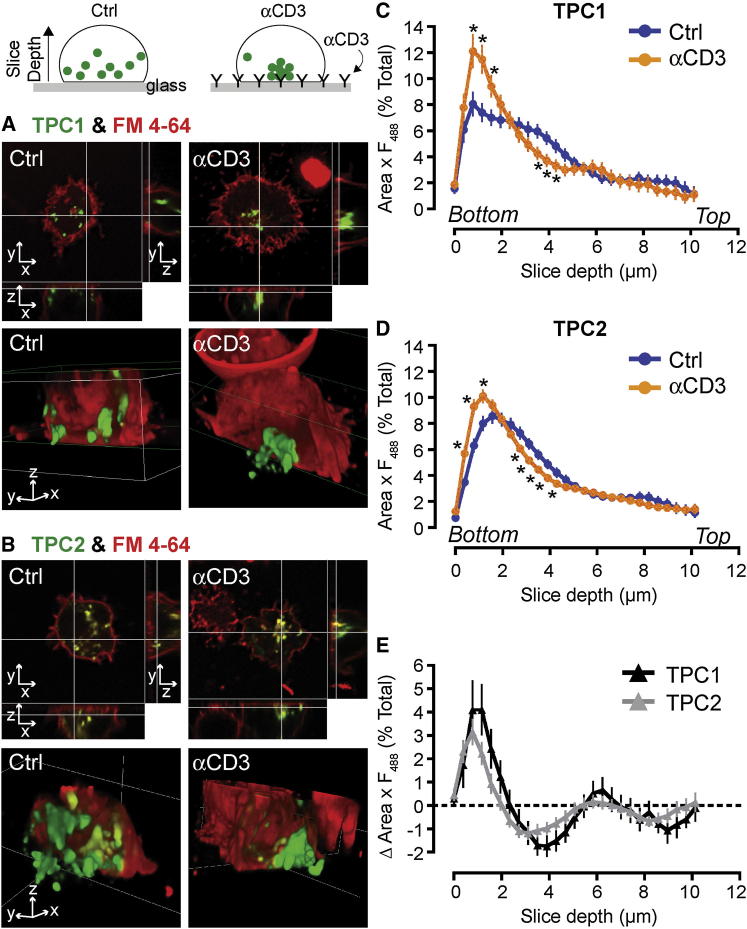
An important characteristic of cytolytic granules is that they rapidly polarize along microtubules toward the immunological synapse (IS) when the TCR is activated (Figures S4J and S4K) [25]. Because TPC1 and TPC2 are expressed on these granules, they should also move to the IS. CTLs, including Jurkat clones [33], can form a pseudo-IS with anti-CD3-coated glass coverslips, and so we examined the 3D distribution of fluorescently tagged TPCs in adherent cells in the presence or absence of antibody coating. Under control conditions without antibody, TPC puncta were distributed throughout the cell (Figures 4A–4D). However, when anti-CD3 was present at the contact surface, a pronounced translocation of both TPC1 and TPC2 from deeper regions of the cell to the contact surface was observed (Figures 4A–4E). Figure 4E shows that TPC1 and TPC2 translocate to the same extent, and their clustering may contribute to the high Ca2+ domains required for lytic granule exocytosis [3]. Moreover, immunolabeled TPC2 in primary CTLs shows endogenous polarization to the IS within a primary CTL:target cell conjugate (Figures S4L and S4M). This is the first report of a directed movement and clustering of TPCs during a specific response.
Figure 4.
TPCs Translocate to an Immunological Synapse upon Stimulation with Anti-CD3
eYFP-tagged HsTPC1 and HsTPC2 (green) were expressed in Jurkat 1G4 cells and added to glass coverslips coated with poly-L-lysine or 10 μg/ml anti-human CD3 antibody mounted in an imaging chamber, as depicted in the cartoon. Cells were labeled with FM4-64 (red) to delineate the plasma membrane edge of the cell.
(A and B) Confocal images of cells attached to poly-L-lysine (Ctrl; left-hand side) or engaged with anti-CD3 antibody (αCD3; right-hand side) showing z stacks through x and y axes (top images) and 3D reconstruction of the z stack (bottom images). TPC localization was assessed through the z stack as a product its area and its fluorescence, as depicted in (C)–(E). A slice depth of 0 indicates the bottom of the cell (i.e., the contact zone between cell and the coverslip).
(C and D) TPC1 (C) and TPC2 (D) localization significantly alters from being present deeper in the cell (slice depth 3–5 μm) toward αCD3, i.e., the bottom of the cells (slice depth 0–2 μm) compared to control.
(E) Comparison of TPC1 and TPC2 movement in cells engaged with anti-CD3. There was no significant difference in the migration of the two isoforms. TPC1 n = 32–43; TPC2 n = 52–69.
Error bars are mean ± SEM. See also Figure S4.
Conclusion
In summary, TCR activation recruits NAADP to activate target TPC channels resident on the exocytotic granules themselves, and TPCs consequently translocate toward the immunological synapse. Therefore, these granules store and release the Ca2+ for their own exocytosis and deliver Ca2+ in an “autocrine” fashion via TPCs, presumably acting in local perigranular Ca2+ nanodomains [8]. In the absence of TCR activation, other Ca2+ signaling pathways, e.g., IP3, fail to mimic NAADP. Although the Orai1-STIM1 complex (CRAC channels) makes a substantial contribution to CTL Ca2+ signals [3], activation of Ca2+ influx per se is insufficient to drive exocytosis, requiring the additional presence of protein kinase activators [2, 9]; the NAADP/TPC pathway is therefore all the more remarkable for being able to drive exocytosis. Clearly, CRAC channels are important for sustaining exocytosis [1, 3], but we propose that local NAADP/acidic store/TPC signaling is an additional important component.
Acknowledgments
We thank F.M. Platt and D.C. Anthony (University of Oxford) for use of the Amaxa Nucelofector II device and use of the Roche LightCycler 480 qPCR machine, respectively. We are also grateful to N. Emptage and Z. Padamsey (University of Oxford) for advice on statistical analyses. We thank the Wellcome Trust, the Medical Research Council, and Cancer Research UK (grant C399/A2291) for financial support.
Published: November 21, 2012
Footnotes
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2012.10.035.
Contributor Information
Lianne C. Davis, Email: lianne.davis@pharm.ox.ac.uk.
Antony Galione, Email: antony.galione@pharm.ox.ac.uk.
Supplemental Information
References
- 1.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 2.Grybko M.J., Pores-Fernando A.T., Wurth G.A., Zweifach A. Protein kinase C activity is required for cytotoxic T cell lytic granule exocytosis, but the theta isoform does not play a preferential role. J. Leukoc. Biol. 2007;81:509–519. doi: 10.1189/jlb.0206109. [DOI] [PubMed] [Google Scholar]
- 3.Pores-Fernando A.T., Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol. Rev. 2009;231:160–173. doi: 10.1111/j.1600-065X.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 4.Lancki D.W., Weiss A., Fitch F.W. Requirements for triggering of lysis by cytolytic T lymphocyte clones. J. Immunol. 1987;138:3646–3653. [PubMed] [Google Scholar]
- 5.Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brailoiu E., Churamani D., Cai X., Schrlau M.G., Brailoiu G.C., Gao X., Hooper R., Boulware M.J., Dun N.J., Marchant J.S., Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermann E., Bucurenciu I., Goswami S.P., Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci. 2012;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J.S., Haydar T.F., Radoja S. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 2008;181:4716–4722. doi: 10.4049/jimmunol.181.7.4716. [DOI] [PubMed] [Google Scholar]
- 10.Gasser A., Bruhn S., Guse A.H. Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J. Biol. Chem. 2006;281:16906–16913. doi: 10.1074/jbc.M601347200. [DOI] [PubMed] [Google Scholar]
- 11.Guse A.H., da Silva C.P., Berg I., Skapenko A.L., Weber K., Heyer P., Hohenegger M., Ashamu G.A., Schulze-Koops H., Potter B.V., Mayr G.W. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 12.Guse A.H. Second messenger signaling: multiple receptors for NAADP. Curr. Biol. 2009;19:R521–R523. doi: 10.1016/j.cub.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Morgan A.J., Platt F.M., Lloyd-Evans E., Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 14.Fasolato C., Zottini M., Clementi E., Zacchetti D., Meldolesi J., Pozzan T. Intracellular Ca2+ pools in PC12 cells. Three intracellular pools are distinguished by their turnover and mechanisms of Ca2+ accumulation, storage, and release. J. Biol. Chem. 1991;266:20159–20167. [PubMed] [Google Scholar]
- 15.Goeger D.E., Riley R.T., Dorner J.W., Cole R.J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem. Pharmacol. 1988;37:978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- 16.Galione A., Morgan A.J., Arredouani A., Davis L.C., Rietdorf K., Ruas M., Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem. Soc. Trans. 2010;38:1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- 17.Menteyne A., Burdakov A., Charpentier G., Petersen O.H., Cancela J.M. Generation of specific Ca2+ signals from Ca2+ stores and endocytosis by differential coupling to messengers. Curr. Biol. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Naylor E., Arredouani A., Vasudevan S.R., Lewis A.M., Parkesh R., Mizote A., Rosen D., Thomas J.M., Izumi M., Ganesan A. Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galione A. NAADP receptors. Cold Spring Harb. Perspect. Biol. 2011;3:a004036. doi: 10.1101/cshperspect.a004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dammermann W., Guse A.H. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J. Biol. Chem. 2005;280:21394–21399. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- 21.Cancela J.M., Churchill G.C., Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 22.Bennett D.L., Cheek T.R., Berridge M.J., De Smedt H., Parys J.B., Missiaen L., Bootman M.D. Expression and function of ryanodine receptors in nonexcitable cells. J. Biol. Chem. 1996;271:6356–6362. doi: 10.1074/jbc.271.11.6356. [DOI] [PubMed] [Google Scholar]
- 23.Bootman M.D., Collins T.J., Mackenzie L., Roderick H.L., Berridge M.J., Peppiatt C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 24.Chen J.L., Morgan A.J., Stewart-Jones G., Shepherd D., Bossi G., Wooldridge L., Hutchinson S.L., Sewell A.K., Griffiths G.M., van der Merwe P.A. Ca2+ release from the endoplasmic reticulum of NY-ESO-1-specific T cells is modulated by the affinity of TCR and by the use of the CD8 coreceptor. J. Immunol. 2010;184:1829–1839. doi: 10.4049/jimmunol.0902103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page L.J., Darmon A.J., Uellner R., Griffiths G.M. L is for lytic granules: lysosomes that kill. Biochim. Biophys. Acta. 1998;1401:146–156. doi: 10.1016/s0167-4889(97)00138-9. [DOI] [PubMed] [Google Scholar]
- 26.Esser M.T., Krishnamurthy B., Braciale V.L. Distinct T cell receptor signaling requirements for perforin- or FasL-mediated cytotoxicity. J. Exp. Med. 1996;183:1697–1706. doi: 10.1084/jem.183.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Togashi K., Kataoka T., Nagai K. Characterization of a series of vacuolar type H+-ATPase inhibitors on CTL-mediated cytotoxicity. Immunol. Lett. 1997;55:139–144. doi: 10.1016/s0165-2478(97)02698-9. [DOI] [PubMed] [Google Scholar]
- 28.Hosoi E., Nishizaki C., Gallagher K.L., Wyre H.W., Matsuo Y., Sei Y. Expression of the ryanodine receptor isoforms in immune cells. J. Immunol. 2001;167:4887–4894. doi: 10.4049/jimmunol.167.9.4887. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt J.K., Hester S., Lapham C.K., Argon Y. The lytic granules of natural killer cells are dual-function organelles combining secretory and pre-lysosomal compartments. J. Cell Biol. 1990;111:2327–2340. doi: 10.1083/jcb.111.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey T.M., Meade J.L., Hewitt E.W. Organelle proteomics: identification of the exocytic machinery associated with the natural killer cell secretory lysosome. Mol. Cell. Proteomics. 2007;6:767–780. doi: 10.1074/mcp.M600365-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Petersen O.H. Can Ca2+ be released from secretory granules or synaptic vesicles? Trends Neurosci. 1996;19:411–413. [PubMed] [Google Scholar]
- 32.Mitchell K.J., Lai F.A., Rutter G.A. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic beta-cells (MIN6) J. Biol. Chem. 2003;278:11057–11064. doi: 10.1074/jbc.M210257200. [DOI] [PubMed] [Google Scholar]
- 33.Yi J., Wu X.S., Crites T., Hammer J.A., 3rd Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol. Biol. Cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.