Abstract
BACKGROUND AND PURPOSE
URB937 is a peripherally restricted inhibitor of the anandamide-deactivating enzyme fatty-acid amide hydrolase (FAAH). Despite its limited access to the CNS, URB937 produces marked antinociceptive effects in rodents. URB937 is actively extruded from the CNS by the ATP-binding cassette (ABC) membrane transporter, Abcg2. Tissue Abcg2 levels are markedly different between males and females, and this transporter is known to limit the access of xenobiotics to the fetoplacental unit in gestating female rodents. In the present study, we investigated the tissue distribution and antinociceptive properties of URB937 in female mice and rats.
EXPERIMENTAL APPROACH
We studied the systemic disposition of URB937 in female mice and the antinociceptive effects of this compound in models of visceral (acetic acid-induced writhing) and inflammatory nociception (carrageenan-induced hyperalgesia) in female mice and rats. Furthermore, we evaluated the interaction of URB937 with the blood-placenta barrier in gestating mice and rats.
KEY RESULTS
Abcg2 restricted the access of URB937 to the CNS of female mice and rats. Nevertheless, URB937 produced a high degree of antinociception in female mice and rats in models of visceral and inflammatory pain. Moreover, the compound displayed a restricted access to placental and fetal tissues in pregnant mice and rats.
CONCLUSIONS AND IMPLICATIONS
Peripheral FAAH blockade with URB937 reduces nociception in female mice and rats, as previously shown for males of the same species. In female mice and rats, Abcg2 limits the access of URB937, not only to the CNS, but also to the fetoplacental unit.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.167.issue-8
Keywords: fatty-acid amide hydrolase, URB937, Abcg2, blood-placental barrier, female, pain
Introduction
The endocannabinoid anandamide exerts a powerful inhibitory control over pain initiation by activating CB1-type cannabinoid receptors in peripheral tissues (Calignano et al., 1998; Clapper et al., 2010). This regulatory action can be magnified by blocking anandamide deactivation, which is mediated in part by the intracellular enzyme fatty-acid amide hydrolase (FAAH) (Cravatt et al., 1996). The peripherally restricted FAAH inhibitor, URB937, enhances anandamide signalling at CB1 receptors and, by doing so, exerts profound antinociceptive effects in animal models of pain (Clapper et al., 2010). These effects are greater than those observed with globally active FAAH inhibitors, such as URB597 or PF-04457845 (Sasso et al., 2012), highlighting the potential therapeutic significance of peripheral FAAH blockade.
We have recently shown that URB937 is extruded from the CNS by the action of the blood-brain barrier (BBB) transporter Abcg2 (ABCG2 in humans), a member of the ATP-binding cassette (ABC) superfamily of efflux transporters (Moreno-Sanz et al., 2011). Abcg2 is found on the apical surface of endothelial cells of the BBB, where it participates in the active transport of various clinically used drugs (Cole et al., 2012). Shortly after its identification (Doyle et al., 1998), the Abcg2 gene was found in human placenta and named ABCP (ABC transporter in placenta) to reflect its high level of expression in that tissue (Allikmets et al., 1998). The apical-side location of ABCG2 in human and Abcg2 in murine placental syncytiotrophoblasts, along with the tight control of its expression during pregnancy, suggests an important role for this transporter in protecting the fetus from potentially toxic chemicals (Myllynen et al., 2010; Hahnova-Cygalova et al., 2011). Like Abcg2, FAAH is also expressed in placental syncytiotrophoblasts, where its levels are strictly controlled during pregnancy (Helliwell et al., 2004), probably because of the critical roles played by anandamide in orchestrating blastocyst implantation (Wang et al., 2003). The fact that FAAH expression remains elevated during early human placenta development suggests that this enzyme might protect the fetus from maternal circulating anandamide before the placental barrier becomes functional (Habayeb et al., 2008).
Substantial gender differences in Abcg2 gene expression have been described in both mice and rats (Tanaka et al., 2005). These differences could affect the systemic disposition and pharmacological activity of URB937 in a gender-specific manner. Therefore, the primary objective of the present study was to determine whether gender differences in Abcg2 expression might alter the tissue distribution and pharmacodynamic properties of URB937. We first determined the tissue distribution of URB937 in female mice. Next, we examined the antinociceptive effects of URB937 in two models of nociception in which the compound has previously demonstrated activity when tested in male mice and rats. Finally, we studied the interaction of URB937 with Abcg2 in the blood-placenta barrier (BPB) by assessing the placental and fetal distribution of this compound after administration to pregnant mice and rats.
Methods
Animals
One hundred and fifty adult male (12) and female (138) C57BL6 mice (25–30 g; Jackson, Bar Harbor, ME, USA) and sixty eight adult male (12) and female (56) Sprague-Dawley (SD) rats (225–250 g; Charles River, Wilmington, MA, USA and Calco, Italy) were kept in a temperature-controlled environment with a 12 h light/12 h dark cycle and received a standard chow and water ad libitum. All procedures were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and the Ethical Guidelines of the International Association for the Study of Pain, and were approved by the Institutional Animal Care and Use Committee of the University of California and the Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with European Economic Community regulations (O.J. of E.C. L 358/1 12/18/1986). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Chemicals
URB937 was synthesized as described by Clapper et al. (2010). Anandamide-[ethanolamine-3H] was from American Radiolabeled Chemicals, Inc. (St. Louis, MO, USA). AM251, anandamide, palmitoylethanolamide (PEA), anandamide-[ethanolamine-2H4] and PEA-[ethanolamine-2H4] were from Cayman Chemical (Ann Arbor, MI, USA). Dimethyl sulfoxide (DMSO), 2,6-dichloro-4-nitrophenol (DCNP), indomethacin and λ-carrageenan were from Sigma (St. Louis, MO, USA). Ko143 was from Tocris (Ellisville, MO, USA). Rimonabant and N-cyclohexyl biphenyl-3-ylacetamide were kind gifts of the National Institute on Drug Abuse and Kadmus Pharmaceuticals Inc. respectively. All other chemicals were of analytical grade and available from commercial sources.
Tissue processing
Animals were killed by decapitation under anaesthesia with isoflurane. Blood was collected through a left cardioventricular puncture and centrifuged at 2000×g for 30 min to obtain plasma. Tissues were collected, washed with PBS and snap frozen in liquid nitrogen.
FAAH activity
Tissues were weighed, homogenized in ice-cold Tris–HCl buffer (50 mM, 5–9 vol, pH 7.5) containing 0.32 M sucrose and centrifuged at 1000×g for 10 min at 4°C. Supernatants were collected and protein concentrations determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). FAAH activity was measured at 37°C for 30 min in 0.5 mL of Tris–HCl buffer (50 mM, pH 7.5) containing fatty acid-free BSA (0.05%, w v−1), tissue homogenates (50 µg protein from brain, placenta, and fetus, and 10 µg from liver), 10 µM anandamide and anandamide-[ethanolamine-3H] (10 000 cpm, specific activity 60 Ci·mmol−1). The reactions were stopped with chloroform/methanol (1:1, 1 mL) and radioactivity was measured in the aqueous layers by liquid scintillation counting.
Quantification of anandamide, PEA and URB937 by LC/MS
Frozen tissues were weighed and homogenized in methanol (1 mL) containing [2H4]-anandamide and [2H4]-PEA as internal standards. Analytes were extracted with chloroform (2 vol) and washed with water (1 vol). Organic phases were collected and dried under nitrogen. The organic extract was fractionated by open-bed silica gel column chromatography (Cadas et al., 1997). Briefly, the extract was dissolved in chloroform and loaded onto small glass columns packed with Silica Gel G (60∼A 230–400 Mesh ASTM; Whatman, Clifton, NJ, USA). Anandamide and PEA were eluted with chloroform/methanol (9:1 w v−1). Organic phases were evaporated under nitrogen and reconstituted in 60 µL of methanol. Levels of anandamide and PEA were analysed using an 1100-LC system coupled to a 1946A-MS detector (Agilent Technologies, Inc., Palo Alto, CA, USA) equipped with an electrospray ionization interface as previously described (Astarita et al., 2008). URB937 was extracted from aqueous tissue homogenates and plasma samples with methanol/chloroform (1:2) containing cyclohexyl biphenyl-3-ylacetamide as internal standard. Organic phases were evaporated under nitrogen and reconstituted in 60 µL of methanol.
RNA extraction and gene expression analysis
Total mRNA was extracted from mice and rat tissues with Trizol® (Life Technologies, Grand Island, NY, USA) and purified by using the RNeasy® Plus Mini Kit (Quiagen, Valencia, CA, USA). mRNA (2 µg) was then reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). Relative expression of Abcg2 was determined by quantitative RT-PCR in an Mx3000P plate thermocycler (Stratagene, La Jolla, CA, USA) and normalized to GAPDH expression in every tissue. Taqman® PCR Master Mix and primers for mAbcg2, rAbcg2, mGAPDH and rGAPDH were commercially available from Applied Biosystems-Roche (Carlsbad, CA, USA). Inter-species differences were calculated by the Δ-Δ Ct method.
Experimental design
Ko-143 was dissolved in saline/PEG400/Tween-80 (18:1:1) with DMSO (30%). All other drugs were dissolved in saline/PEG400/Tween-80 (18:1:1). Drug solutions were prepared immediately before use and administered in a volume of 10 mL·kg−1 to mice and 1 mL·kg−1 to rats.
Experiment 1
To study the systemic distribution of URB937, C57BL6 female mice that had received various doses of URB937 (0.03–100 mg·kg−1, s.c.), were killed after 1 h under light anaesthesia, and tissues collected for FAAH activity determination. To test the contribution of Abcg2 to the peripheral segregation of URB937, a second group of animals received a single dose of URB937 (25 mg·kg−1, s.c.) 20 min after a pretreatment with either vehicle or the selective Abcg2 inhibitor Ko-143 (10 mg·kg−1, i.p.). As phenol sulfotransferase (SULT1) is involved in the transport of xenobiotics by Abcg2, the effects of non-selective SULT1 inhibitor DCNP (30 mg·kg−1, i.p.), alone or in combination with Ko-143, were also determined. Animals were killed after 1 h and their brains collected for FAAH activity determination. Finally, a third set of C57BL6 female mice received URB937 (1 mg·kg−1, i.p.) and was killed 2 h later for determination of fatty-acid ethanolamides levels.
Experiment 2
It was previously shown that URB937 attenuates behavioural responses to various noxious stimuli in male mice and rats (Clapper et al., 2010). To test whether these antinociceptive effects are gender specific, we investigated the ability of URB937 to reduce nocifensive responses evoked by acetic acid injection into the peritoneal cavity of female C57BL6 mice. Animals were acclimatized to the experimental room for 2 h. Each animal received an acetic acid injection (150 µL, 0.6% in saline, i.p.) and was placed into a 2 L glass beaker. Abdominal stretches (extension of body and hind limbs) were counted for 20 min, starting 5 min after acetic acid injection. URB937 (0.3–3 mg·kg−1, s.c.) and the COX inhibitor indomethacin (1 mg·kg−1, s.c.) were injected 1 h before acetic acid. The selective CB1 inverse agonist rimonabant (1 mg·kg−1, s.c.) was administered 20 min before URB937 (1 mg·kg−1, s.c). Behaviour was scored by an observer blinded to the treatment conditions.
Experiment 3
We studied the distribution of URB937 into the fetoplacental unit in gestating mice. Female C57BL6 mice were exposed to C57BL6 males in a 2:1 ratio and visually inspected for sperm plugs the next morning. This was considered as embryonic day 1 (E1). Females were then housed in groups of four and monitored daily for weight gain to determine successful pregnancies. Pregnant females were used on day 15 (E15), at the highest point of Abcg2 gene expression during pregnancy (Myllynen et al., 2010). Animals received three different doses of URB937 (0.3, 1, 3 mg·kg−1, s.c.). To check for the role of Abcg2 in the interaction of URB937 with the BPB, the selective inhibitor Ko-143 (10 mg·kg−1, s.c.) was injected to a different group of animals 20 min before URB937 (1 mg·kg−1, s.c.). Females were killed under light anaesthesia 1 h later and blood and tissues were collected. Brain, liver, kidney, fetuses and placentas (three to four per animal) were analysed for URB937 content and/or FAAH activity, as described above. Because URB937 is an irreversible inhibitor, diminution of FAAH activity can be considered directly proportional to the amount of compound reaching the tissue.
Experiment 4
Adult female SD rats were used to assess the antinociceptive effects of URB937 in the carrageenan-induced inflammation model. Baseline assessments of nociceptive sensitivity and hindpaw volume were taken for all animals 24 h before the hindpaw injection of λ-carrageenan. On the day of the experiment, rats received an intraplantar injection of 50 µL of 2% (w v−1) carrageenan into the right hindpaw, using a 1 cm3 syringe and a 27 gauge needle. Animals were pretreated orally 1 h before carrageenan injection with either vehicle, URB937 (0.3–3 mg·kg−1, oral gavage, o.g.) or indomethacin (30 mg·kg−1, o.g.). The CB1 inverse agonist AM251 (1 mg·kg−1, i.p.) was given 30 min before URB937 (3 mg·kg−1, o.g.). Pain behaviour and paw oedema were assessed 2 h after carrageenan injection, at the peak of the acute phase of the inflammatory response. Paw volumes were measured with a plethysmometer (Ugo Basile, Comerio, Italy). The increase in paw volume was evaluated as differences between ipsi- and contralateral hindpaw. Pain sensitivity to a noxious thermal stimulus was measured using the radiant heat source method (Hargreaves et al., 1988). Briefly, each animal was placed in a clear acrylic cubicle (22 × 16.5 × 14 cm) on top of a glass floor in a temperature-controlled room (22°C) and allowed to acclimatize for 15 min before testing. The withdrawal latencies were averaged over three consecutive tests, at least 5 min apart, in response to the thermal challenge from a calibrated (output of 190 mW·cm2) radiant light source. A cut-off of 20 s was imposed to prevent any significant tissue damage.
Experiment 5
To study the access of URB937 to the fetoplacental unit in gestating rats, we repeated the experimental design described in Experiment 3 on female SD rats. URB937 was administered orally.
Experiment 6
Relative levels of Abcg2 gene expression in pregnant female mice and rats were compared with quantitative RT-PCR in kidney, liver, brain, fetuses and placentas of vehicle-treated animals from Experiments 3 and 5.
Statistical analysis
Results are expressed as mean ± SEM and the significance of differences was determined using one-way anova followed by Dunnett's test as post hoc, and Student's t-test. Differences were considered significant if P < 0.05. Statistical analyses were conducted using GraphPad Prism Version 4.0 (San Diego, CA, USA).
Results
Experiment 1. URB937 is peripherally restricted in female mice
A dose-exploration study in female C57BL6 mice showed that the ED50 of URB937 for FAAH inhibition in the brain (48 mg·kg−1, s.c.) was approximately 250 times higher than the ED50 for FAAH inhibition in the liver (0.2 mg·kg−1, s.c.) (Figure 1A). Systemic administration of the selective Abcg2 inhibitor Ko-143 (10 mg·kg−1, i.p.) 20 min before the injection of URB937 (25 mg·kg−1, s.c.) increased the ability of URB937 to inhibit brain FAAH activity, indicating increased access to the CNS. Similar results were obtained with the non-selective SULT1 inhibitor DCNP (30 mg·kg−1, i.p.), which might be acting by blocking the sulfate conjugation of URB937 (Clapper et al., 2010). Ko-143 and DCNP produced additive effects when administered in combination (F4,22= 108.7, P < 0.001; Figure 1B). As expected from previous results (Clapper et al., 2010), URB937 treatment was associated with an increase in anandamide and PEA levels in peripheral tissues, but not in the CNS (Figure 1C, D). Levels of both anandamide and PEA remained unaffected in the ovary of treated animals (Figure 1C, D) suggesting a restrictive effect of Abcg2 in female gonads, similar to what has been described in testis (Maliepaard et al., 2001; Vlaming et al., 2009).
Figure 1.
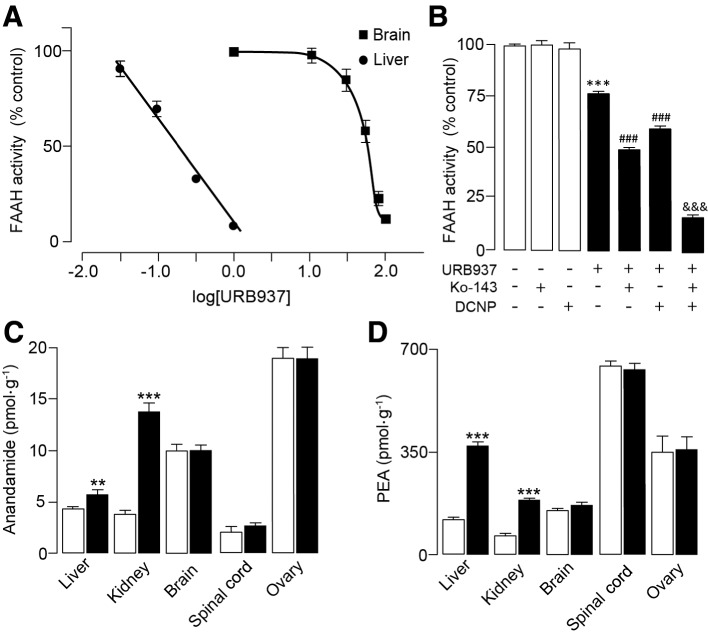
URB937 is peripherally restricted in female mice. (A) FAAH activity in liver and brain 1 h after injection of URB937 (0.03–100 mg·kg−1, s.c.) in female C57BL6 mice. (B) Brain FAAH activity after systemic administration of vehicle or a supra-maximal dose of URB937 (25 mg·kg−1, s.c.); URB937 was administered alone or in combination with the Abcg2 inhibitor Ko-143 (Ko, 10 mg ·kg−1, i.p.), the SULT1 inhibitor DCNP (30 mg·kg−1, i.p.) or both. Effects of vehicle (open columns) or URB937 (1 mg·kg−1, i.p., solid columns) on (C) anandamide and (D) PEA levels in liver, kidney, forebrain, spinal cord and ovary of female C57BL6 mice 2 h after administration. Results are expressed as mean ± SEM; n= 3–7; **P < 0.01 and ***P < 0.001 versus vehicle; ###P < 0.001 versus URB937 alone; &&&P < 0.001 versus URB937 plus Ko-143 and URB937 plus DCNP.
Experiment 2. Peripheral FAAH blockade exerts antinociceptive effects in female mice
Administration of URB937 s.c. attenuated acetic acid–induced writhing in a dose-dependent manner with an ED50 of approximately 1 mg·kg−1 (F3,28= 5.182, P= 0.0057, Figure 2A), which is identical to the ED50 reported for male CD57BL6 mice (Clapper et al., 2010). This antinociceptive effect was comparable to that elicited by the COX inhibitor indomethacin (1 mg·kg−1, s.c.; Figure 2A), and was blocked by the CB1 inverse agonist rimonabant (1 mg·kg−1, s.c.; Figure 2B).
Figure 2.
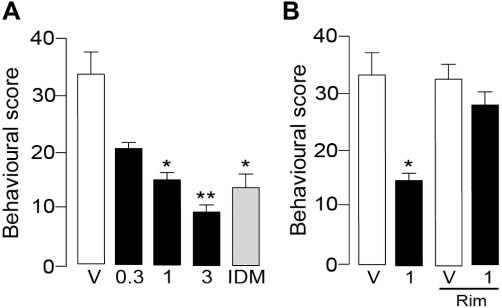
URB937 suppresses nocifensive behaviour elicited by injections of acetic acid (0.6%, 150 µL, i.p.) in female C57BL6 mice. (A) Behavioural score (number of writhing episodes in 20 min) assessed 1 h after administration of vehicle (V), URB937 (0.3–3 mg·kg−1, s.c.) or indomethacin (IDM, 1 mg·kg−1, s.c.). (B) Co-administration of the CB1 inverse agonist rimonabant (Rim, 1 mg·kg−1, s.c.) prevented the antinociceptive effects of URB937 (1 mg·kg−1, s.c.). Results are expressed as mean ± SEM.; n= 6–8. *P < 0.05 and **P < 0.01 versus vehicle.
Experiment 3. Abcg2 limits the access of URB937 to the fetoplacental unit in gestating mice
To assess the interaction of URB937 with the BPB, we administered the compound to pregnant C57BL6 female mice (E15). URB937 (1 mg·kg−1, s.c.) displayed an uneven distribution among tissues. The tissue:blood ratios of URB937 in fetus and placenta were significantly lower than the tissue:blood ratio of URB937 in liver while, as expected, the compound did not reach the brain (F3,31= 49.08, P < 0.001; Figure 3A). Pharmacological blockade of Abcg2 with the selective inhibitor Ko-143 (10 mg·kg−1, s.c.) increased access of URB937 to the placenta and fetus (Table 1) and elevated its tissue:blood ratios (Figure 3B). URB937 (0.3–3 mg·kg−1, s.c.) reduced FAAH activity in the placenta (F3,24= 187.3, P < 0.001; Figure 3C) and fetus (F3,31= 119.8, P < 0.001; Figure 3D) of female mice in a dose-dependent manner. Pretreatment with Ko-143 (10 mg·kg−1, s.c.) 20 min before URB937 (1 mg·kg−1, s.c) increased the access of URB937 to the fetoplacental unit, resulting in inhibition of FAAH activity both in the placenta (P= 0.009; Figure 3C) and fetus (P < 0.001; Figure 3D).
Figure 3.
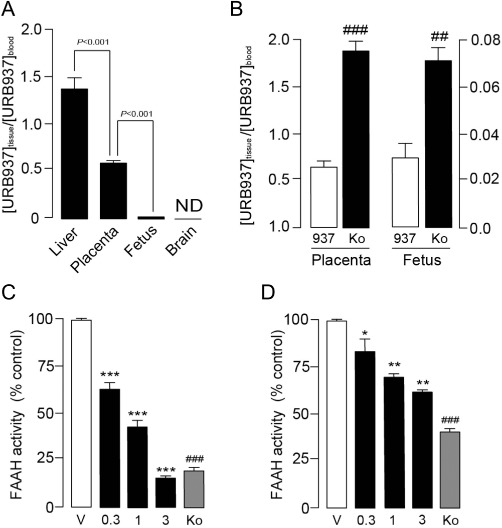
Abcg2 controls the access of URB937 to the fetus and placenta in pregnant mice. (A) Tissue:blood ratios of URB937 (1 mg·kg−1, s.c.) in various tissues of gestating (E15) C57BL6 female mice (ND, not detected). (B) Effects of pretreatment with the Abcg2 inhibitor Ko-143 (Ko, 10 mg·kg−1, s.c.) on the tissue:blood ratios of URB937 (937, 1 mg·kg−1, s.c.) in mouse placenta and fetus. FAAH activity in (C) placenta and (D) fetus of pregnant (E15) C57BL6 mice 1 h after administration of vehicle (V), URB937 (0.3–3 mg·kg−1, s.c.). The contribution of Abcg2 to fetoprotection was assessed by administration of Ko-143 (Ko, 10 mg·kg−1, s.c.) 20 min before URB937 (1 mg·kg−1, s.c.). Results are expressed as mean ± SEM; n= 6–12. *P < 0.05, **P < 0.01 and ***P < 0.001 versus vehicle; ##P < 0.01 and ###P < 0.001 versus URB937 (1 mg·kg−1, s.c.).
Table 1.
URB937 levels in the plasma, placenta and fetus of female mice and rats 1 h after drug administration (1 mg·kg−1, s.c. in mice and o.g. in rats) to animals pretreated with either vehicle or Ko-143 (10 mg·kg−1, s.c.; 20 min before URB937)
| Plasma (pmol·mL−1) | Placenta (pmol·g−1) | Fetus (pmol·g−1) | ||||
|---|---|---|---|---|---|---|
| 937 | 937 + Ko | 937 | 937 + Ko | 937 | 937 + Ko | |
| Mouse | 85.6 ± 6.8 | 80.5 ± 12.2 | 48.9 ± 6.1 | 144.8 ± 35.0** | 2.4 ± 0.5 | 6.9 ± 1.2** |
| Rat | 32.4 ± 8.2 | 30.3 ± 15.7 | 31.7 ± 1.2 | 64.5 ± 10.9** | 4.4 ± 0.3 | 8.2 ± 1.1** |
Results are expressed as mean ± SEM; n= 3–12.
P < 0.01 versus URB937 (1 mg·kg−1).
Experiment 4. Peripheral FAAH blockade exerts antinociceptive effects in female rats
We produced peripheral inflammation in female SD rats by injecting the pro-inflammatory polysaccharide carrageenan into the right hindpaw. This resulted in the development of heat hyperalgesia as well as local oedema (Figure 4A, C). A single administration of URB937 (1 and 3 mg·kg−1, o.g.), given together with carrageenan, decreased heat hyperalgesia at the peak of the acute phase of the inflammatory response (2 h after carrageenan treatment) (F3,128= 8.759, P < 0.001; Figure 4A). URB937 (0.3–3 mg·kg−1, o.g.) also reduced paw swelling 2 h after carrageenan injection (F3,60= 219.6, P < 0.001; Figure 4C). These effects were comparable to those elicited by a 10-fold higher dose of indomethacin (30 mg·kg−1, o.g.; Figure 4A, C). The anti-hyperalgesic and anti-inflammatory effects of URB937 (3 mg·kg−1, o.g.) were prevented by the CB1 inverse agonist AM251 (1 mg·kg−1, i.p.) (Figure 4B, D).
Figure 4.
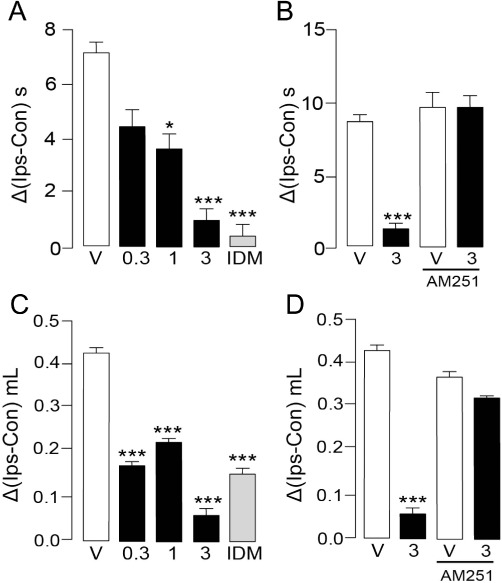
URB937 attenuates nocifensive behaviour and paw oedema elicited by carrageenan in female rats. Effects of vehicle (V, open bars), URB937 (0.3–3 mg·kg−1, o.g.) and indomethacin (IDM, 30 mg·kg−1, o.g.) on carrageenan-induced (A) thermal hyperalgesia and (C) paw oedema, expressed as the difference between the ipsilateral (Ips, carrageenan-injected) and contralateral (Con) paw responsiveness. (B,D) The CB1 inverse agonist AM251 (1 mg·kg−1, s.c.) prevented the antinociceptive effects of URB937 (3 mg·kg−1, o.g.). Heat hyperalgesia and paw oedema were measured 2 h after carrageenan (50 µL, 2% w v−1), at the peak of the acute inflammatory response. Results are expressed as mean ± SEM; n= 8. *P < 0.05 and ***P < 0.001 versus vehicle.
Experiment 5. Abcg2 limits the access of URB937 to the fetoplacental unit in gestating rats
To assess the interaction of URB937 with the BPB, we administered the compound to pregnant female rats (E15). URB937 (1 mg·kg−1, o.g.) displayed an uneven distribution among rat tissues, as previously shown in mice. The tissue:blood ratio was lower in the fetus than the placenta and both of them were significantly lower than in the liver. URB937 was not detectable in brain (F3,30= 70.85, P < 0.001; Figure 5A). Pharmacological blockade of Abcg2 with Ko-143 (10 mg·kg−1, s.c.) increased the access of URB937 to the placenta and fetus (Table 1) and elevated the compound's tissue:blood ratio (Figure 5B). URB937 (0.3–3 mg·kg−1, o.g.) reduced FAAH activity in the placenta (F3,28= 45.70, P < 0.001; Figure 5C) and fetus (F3,28= 19.69, P < 0.001; Figure 5D) of female rats in a dose-dependent manner. Pretreatment with Ko-143 (10 mg·kg−1, s.c.) 20 min before URB937 (1 mg·kg−1, s.c.) increased the access of URB937 to the fetoplacental unit, resulting in an increased inhibition of FAAH activity both in the placenta (P= 0.031; Figure 5C) and fetus (P= 0.007; Figure 5D).
Figure 5.
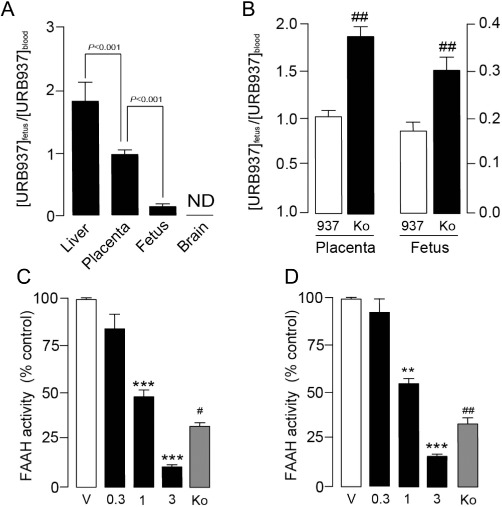
Abcg2 controls the access of URB937 to the fetus and placenta in pregnant rats. (A) Tissue:blood ratios of URB937 (1 mg·kg−1, o.g.) in various tissues of gestating (E15) female rats (ND, not detected). (B) Effect of pretreatment with the Abcg2 inhibitor Ko-143 (Ko, 10 mg·kg−1, s.c.) on the tissue:blood ratio of URB937 (937, 1 mg·kg−1, o.g.) in rat placenta and fetus. FAAH activity in (C) placenta and (D) fetus of pregnant (E15) rats 1 h after administration of vehicle (V), URB937 (0.3–3 mg·kg−1, s.c.). Abcg2 contribution to fetoprotection was assessed by injecting Ko-143 (Ko, 10 mg·kg−1, s.c) 20 min before URB937 (1 mg·kg−1, o.g.) administration. Results are expressed as mean ± SEM; n= 6–12. **P < 0.01 and ***P < 0.001 versus vehicle; #P < 0.05 and ##P < 0.01 versus URB937 (1 mg·kg−1, o.g.).
Experiment 6. Abcg2 gene expression displays tissue and species specificity in female mice and rats
We extracted total RNA from various tissues of pregnant female mice and rats and measured Abcg2 gene transcription by quantitative RT-PCR (Figure 6). No differences were detected in brain between species (P= 0.854). In mice, Abcg2 expression was significantly higher in kidney (P < 0.001), fetus (P= 0.0006) and placenta (P= 0.0089) compared with the same tissues of rats. By contrast, liver levels of Abcg2 were slightly higher in female rats than mice (P= 0.0308). This expression profile is consistent with results from previous reports (Tanaka et al., 2005), and correlates with the inter-species differences we observed in the fetoprotective function of Abcg2, which point to a higher exposure of rat fetoplacental unit to URB937: (i) the tissue:blood ratio was found to be higher in rat fetus and placenta compared with mice (Figures 3B, 5B); and (ii) pharmacological blockade of Abcg2 increased FAAH inhibition in placenta (Figure 5C, P < 0.05) and fetus (Figure 5D, P < 0.01) induced by URB937 at 1 mg·kg−1 contrary to what was shown in mice (Figure 3C, P < 0.001 and Figure 3D, P < 0.001 respectively).
Figure 6.
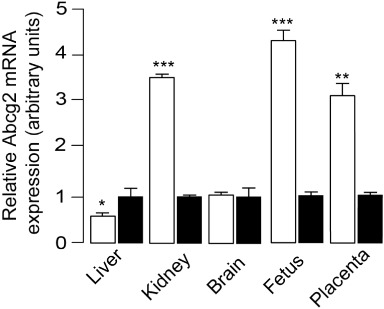
Differences in Abcg2 transcription in female mice and rats. Levels of Abcg2 mRNA in various tissues of mice (open columns) relative to those found in rats (solid columns). mRNA levels were measured by RT-PCR and normalized to GAPDH expression. Abcg2 mRNA levels in mouse tissues are normalized to Abcg2 levels in the same tissue of the rat to highlight inter-species differences. Results are expressed as mean ± SEM; n= 6. *P < 0.05, **P < 0.01 and ***P < 0.001 versus rat.
Discussion and conclusions
URB937 is a potent and selective FAAH inhibitor, which has restricted access to the CNS (Clapper et al., 2010). Despite this limited distribution, URB937 exerts substantial antinociceptive effects in male mice and rats, which are probably caused by increased anandamide signalling at peripheral CB1 receptors (Richardson et al., 1998). The restricted penetration of URB937 into the brain has been attributed to the ability of this compound to serve as a substrate for the transporter Abcg2 (ABCG2 in humans) (Moreno-Sanz et al., 2011). Abcg2 is found on the apical side of endothelial cells in the BBB, where its expression levels show species-specific (Warren et al., 2009) and gender-specific differences (Tanaka et al., 2005). Despite such differences, our results show that URB937 does not readily access the CNS of female mice and rats, as previously shown for males of the same species (Clapper et al., 2010). Consistent with this finding, we found that systemic URB937 administration inhibits FAAH activity and increases anandamide levels in peripheral tissues, but not brain or spinal cord. Furthermore, we showed that URB937 exerts marked antinociceptive effects in female mice and rats, which were quantitatively similar to those previously found in males (Clapper et al., 2010). These results indicate that species and gender differences in Abcg2 expression do not significantly influence the key pharmacological properties of URB937 in mice and rats.
Several factors can cause sexual dimorphism in pain sensitivity and analgesia (Mogil and Bailey, 2010). Gender differences in morphine potency have been related to anatomical and physiological sex dimorphisms of the periaqueductal grey (PAG) and its descending projections to the rostral ventromedial medulla (RVM) (Loyd and Murphy, 2009). Furthermore, an inverse relationship has been reported for 17 β-oestradiol and spinal levels of substance P in female rats (Duval et al., 1996). As for the endocannabinoid system, gonadal hormones have been shown to regulate expression and signal transduction of CB1 receptors (Rodriguez de Fonseca et al., 1994; Gonzalez et al., 2000) in various brain regions, suggesting that CB1 function may be sexually dimorphic (Fattore and Fratta, 2010). The antinociceptive effects of the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) are mediated by multiple areas of the CNS – including the amygdala, thalamus, PAG-RVM and spinal cord (Walker and Hohmann, 2005). Although activation of supraspinal and spinal CB1 receptors modulates nociception in rats of both sexes in a similar manner (Tseng and Craft, 2004), oestradiol has been shown to enhance Δ9-THC-mediated antinociception in gonadectomized female rats (Craft and Leitl, 2008). The present study shows that URB937 inhibits nocifensive responses in female mice and rats as potently as it does in males (Clapper et al., 2010). This result suggests that the peripheral action of URB937 on anandamide signalling might circumvent the central mechanisms that account for gender divergence in pain perception (Mogil and Bailey, 2010). According to this hypothesis, local activation of CB1 receptors in peripheral tissues would prevent emerging pain signals from reaching CNS sites where sex differences might arise.
Abcg2/ABCG2 expression in embryonic and fetal membranes helps ensure proper function of the fetoplacental unit during pregnancy in mammals (Myllynen et al., 2010). Evidence indicates that Abcg2/ABCG2 transfers its substrates in the fetal-to-maternal direction, thus playing an important role in transplacental pharmacokinetics and fetal protection (Hahnova-Cygalova et al., 2011). Our results indicate that Abcg2 limits the passage of URB937 through the BPB in female mice and rats. The fetoprotective effects of Abcg2 showed inter-species differences, pointing to a higher degree of exposure to URB937 of the rat fetoplacental unit compared with the mouse, as shown by the sevenfold higher fetus:blood ratio of URB937 (compare Figures 3B, 5B). This finding correlated with the levels of Abcg2 transcription found in placenta and fetus of the two species. Interestingly, the expression of Abcg2 is much higher in mouse brain compared with the placenta and fetus (Maliepaard et al., 2001), which might explain the different permeability of the BBB and BPB to URB937. This is relevant to a translational interpretation of the present data, because levels of Abcg2 in mouse placenta are markedly lower than levels of ABCG2 in human placenta (Maliepaard et al., 2001). Thus, access of URB937 to the fetoplacental unit might be restricted more effectively in humans than in mice. However, differences in other xenobiotic transporters such as P-glycoprotein (Pgp/Abcb1) (Myllynen et al., 2010), known to overlap with Abcg2 in substrate specificity (Tang et al., 2011), as well as inter-species differences in the BPB architecture (Myllynen et al., 2010), might also affect URB937 penetration. Further experiments using ex vivo human placental perfusion should help to predict the interaction of URB937 with the BPB at different stages of human pregnancy at term and labour (Yeboah et al., 2008).
In conclusion, the present results indicate that Abcg2 limits the access of URB937 to the CNS and, partially, to the fetoplacental unit in female mice and rats. These findings offer new insights into the gender dimorphism of endocannabinoid-mediated antinociception and might be exploited therapeutically to develop analgesic agents that can be safely used during pregnancy and labour.
Acknowledgments
We thank Ilaria d'Elia and Cataldo Martucci for their help with experiments. This study was partially supported by grants from the National Institutes on Drug Abuse (RO1-DA-012413, DP1DA031387 to D P). The contribution of the Agilent Technologies/UCI Analytical Discovery Facility, Center for Drug Discovery, is gratefully acknowledged.
Glossary
- Δ9-THC
Δ9-tetrahydrocannabinol
- Abcg2/ABCG2
ATP-binding cassette subfamily G member 2
- BBB
blood-brain barrier
- BPB
blood-placenta barrier
- DCNP
2,6-dichloro-4-nitrophenol
- FAAH
fatty-acid amide hydrolase
- o.g
oral gavage
- Ko143
(3S,6S,12aS)-1,2,3,4,6,7,12,12a-Oct ahydro-9-methoxy-6-(2-methylpropyl)-1,4 dioxopyraz ino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester
- PEA
palmitoylethanolamide
- URB937
cyclohexylcarbamic acid 3′-carbamoyl-6-hydroxybiphenyl-3-yl ester
Conflict of interest
The authors declare the following conflict of interest: G M-S and D P are inventors in patent applications that describe peripheral FAAH inhibitors.
References
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- Astarita G, Ahmed F, Piomelli D. Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain. J Lipid Res. 2008;49:48–57. doi: 10.1194/jlr.M700354-JLR200. [DOI] [PubMed] [Google Scholar]
- Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Bagal S, El-Kattan A, Fenner K, Hay T, Kempshall S, et al. Full efficacy with no CNS side-effects: unachievable panacea or reality? DMPK considerations in design of drugs with limited brain penetration. Xenobiotica. 2012;42:11–27. doi: 10.3109/00498254.2011.617847. [DOI] [PubMed] [Google Scholar]
- Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of delta9-tetrahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval P, Lenoir V, Moussaoui S, Garret C, Kerdelhue B. Substance P and neurokinin A variations throughout the rat estrous cycle; comparison with ovariectomized and male rats: II. Trigeminal nucleus and cervical spinal cord. J Neurosci Res. 1996;45:610–616. doi: 10.1002/(SICI)1097-4547(19960901)45:5<610::AID-JNR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol. 2010;160:544–548. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Bell SC, Taylor DJ, Konje JC. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- Hahnova-Cygalova L, Ceckova M, Staud F. Fetoprotective activity of breast cancer resistance protein (BCRP, ABCG2): expression and function throughout pregnancy. Drug Metab Rev. 2011;43:53–68. doi: 10.3109/03602532.2010.512293. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Helliwell RJ, Chamley LW, Blake-Palmer K, Mitchell MD, Wu J, Kearn CS, et al. Characterization of the endocannabinoid system in early human pregnancy. J Clin Endocrinol Metab. 2004;89:5168–5174. doi: 10.1210/jc.2004-0388. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plast. 2009;2009:462–879. doi: 10.1155/2009/462879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res. 2010;186:141–157. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanz G, Barrera B, Guijarro A, d'Elia I, Otero JA, Alvarez AI, et al. The ABC membrane transporter ABCG2 prevents access of FAAH inhibitor URB937 to the central nervous system. Pharmacol Res. 2011;64:359–363. doi: 10.1016/j.phrs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllynen P, Kummu M, Sieppi E. ABCB1 and ABCG2 expression in the placenta and fetus: an interspecies comparison. Expert Opin Drug Metab Toxicol. 2010;6:1385–1398. doi: 10.1517/17425255.2010.514264. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, et al. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res. 2012;65:553–563. doi: 10.1016/j.phrs.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Tang SC, Lagas JS, Lankheet NA, Poller B, Hillebrand MJ, Rosing H, et al. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer. 2011;130:223–233. doi: 10.1002/ijc.26000. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. CB(1) receptor mediation of cannabinoid behavioral effects in male and female rats. Psychopharmacology (Berl) 2004;172:25–30. doi: 10.1007/s00213-003-1620-x. [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61:14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005;168:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci USA. 2003;100:14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MS, Zerangue N, Woodford K, Roberts LM, Tate EH, Feng B, et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59:404–413. doi: 10.1016/j.phrs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Yeboah D, Kalabis GM, Sun M, Ou RC, Matthews SG, Gibb W. Expression and localisation of breast cancer resistance protein (BCRP) in human fetal membranes and decidua and the influence of labour at term. Reprod Fertil Dev. 2008;20:328–334. doi: 10.1071/rd07133. [DOI] [PubMed] [Google Scholar]


