Abstract
Background and Purpose
Two oxidation products of linoleic acid, 9- and 13-hydroxy-octadecadienoic acids (HODEs), have recently been suggested to act as endovanilloids, that is, endogenous agonists of transient receptor potential vanilloid-1 (TRPV1) channels, thereby contributing to inflammatory hyperalgesia in rats. However, HODE activity at rat TRPV1 in comparison with the best established endovanilloid, anandamide, and its enantioselectivity and selectivity towards other TRP channels that are also abundant in sensory neurons have never been investigated.
Experimental Approach
We studied the effect of 9(R)-HODE, 9(S)-HODE, (+/–)13-HODE, 15(S)-hydroxyanandamide and anandamide on [Ca2+]i in HEK-293 cells stably expressing the rat or human recombinant TRPV1, or rat recombinant TRPV2, TRPA1 or TRPM8, and also the effect of 9(S)-HODE in rat dorsal root ganglion (DRG) neurons by calcium imaging.
Key Results
Anandamide and 15(S)-hydroxyanandamide were the most potent endovanilloids at human TRPV1, whereas 9(S)-HODE was approximately threefold less efficacious and 75- and 3-fold less potent, respectively, and did not perform much better at rat TRPV1. The 9(R)-HODE and (+/–)13-HODE were almost inactive at TRPV1. Unlike anandamide and 15(S)-hydroxyanandamide, all HODEs were very weak at desensitizing TRPV1 to the action of capsaicin, but activated rat TRPV2 [only (+/–)13-HODE] and rat TRPA1, and antagonized rat TRPM8, at concentrations higher than those required to activate TRPV1. Finally, 9(S)-HODE elevated [Ca2+]i in DRG neurons almost exclusively in capsaicin-sensitive cells but only at concentrations between 25 and 100 μM.
Conclusions and Implications
The present data suggest that HODEs are less important endovanilloids than anandamide.
Linked Articles
This article is part of a themed section on Cannabinoids. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.167.issue-8
Keywords: vanilloid, TRP channels, endocannabinoids, linoleic acid, lipoxygenases
Introduction
The transient receptor potential vanilloid-1 (TRPV1) channel plays a pivotal role in pain transduction pathways and in pathological conditions accompanied by hyperalgesia and/or allodynia (Caterina et al., 1997), as well as being important for thermoregulation (Caterina et al., 2000; Gavva et al., 2007). TRPV1 is expressed in the peripheral as well as CNS, including several areas involved in nociceptive transmission (Mezey et al., 2000; Cristino et al., 2006), with high levels of expression being observed in dorsal root, trigeminal and nodose ganglia (Caterina et al., 1997). TRPV1 is predominantly expressed in small- and medium-diameter unmyelinated fibres, mainly of peptidergic nature, which are important in the development of neurogenic inflammation. TRPV1 is a polymodal channel, activated by physical and chemical stimuli, including noxious heat (with a threshold of approximately 43°C), protons, voltage and plant vanilloids (Latorre et al., 2007; Moran et al., 2011) as well as by endogenous lipids, including some metabolites of lipoxygenases (i.e. 12-HETE and 15-HETE) (Hwang et al., 2000; Wen et al. 2012) and PLD (i.e. lysophosphatidic acid) (Nieto-Posadas et al., 2011). Some of these compounds share a structural similarity with endogenous fatty acid derivatives that have also been identified as TRPV1 agonists (Di Marzo et al., 1998; De Petrocellis et al., 2004), such as anandamide, N-arachidonoyldopamine and N-oleoyldopamine (Zygmunt et al., 1999; Huang et al., 2002; Chu et al., 2003), and 18–20 carbon unsaturated N-acylethanolamines (Movahed et al., 2005), which seem to bind to intracellular domains of TRPV1 (Jung et al., 1999; De Petrocellis et al., 2001). Interestingly, the systemic/spinal administration of TRPV1 antagonists blocks inflammation, heat hyperalgesia and mechanical allodynia (Kanai et al., 2007; Tang et al., 2007; Gavva, 2008). Accordingly, spinal TRPV1 activation leads to central sensitization to both thermal and mechanical stimuli (Cui et al., 2006; Pitcher et al., 2007). However, in the continuous presence of an activating stimulus, TRPV1 undergoes a Ca2+-dependent desensitization (Mohapatra and Nau, 2005), which explains why agonists at this channel can also produce anti-hyperalgesic effects (Starowicz et al., 2012).
Another channel that is emerging as a major player in inflammatory hyperalgesia is the transient receptor potential ankyryn-1 (TRPA1) channel, which is very often co-expressed with TRPV1 (McMahon and Wood, 2006). Activation of TRPA1 enhances inflammatory pain not only via well-demonstrated peripheral sensitization (Bautista et al., 2006; Dai et al., 2007) but also through central sensitization, by increasing glutamate release from primary afferent terminals to enhance synaptic transmission (Kosugi et al., 2007). Furthermore, TRPA1 activation can result in TRPV1 cross-activation and cross-desensitization (Akopian,2011) as well as producing analgesia via inhibition of voltage-gated calcium and sodium currents (Andersson et al., 2011). Finally, transient receptor potential vanilloid-2 (TRPV2) and melastatin-8 (TRPM8) channels are also expressed in sensory neurons and it has been suggested they play a role in pain. Interestingly, all these channels, like TRPV1, are known to be modulated by plant cannabinoids and endocannabinoids, and are thought to act as ‘ionotropic cannabinoid receptors’ (De Petrocellis et al., 2011; see Akopian et al., 2009 and De Petrocellis and Di Marzo, 2010, for reviews).
It was hypothesized that peripheral tissue injury leads to the generation of endogenous TRPV1 ligands in the spinal cord, which then activate TRPV1 in the CNS, resulting in central sensitization contributing to mechanical allodynia. These studies led to the recent discovery of a new family of endogenous TRPV1 ligands, 9-hydroxyoctadecadienoic (9-HODE) and 13-hydroxyoctadecadienoic (13-HODE) acid. Indeed, calcium influx caused by peripheral inflammation may activate lipoxygenases that can oxidize the ω-6 fatty acid, linoleic acid (Brinckmann et al., 1998), and lead to the production of 9- and 13-HODE, which evoke mechanical allodynia following spinal administration (Patwardhan et al., 2009). These putative endogenous TRPV1 agonists are also formed on exposure of cell plasma membranes to noxious heat, thus contributing to the thermal responsiveness of this channel (Patwardhan et al., 2010). As they are formed near the plasma membrane, the intracellular concentrations of these substances in the vicinity of TRPV1 could become very high and get over the threshold for TRPV1 activation in sensory neurons, with subsequent release of neuropeptides such as calcitonin gene-related peptide (CGRP) and/or substance P, which in turn would result in neurogenic inflammation (Patapoutian et al., 2009).
The purpose of the present work was to explore the activity at rat and human TRPV1 of 9- and 13-HODE by means of fluorescence-based intracellular Ca2+ assays in HEK-293 cells overexpressing these proteins and calcium imaging experiments in DRG neurons. In particular, we assessed for the first time (1) which of the two enantiomers of 9-HODE exhibits highest potency and efficacy, in comparison with a lipoxygenase metabolite of anandamide, and (2) the selectivity of 9- and 13-HODE at rat TRPV1 versus rat recombinant TRPA1, TRPV2 and TRPM8 channels, also overexpressed in HEK-293 cells.
Methods
Chemicals
9(R)-HODE, 9(S)-HODE, (+/–)9-HODE [an approximately equimolar mixture of 9(R)- and 9(S)-HODE)], (+/–)13-HODE [an approximately equimolar mixture of 13(R)- and 13(S)-HODE] and 15(S)-HETE ethanolamide [15(S)-hydroxy-N-(2-hydroxyethyl)-5Z,8Z,11Z,13E-eicosatetraenamide] were purchased from Cayman Chemical (Ann Arbor, MI). DRG neurons were purchased from Lonza (Lonza Walkersville, MD). Drug, receptor and channel nomenclature has been used according to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
TRPV1 channel assays
HEK-293, wild-type and stably transfected clones containing either the human or rat TRPV1-cDNA cells, were grown as monolayers in EMEM supplemented with nonessential amino acids, 10% FBS and 2 mM glutamine, maintained under 5% CO2 at 37°C plated on 100 mm diameter Petri dishes. The effect of the substances on intracellular Ca2+ concentration ([Ca2+]i) was determined by using Fluo-4-AM, a selective intracellular fluorescent probe for Ca2+. On the day of the experiment, cells were loaded for 1 h in the dark at room temperature with the methyl ester Fluo-4-AM (4 μM; Invitrogen Life Technologies, Grand Island, NY. USA) in EMEM then were washed twice in Tyrode's buffer (145 mM NaCl, 2.5 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 10 mM d-glucose and 10 mM HEPES, pH 7.4), resuspended in Tyrode's buffer and transferred to the quartz cuvette of the spectrofluorimeter (Perkin-Elmer LS50B; Perkin-Elmer Life, , Waltham, MA) under continuous stirring. [Ca2+]i was determined before and after the addition of various concentrations of test compounds by measuring cell fluorescence (λEX = 488 nm, λEM = 516 nm) at 25°C. Curve fitting (sigmoidal dose–response variable slope) and parameter estimation were performed with GraphPad Prism® (GraphPad Software Inc., San Diego, CA). Potency was expressed as the concentration of test substances exerting a half-maximal agonist effect (i.e. half-maximal increases in [Ca2+]i), EC50. The efficacy of TRPV1 agonists was first determined by normalizing their effect to the maximum Ca2+ influx effect on [Ca2+]i observed after the application of 4 μM ionomycin (Enzo). Antagonist/desensitizing behaviour was evaluated against capsaicin (0.1 μM) by adding the test compounds in the quartz cuvette 5 min before stimulation of cells with agonists. Data are expressed as the concentration exerting a half-maximal inhibition of agonist-induced [Ca2+]i elevation (IC50), which was calculated again using GraphPad Prism® software. The effect on [Ca2+]i exerted by agonist alone was taken as 100%. Dose–response curves were fitted by a sigmoidal regression with variable slope. All determinations were performed at least in triplicate. Statistical analysis of the data was performed by analysis of variance at each point using anova followed by the Bonferroni's test. Although previous studies using Fluo-3 or Fluo-4 only very seldom report the actual changes in the [Ca2+]i caused by compounds in cells, this can be calculated using the following equation: [Ca2+]free = KD [F − Fmin]/[Fmax − F], where Fmin is the fluorescence intensity of the indicator in the absence of calcium (baseline fluorescence), Fmax is the fluorescence of the calcium saturated indicator (e.g. after treatment of cells with 4 μM ionomycin), F is the fluorescence at intermediate calcium levels and KD = 345 nM for Fluo-4 [Gee et al., 2000].
TRPV2, TRPA1 and TRPM8 assays
HEK-293 cells stably overexpressing recombinant rat TRPA1, rat TRPM8 or rat TRPV2 were selected by G-418 (Geneticin, 600 μg mL−1; Invitrogen), grown on 100 mm diameter Petri dishes as monolayers in minimum essential medium supplemented with non-essential amino acids, 10% FBS and 2 mM glutamine, and maintained under 5% CO2 at 37°C. The assays were conducted as described above for TRPV1, except for TRPM8-HEK-293 cells, for which a Peltier System (PTP-1 Fluorescence Peltier System; Perkin-Elmer Life and Analytical Sciences) helped us to maintain temperature at 25°C. The effects of the compounds on TRPA1 are expressed as a percentage of the effect obtained with 100 μM allylisothiocyanate (AITC). Antagonist/desensitizing behaviour was evaluated against icilin (0.25 μM) for TRPM8, AITC (100 μM) for TRPA1 or lysophosphatidylcholine (LPC) (3 μM) for TRPV2, by adding the compounds in the quartz cuvette 5 min before stimulation of cells with agonists.
Calcium imaging
Calcium imaging experiments were performed on rat DRG cells cultured in Primary Neuron Basal Medium (PNBM) supplemented with 2 mM L-glutamine, 50 μg mL−1 gentamicin/37 ng mL−1 amphotericin and 2% neural cell-survival factor (NSF-1). Briefly, from liquid nitrogen, the cells were placed in a water bath preheated to 37°C, plated onto a 24-well plate culture dish containing poly-L-lysine (33μg mL−1) pre-coated coverslips and incubated for 4 h at 37°C and 5% CO2. For inhibition of Schwann cell proliferation, mitotic inhibitors uridine (17.5 μg mL−1) and 5-fluoro-2-deoxyuridine (7.5 μg mL−1) were added to the wells 4 h after the plating and every time the media was changed. On the day of the experiment, the cells were loaded for 1 h at 37°C with the cytoplasmic calcium indicator Fluo-4AM (Invitrogen), 4 μM in DMSO containing 0.02% Pluronic F-127 (Invitrogen) in PNBM medium. Experiments were carried out in the following extracellular solution: 145 mM NaCl, 2.5 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 10 mM d-glucose and 10 mM HEPES (pH 7.4). The coverslips were placed into a perfusion chamber (Leica Microsystems, Wetzlar, Germany) mounted on the stage of an inverted fluorescent Leica Digital microscope DMI6000 (Leica Microsystems) equipped with a 20× objective lens. Experiments were carried out with a digital imaging system composed of Leica DFC320 cooled digital CCD camera (Leica Microsystems), with the appropriate filter wheel and a LAS AF 2.2 Live Data Mode software for calcium imaging (Leica Microsystems). The excitation light for Fluo-4 was filtered through a 460–495 nm excitation filter, and the emitted light was collected through a 510–550 nm filter. Images were digitized and analysed using LAS-AF Analyses software and the fluorescence intensity in specific regions of interest was measured before and after the addition of various concentrations of 9(S)-HODE or capsaicin. To identify neurons responding to both 9(S)-HODE and capsaicin, 9(S)-HODE was added to DRG cultures 5 min before capsaicin 1 μM. At the end of the experiment, the maximal increase in fluorescence (Fmax) was obtained by application of the calcium ionophore ionomycin 4 μM. Calcium imaging data are expressed as F/Fmax, where F is the increase of fluorescence after 9(S)-HODE addition. A simple normalization procedure was utilized to compare the relative fluorescent signals between experiments and correlate them to calcium. For single wavelength excitation/emission, the simplest procedure is to divide changes in the fluorescent signal by the average resting fluorescence ΔCa2+ = ΔF/F = (F − Frest)/Frest, where F = dye fluorescence at any given time, Frest = average fluorescence signal prior to addition of agonist.
Results
Effect of HODEs at human and rat TRPV1
As shown in Figure 1, the addition of 9-HODE activated TRPV1-mediated intracellular Ca2+ elevation, although with very low efficacy (between ∼14% and ∼38%) compared with that induced by anandamide. Importantly, the potency and efficacy of 9-HODE depend on the configuration of the C-9 chiral centre, with higher efficacy being exhibited by 9(S)-HODE (i.e. the likely naturally occurring enantiomer) (Table 1). The 9(R) enantiomer exhibited very low efficacy (<10% of 4 μM ionomycin), thus rendering inaccurate any measurement of its potency (Table 1). The (S) isomer also proved to be the most active enantiomer at rat TRPV1, although in this case the potency was higher and the efficacy lower than those observed with the human TRPV1 (Table 1). Racemic 13-HODE was less efficacious (12.7 ± 0.5%) and potent (27.5 ± 4.2 μM) than 9(S)-HODE at human TRPV1 (Figure 1; Table 1). All responses were attenuated by at least 50% by pre-incubation of cells with the selective TRPV1 antagonist iodo-resiniferatoxin (I-RTX, 0.1 μM) (Wahl et al., 2001) and were not observed in wild-type (i.e. non-transfected) HEK-293 cells (data not shown).
Figure 1.
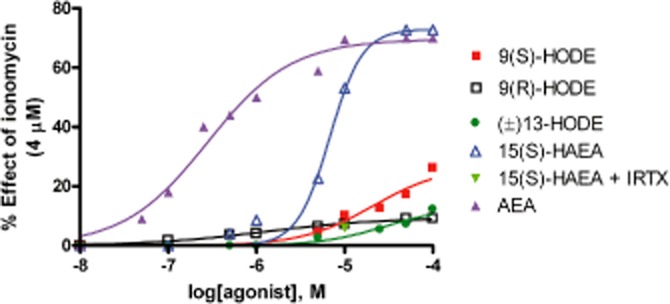
Effect of 9(S)-HODE, 9(R)-HODE, (+/–)13-HODE and 15(S)-hydroxy-anandamide [15(S)-HAEA] on intracellular Ca2+ elevation in HEK-293 cells overexpressing the human recombinant TRPV1 channel. The effect of a 5 min pre-incubation with the TRPV1 antagonist iodo-resiniferatoxin (IRTX) on the response to 10 μM 15(S)-HAEA is also shown. Also the effect of AEA is shown. Data are means of n = 3 separate determinations. SEM bars are not shown for the sake of clarity and were never higher than 10% of the means.
Table 1.
Effect of linoleic acid and anandamide oxidative metabolites on various transient receptor potential channels
| TRPV1 | TRPM8 | TRPA1 | TRPV2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Efficacy (% ionomycin, 4 μM) | Potency (EC50, μM) | Efficacy of des. (% at max tested conc.) | Des. vs. Caps. (0.1 μM) (IC50, μM) | Efficacy of inhib. (% at max tested conc.) | Inh. icilin (0.25 μM) (IC50, μM) | Efficacy (% AITC, 100 μM) | Potency (EC50, μM) | Efficacy of des. (% at max tested conc.) | Des. versus AITC (100 μM) (IC50, μM) | Efficacy (% ionomycin 4 μM) | Potency (EC50, μM) | Efficacy of des. (% at max tested conc.) | Des. versus LPC (3 μM) (IC50, μM) | |
| 9(S)-HODE | 26.7 ± 1.0 | 21.0 ± 3.2 | 51.5 ± 1.5 | 86.2 ± 5.1 | 86.6 ± 2.5 | 43.6 ± 0.9 | 44.9 ± 1.8 | 31.9 ± 4.2 | 58.8 ± 0.6 | 43.6 ± 0.9 | <10 | NM | 40.8 ± 0.7 | >100 |
| 9(R)-HODE | <10 | NM | 29.0 ± 1.0 | >100 | 86.6 ± 1.5 | 55.7 ± 0.1 | ||||||||
| (+/-)13-HODE | 12.7 ± 0.5 | 27.5 ± 4.2 | 14.7 ± 0.5 | >100 | 79.8 ± 0.5 | 69.8 ± 3.6 | 40.0 ± 1.4 | 12.6 ± 2.2 | 61.6 ± 1.3 | 72.5 ± 0.8 | 12.5 ± 1.3 | 43.5 ± 1.6 | 47.4 ± 0.9 | >100 |
| 15(S)-HAEA | 72.9 ± 1.2 | 6.8 ± 0.4 | 100 | 6.3 ± 0.4 | ||||||||||
| Anandamide | 69.5 ± 1.6 | 0.28 ± 0.03 | 100 | 0.21 ± 0.01 | 100 | 0.15 ± 0.08 | 158.7 ± 11.1 | 10.1 ± 1.9 | 100 | 21.0 ± 1.6 | NM | NM | NM | NM |
| 9(S)-HODE(rTRPV1) | 13.9 ± 0.6 | 11.6 ± 3.0 | 38.6 ± 0.5 | >100 | ||||||||||
| 9(R)-HODE(rTRPV1) | <10 | NM | >100 | |||||||||||
Effect on TRPV1: The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods, in HEK-293 cells stably over-expressing the human or the rat recombinant TRPV1 channel, and as a control, in wild-type HEK-293 cells. Efficacy was calculated as % of the effect obtained with ionomycin (4 μM). Capsaicin efficacy in this test is ∼75% of the effect of ionomycin. In the antagonism-desensitization experiments, the compounds were given to cells 5 min before capsaicin (0.1 μM), and data for the efficacy of desensitization (inhibition obtained at the maximum tested concentration) and potency of desensitization are provided. Data obtained for 9(S)- and 9(R)-HODE in HEK-293 cells overexpressing the rat recombinant TRPV1 (rTRPV1) are also shown in the last two lines of the Table. Data are means ± SD of n = 3 separate experiments. NM, not measurable.
Effect on TRPM8: The effect of the compounds and anandamide on the elevation of intracellular calcium was measured by fluorescence as described in Methods, in HEK-293 cells stably over-expressing the rat recombinant TRPM8 channel, and as a control, in wild-type HEK-293 cells. Data are means ± SD of n = 3 separate experiments in which various concentrations of the compounds were given to cells 5 min before icilin (0.25 μM). None of the compounds exerted any significant TRPM8-mediated effect on intracellular calcium per se (not shown).
Effect on TRPA1: The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods, in HEK-293 cells stably over-expressing the rat recombinant TRPA1 channel, and as a control, in wild-type HEK-293 cells. Efficacy was calculated as % of the effect obtained with allyl isothiocyanate (AITC, 100 μM), the effect of which was ∼30% of that of ionomycin (4 μM). In the antagonism-desensitization experiments, the compounds were given to cells 5 min before AITC (100 μM), and data for the efficacy of desensitization (inhibition obtained at the maximum tested concentration) and potency of desensitization are provided. Data are means ± SD of n = 3 separate experiments.
Effect on TRPV2: The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods, in HEK-293 cells stably over-expressing the rat recombinant TRPV2 channel, and as a control, in wild-type HEK-293 cells. Efficacy was calculated as % of the effect obtained with ionomycin (4 μM). In the antagonism-desensitization experiments, the compounds were given to cells 5 min prior to LPC (3 μM) and data for the efficacy of desensitization (inhibition obtained at the maximum tested concentration), and potency of desensitization are provided. NM, not measurable. Data are means ± SD of n = 3 separate experiments.
The HODEs were much less potent and efficacious than anandamide (EC50 = 0.28 ± 0.03 μM) (Table 1) at human TRPV1. Also the 15-lipoxygenase oxidation product of anandamide, 15(S)-hydroxy-AEA [15(S)-HAEA], a fatty acid amide hydrolase inhibitor (van der Stelt et al., 2002), was more efficacious (72.9 ± 0.2% of the maximal effect observed with 4 μM ionomycin) and potent (6.8 ± 0.4 μM) at human TRPV1 than all HODEs (Table 1), its effect being antagonized by I-RTX (Figure 1).
To further characterize the TRPV1 activity of these compounds, we assessed whether or not they are able to desensitize the capsaicin (0.1 μM)-induced and TRPV1-mediated effects on [Ca2+]i in HEK-293 stably expressing the human TRPV1. 15(S)-HAEA was able to desensitise TRPV1 with an IC50 = 6.3 ± 0.4 μM (Figure 2), whereas among the two 9-HODE enantiomers, only 9(S)-HODE was able to desensitize to some extent the channels, although with much lower potency (IC50 = 86.2 ± 5.1 μM) (Figure 2; Table 1).
Figure 2.
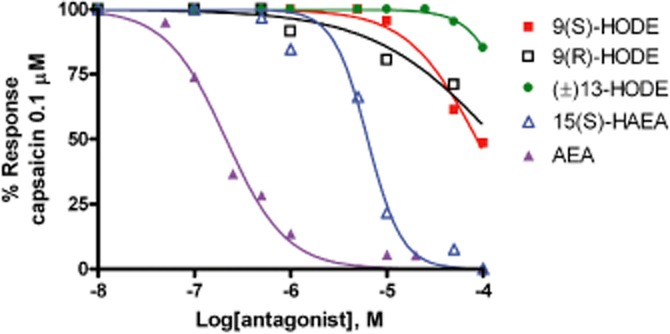
Desensitization by 5 min pre-incubation with 15(S)-hydroxy-anandamide [15(S)-HAEA] or 9(S)-HODE, 9(R)-HODE, (+/–)13-HODE and AEA, of capsaicin (0.1 μM)-induced Ca2+ elevation in HEK-293 cells over-expressing the human recombinant TRPV1 channel. Data are means of n = 3 separate determinations. SEM bars are not shown for the safe of clarity and were never higher than 10% of the means. The curves were fitted by considering 100% inhibition at 1 mM.
Effect of HODEs on other rat TRP channels
Both 9(S)-HODE and (+/–)13-HODE also antagonized TRPM8-mediated elevation of [Ca2+]i by icilin in HEK-293 cells stably expressing the rat TRPM8, but with much lower potency (IC50 ∼ 40–70 μM) than anandamide (IC50 ∼ 0.2 μM), when this latter was tested under the same conditions (De Petrocellis et al., 2007) (see Table 1). However, this antagonism was exerted at concentrations not too dissimilar (fourfold higher) than those required for rat TRPV1 activation (Table 1).
Racemic 13-HODE and 9(S)-HODE also activated TRPA1-mediated elevation of [Ca2+]i in HEK-293 cells stably expressing the rat TRPA1, with similar potency (EC50 = 13–32 μM) to anandamide (EC50 = 10.1 ± 1.9 μM), when this latter was tested under the same conditions (De Petrocellis and Di Marzo, 2009) (Table 1). Also in this case, the compounds desensitized the channel to activation by its agonist, AITC (Table 1).
Finally, racemic 13-HODE, but not 9(S)-HODE, also activated TRPV2-mediated elevation of [Ca2+]i in HEK-293 cells stably expressing rat TRPV2, with higher potency (EC50 = 43 μM) than anandamide (EC50 > 50 μM), when this latter was tested under the same conditions (Di Marzo and De Petrocellis, unpublished observations) (Table 1) (see also Qin et al., 2008). Again, the potency of (+/–)13-HODE was similar to that observed at TRPV1 (EC50 = 27.5 ± 4.2 μM; Table 1).
Effect of 9(S)-HODE on [Ca2+]i in DRG neurons
Since 9(S)-HODE was the most potent and efficacious of the HODEs tested here, we determined whether this compound also exhibited activity in primary cells constitutively expressing TRP channels. Results obtained with Ca2+ imaging experiments carried out on rat DRG neurons (n = 40), using calcium imaging and employing Fluo-4 as the fluorimetric probe, showed that both 50 μM 9(S)-HODE and 1 μM capsaicin increased intracellular Ca2+ levels in 96.2% of the responsive cells. 9(S)-HODE exhibited a low efficacy (24.7 ± 3.5% of the effect of 4 μM ionomycin and 27.4 ± 5.2% of the effect of 1 μM capsaicin; mean ± SD, n = 30) not different from that observed in rat recombinant TRPV1 transfected HEK-293 cells, but appeared to be less potent, since the 25 μM concentration was almost inactive, and only the 50 μM concentration exhibited full activity (Figure 3).
Figure 3.
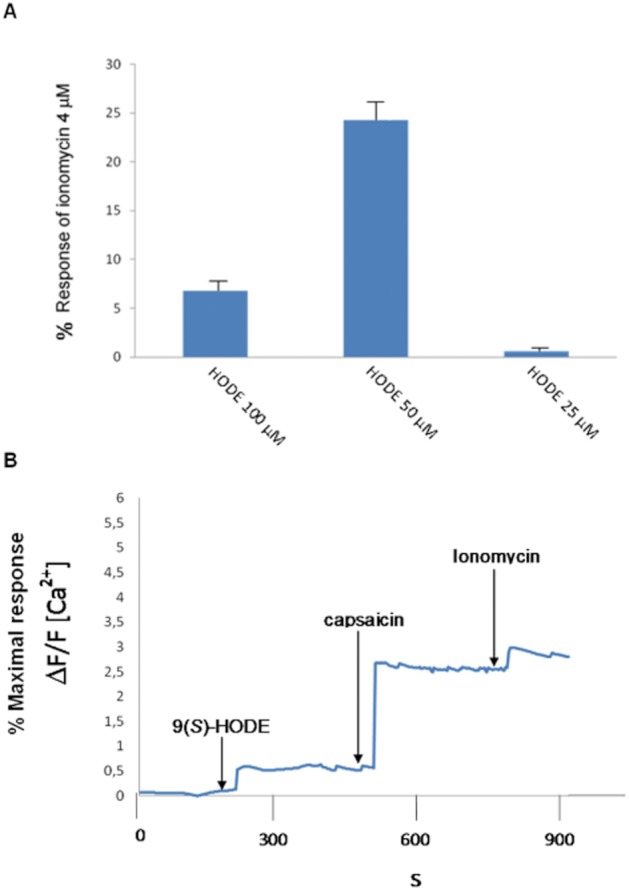
9(S)-HODE 50 μM induces an increase in [Ca2+]i in rat DRG neurons. (A) Normalized increase in Fluo-4 fluorescence (mean ± SD) induced by 9(S)-HODE, 25, 50 and 100 μM. The application of 9(S)-HODE in the bath triggered a calcium response in a concentration-dependent manner. The 25 and 100 μM concentrations of 9(S)-HODE were less efficacious than the 50 μM concentration. Data are means ± SD of measures made in n = 30 cells for each concentration tested. (B) Shows the representative time course of the Fluo-4 signals recorded from 20 to 40 cells as response to 9(S)-HODE (50 μM), capsaicin (1 μM) and ionomycin (4 μM). Arrows indicate when compounds were added. Images were collected continuously for 30 min. Note that 9(S)-HODE exhibited a low efficacy (on average 24.7 ± 3.5% of the effect of 4 μM ionomycin and 27.4 ± 5.2% of the effect of 1 μM capsaicin; means ± SD of n = 30).
Discussion
We have shown in this study that the previously suggested endogenous TRPV1 agonist, 9-HODE, when tested in HEK-293 cells overexpressing human or rat recombinant TRP channels, is an ‘endovanilloid’ significantly less potent, efficacious and selective towards TRPV1 channels than anandamide. Furthermore, 9-HODE is a weak agonist in rat DRG neurons and only at concentrations higher than 25 μM, although the 100 μM concentration of this compound was less efficacious than the 50 μM concentration, in agreement with the frequent observation that high concentrations of agonists at TRPV1 might quickly desensitize this channel (Touska et al., 2011). We have demonstrated for the first time that only the S enantiomer of 9-HODE, which is the one more likely to be produced in vivo from the action of a mammalian 8(S)-lipoxygenase, exhibits measurable activity at TRPV1. The putative 15(S)-lipoxygenase derivative of linoleic acid, 13-HODE, instead, was nearly inactive, unlike the corresponding 15(S)-lipoxygenase derivative of anandamide, 15(S)-HAEA, the high potency and, particularly, efficacy of which at human recombinant TRPV1 we report here for the first time. These data suggest that if 15(S)-lipoxygenase does contribute to TRPV1-mediated inflammatory hyperalgesia, it is more likely to do so through the oxidation of anandamide, or arachidonic acid (see Hwang et al., 2000), than linoleic acid.
Although the potency and efficacy of 9(S)-HODE at TRPV1 reported here might still be sufficient for this compound to activate TRPV1 in vivo, since its local concentration might be higher than that of the more potent and efficacious anandamide, it remains to be explained why the effects observed here for this compound were weaker than those previously reported. However, it must be noted that (1) Patwardhan et al. (2009) reported TRPV1-mediated effects of 9-HODE that were mostly exerted at a 100 μM concentration and stated that these effects could be observed also at much lower concentrations, although they did not show the data; (2) Patwardhan et al. (2010), instead, showed TRPV1-mediated effects starting from 100 nM or 1 μM, for 9-HODE and 13-HODE respectively. However, the respective assessed endpoints (release of CGRP from cultured rat trigeminal ganglion neurons, assessed by radioimmunoassay, and membrane currents in TRPV1-transfected CHO cells, assessed by patch clamp) were different from those measured in this study (i.e. [Ca2+]i elevation in HEK-293 cells stably expressing rat or human TRPV1), and in rat DRG neurons. Nevertheless, intracellular calcium-based assays are supposed to be a more sensitive measure of TRPV1-mediated effects, as shown by the fact, for example, that anandamide can be 10- to 20-fold more potent at elevating [Ca2+]i than at producing cation currents measured by patch clamp electrophysiology (De Petrocellis and Di Marzo, 2009). Since Patwardhan et al. (2010) also reported the occurrence of synergistic TRPV1-mediated effects between various oxidative metabolites of linoleic acid, or between high temperature and these compounds, it is possible that the presence of other ‘endovanilloids’ in one type of assay and not in another, or the use of a higher assay temperature, explains in part the discrepancies between the potencies of HODEs found here and previously.
Another original finding of this study was the observation that HODEs, much in the same way as TRPV1-active plant cannabinoids and endocannabinoids (Di Marzo and De Petrocellis, 2010; De Petrocellis et al., 2011), also activate TRPA1 and/or TRPV2. However, unlike anandamide, which exhibits a strong selectivity for TRPV1 versus TRPA1 and TRPV2, 9- and 13-HODE were shown here, again using [Ca2+]i elevation measurements in transfected HEK-293 cells, to produce effects via these channels at concentrations comparable with those needed to activate TRPV1; and, in fact, 13-HODE was even more potent at TRPA1 than TRPV1. Therefore, these linoleic acid metabolites cannot be regarded as selective ‘endovanilloids’. As to their effects as functional antagonists at TRPM8, this may merely reflect the fact that most of the TRPV1 agonists investigated so far exhibit functional antagonism at this channel (De Petrocellis et al., 2007).
In conclusion, from the present data, it seems unlikely that 9(S)-HODE or (+/–)13-HODE, rather than arachidonic acid derivatives, such as anandamide or 15(S)-HAEA, are mostly responsible for tonic TRPV1 activity in the spinal cord during inflammatory pain, even accounting for their potentially higher concentrations or the presence of TRPV1-sensitizing factors. Indeed, other oxidation products of arachidonic acid, such as the fungal metabolite 3-HETE (De Petrocellis et al., 2009), and the P450 arachidonic acid metabolite, 20-HETE (Wen et al., 2012), activate native and heterologously expressed TRPV1 with EC50 values in the low micromolar range; whereas low nanomolar concentrations of derivatives from the oxidation of docosahexaenoic acid seem instead to act as indirect endogenous inhibitors of TRPV1 and TRPA1 (Park et al., 2011). However, when these oxidative metabolites are generated in the periphery in inflammation, they might have different effects at primary afferent nerve terminals, when compared with those observed here on DRG cell bodies or transfected cells. Clearly, several other factors, which cannot be always investigated in vitro, need to be taken into account when trying to understand whether or not lipid mediators such as those investigated here do effectively interact with a given molecular target in vivo. These include the availability of biosynthetic precursors for the ligand and the relative abundance of enzymes catalysing its metabolism. For example, several conditions might increase the release and lipoxygenation of linoleic acid rather than arachidonic acid or anandamide, and the amounts of these biosynthetic precursors might also be affected by the intake of certain dietary fats (Batetta et al., 2009). However, while anandamide is certainly less abundant than arachidonic and linoleic acid, several ganglia from the rat were shown to contain less of the latter as compared to arachidonic acid (Hara et al., 1988). On the other hand, anandamide is clearly metabolised through many more catabolic pathways (including both hydrolytic and oxidative pathways) (Di Marzo, 2008) than 9(S)-HODE and 13-HODE, which are almost uniquely converted to the even less potent TRPV1 agonists, 9-oxoODE and 13-oxoODE (Patwardhan et al., 2009). This makes it less likely that the concentrations of anandamide and 15(S)-HAEA reached in tissues are higher than those of 9(S)-HODE and 13-HODE. Indeed, although its metabolism has not been investigated, 15(S)-HAEA, due to the presence of several double bonds in its chemical structure, is also likely to be oxidised in a more extensive way than 9(S)-HODE and 13-HODE, as shown previously for 15-hydroxy-eicosatetraenoic acid (Bergholte et al., 1987; Wei et al., 2009). Finally, the picture may also change following treatment with drugs affecting arachidonic acid and anandamide metabolism. For example, given that endocannabinoids, but not LA, are substrates for COX-2, inhibition of this enzyme may shift the balance between 15(S)-HAEA and 9(S)-HODE/13-HODE towards the former, thus affecting TRPV1 activation. Future studies need to be performed to investigate to what relative extent anandamide and lipoxygenase derivatives of unsaturated fatty acids and fatty acid ethanolamides act as ‘endovanilloids’ in vivo.
Glossary
- AEA
anandamide
- ANOVA
analysis of variance
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglion
- EMEM
Eagle's minimal essential medium
- 15-HAEA
15-hydroxy-anandamide (15(S)-hydroxy-N-(2-hydroxyethyl)-5Z,8Z,11Z,13E-eicosatetraenamide)
- 12-HETE
12-hydroxy-eicosatetraenoic acid
- 15-HETE
15-hydroxy-eicosatetraenoic acid
- 9-HODE
9-hydroxy-octadecadienoic acids
- 13-HODE
13-hydroxy-octadecadienoic acids
- NSF-1
neural cell-survival factor 1
- PNBM
primary neuron basal medium
- TRPA1
transient receptor potential ankyrin-1 channels
- TRPM8
transient receptor potential melastatin-8 channels
- TRPV1
transient receptor potential vanilloid-1 channels
- TRPV2
transient receptor potential vanilloid-2 channels
Conflict of interest
The authors have no conflict of interest to declare with regard to the contents of this article.
References
- Akopian AN. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr Pharm Biotechnol. 2011;12:89–94. doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2009;30:79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat Commun. 2011;2:551. doi: 10.1038/ncomms1559. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- Batetta B, Griinari M, Carta G, Murru E, Ligresti A, Cordeddu L, et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J Nutr. 2009;139:1495–1501. doi: 10.3945/jn.109.104844. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bergholte JM, Soberman RJ, Hayes R, Murphy RC, Okita RT. Oxidation of 15-hydroxyeicosatetraenoic acid and other hydroxy fatty acids by lung prostaglandin dehydrogenase. Arch Biochem Biophys. 1987;257:444–450. doi: 10.1016/0003-9861(87)90589-3. [DOI] [PubMed] [Google Scholar]
- Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kühn H. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91:64–74. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Cristino L, De Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium. 2009;45:611–624. doi: 10.1016/j.ceca.2009.03.003. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143:251–256. doi: 10.1038/sj.bjp.0705924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res. 2007;313:1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Deva R, Mainieri F, Schaefer M, Bisogno T, Ciccoli R, et al. Chemical synthesis, pharmacological characterization, and possible formation in unicellular fungi of 3-hydroxy-anandamide. J Lipid Res. 2009;50:658–666. doi: 10.1194/jlr.M800337-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, et al. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- Hara A, Taketomi T, Iwata M, Ando M, Nagata Y. Differences in neuronal lipid composition between superior cervical ganglia and nodose ganglia of the rat. Biochim Biophys Acta. 1988;960:427–434. [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;1999:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hara T, Imai A, Sakakibara A. Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia. J Pharm Pharmacol. 2007;59:733–738. doi: 10.1211/jpp.59.5.0015. [DOI] [PubMed] [Google Scholar]
- Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci. 2007;27:4443–4451. doi: 10.1523/JNEUROSCI.0557-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42:427–438. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124:1123–1125. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, Ermund A, et al. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Nieto-Posadas A, Picazo-Juárez G, Llorente I, Jara-Oseguera A, Morales-Lázaro S, Escalante-Alcalde D, et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol. 2011;8:78–85. doi: 10.1038/nchembio.712. [DOI] [PubMed] [Google Scholar]
- Park C-K, Xu Z-Z, Liu T, Lü N, Serhan CN, Ji R-R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher MH, Price TJ, Entrena JM, Cervero F. Spinal NKCC1 blockade inhibits TRPV1-dependent referred allodynia. Mol Pain. 2007;3:17. doi: 10.1186/1744-8069-3-17. doi: 10.1186/1744-8069-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Makuch W, Osikowicz M, Piscitelli F, Petrosino S, Di Marzo V, et al. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology. 2012;62:1746–1755. doi: 10.1016/j.neuropharm.2011.11.021. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, van Kuik JA, Bari M, van Zadelhoff G, Leeflang BR, Veldink GA, et al. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J Med Chem. 2002;45:3709–3720. doi: 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- Tang L, Chen Y, Chen Z, Blumberg PM, Kozikowski AP, Wang ZJ. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N'-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- Touska F, Marsakova L, Teisinger J, Vlachova V. A ‘cute’ desensitization of TRPV1. Curr Pharm Biotechnol. 2011;12:122–129. doi: 10.2174/138920111793937826. [DOI] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Wei C, Zhu P, Shah SJ, Blair IA. 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol Pharmacol. 2009;76:516–525. doi: 10.1124/mol.109.057489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Ostman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, et al. 20-hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of TRPV1. J Biol Chem. 2012;287:13868–13876. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


