Abstract
In 2009 canine filarial infections were investigated in two northern areas of Serbia (Pančevo and Veliko Gradište), applying morphometry, biochemical staining, and immunological kit to detect Dirofilaria immitis antigens, and two home-made ELISAs to detect antibodies to D. repens and D. immitis somatic/metabolic polyproteins. Moreover, molecular tools were applied to analyze the phylogenetic relationships of the isolates. The microfilariae detected in 21/122 dogs (17.2%) were identified as D. repens (n=21) and D. immitis (n=2). D. immitis antigens were found in another 13 animals with occult infection. All of the 15 heartworm-positive dogs also had antibodies to this parasite, which were detected in another 13 subjects, indicating an overall D. immitis seroprevalence rate of 22.9%. Serology for D. repens revealed evidence of antibodies in 42.6% of the dogs, but was negative for 4 microfilaremic dogs. As for the two different areas, the prevalence of microfilariae and/or D. immitis antigens, mainly due to D. repens microfilaremic animals, was not significantly higher in Veliko Gradište (33.3%) than in Pančevo (22%). However, serology showed a different epidemiological picture. Heartworm infection occurred more often in both areas, and antibodies to dirofilarial nematodes were detected in 72.9% of dogs living in Pančevo, a rate higher than in those living in Veliko Gradište (57.1%). No risk factors for infection were found, confirming the uselessness of prophylactic drugs against D. repens, and suggesting the presence in these areas of sunrise- or sunset-biting mosquitoes as important vectors. The results indicate the need for both appropriate entomological studies and further research on the intra-species variability shown by D. repens.
Key Words: Dirofilaria, Epidemiology, Genetics, Serbia
Introduction
Dirofilaria cause mosquito-borne zoonoses endemic in areas with temperate, tropical, and subtropical climatic conditions. The species of major interest to veterinary and public health officials are Dirofilaria immitis (cosmopolitan) and Dirofilaria repens (found only in the Old World). D. immitis, also known as heartworm due to its location in the vertebrate host (it inhabits the right ventricle and pulmonary arteries), can cause infections in dogs, cats, and foxes, but also affects lions, ferrets, sea lions, and even horses, bears, orangutans, and humans (Duran-Struuck and Hernandez 2001). Most infected dogs do not show symptoms for months or years, but the infection can become fatal when the parasitic burden causes severe damage to arteries and to the cardiac chambers (Bolio-Gonzales et al. 2007). On the contrary, D. repens mainly resides in cutaneous/subcutaneous tissues of animals, where it induces mild infection, with the severity related to the parasitic load and to the workload of the animal (Cringoli et al. 2001). Aside from abortive infections that cannot be ascribed to any species, immature specimens of D. repens, migrating (often at an ocular level) or trapped in inflammatory nodules, are the most frequently identified etiological agents of human infections (Pampligione and Rivasi 2000; Džamić et al. 2009; Genchi et al. 2011). Superficial forms (in subcutaneous or submucosal tissues, rarely in the dermis or muscles) are the most easily detected and therefore the most commonly reported, whereas internal infections (lungs or other internal organs) are rarely noticed.
In many hosts dirofilariae produce microfilariae circulating in the peripheral blood, which is the source of infection for female vector mosquitoes, and if useful for diagnostic purposes. Searching for microfilariae may be fruitless in cases of low parasitemia or of occult infection; however, heartworm infections can be immunologically detected by using commercial kits available for the detection of both parasite antigens and host antibodies against the filarial worm. Such diagnostic tools do not exist for D. repens, which can be found only by home-made assays (Cancrini et al. 2001; Marcos-Atxutegi et al. 2004).
In Serbia, dogs are affected by both Dirofilaria species, and by Acanthocheilonema reconditum (Tasić et al. 2008). The severity of heartworm disease, the need for data about the geographical areas involved in canine filarioses, and recent reports of human infections (Tasić et al. 2011), urged us to carry out investigations on filarial prevalence in Serbia, applying microscopy and both commercial and home-made immunological diagnostics, and estimating in asymptomatic dogs the infection risk factors. Another aim was the genetic characterization of the isolates and their phylogenetic relationships with those available in GenBank.
Study Area
The study was conducted in northern Serbia, which proved endemic for dirofilarioses. The chosen areas were Pančevo (44.86°N, 20.64°E, in the Vojvodina region), and Veliko Gradište (44.76°N, 21.51°E, at the boundary between Vojvodina and Central Serbia; Fig. 1). The cities are in areas considered at risk for dirofilariosis on the basis of climate, geographical characteristics, and the presence of mosquito species possibly implicated in parasite transmission.
FIG. 1.
Map showing the study area.
Dog population
From June until September 2009, a total of 122 animals (59 resident in Pančevo and 63 in Veliko Gradište areas) were enrolled at random in the study. For each animal, dog owners and shelter attendants completed an anamnestic form to gather data on the dog's age, sex, breed, type of housing, profile, and anti-heartworm prophylaxis. Dogs included in the study population never moved away from the study area.
Blood samples
Blood samples (10 mL) were drawn from the cephalic vein of each animal between 9 am and 3 pm.
Methods
Detection and identification of microfilariae
The Knott technique and a commercial filtration test (Difil-test®; Evsco Pharmaceuticals, Buena, NJ) were applied to concentrate, respectively, a volume of 1 mL and 4 mL of blood before the microscopic analysis. The remaining 5 mL were used for the antigen detection, and after blood coagulation, for serological tests. Clots of positive samples were submitted to genetic analyses.
Microfilariae were identified on the basis of morphological and morphometric characteristics calculated by the Laboratory Universal Computer Image Analysis system (Lucia M., 1996, Czechoslovakia). Then they were further analyzed to assess the somatic distribution of acid phosphatase activity (Chalifoux and Hunt, 1971), and to biochemically discriminate between D. repens and D. immitis.
Detection of antigens
Blood from each dog was examined using a commercial kit to detect adult female D. immitis circulating antigens (Witness Dirofilaria®; Synbiotics, Lyon, France), according to the manufacturer's instructions.
Detection of specific antibodies
Two home-made ELISAs that use as antigen D. repens and D. immitis somatic/metabolic polyproteins (Cancrini et al. 2001) were used to detect IgG against dirofilarial species.
Molecular analyses
Genomic DNA was extracted from positive blood clots (NucleoSpin tissue; Macherey-Nagel, Duren, Germany) and submitted to PCR amplification with specific primers for D. repens (DNA repeat region) and D. immitis (5S ribosomal RNA) (Favia et al. 1996), and with filarioid-generic primers (cox1 gene fragment) (Casiraghi et al. 2004). Amplicons were purified (SureClean; Bioline, London, U.K.), and then sequenced. The sequences were corrected by visual analysis of the electropherogram and aligned using ClustalW. Then they were compared with those available in the GenBank database by BLAST analysis in order to construct phylogenetic trees (neighbor-joining method, 2000 bootstrap replicates) that were rooted by including as an outgroup Acanthocheilonema vitae (MEGA 5.0 software package).
Statistical analyses
Data are presented as mean±standard deviation (SD) or median and range, with p≤0.05 indicating statistical significance. Comparisons between groups were made using the Mann-Whitney U test and the Fisher exact test (the chi-square test for more than two groups) for nominal variables and for non-parametric data, respectively. The individual dog data were analyzed by the logistic regression model, using both D. repens and D. immitis status as dependent variables. The independent variables were tested in a multivariate model by the stepwise forward method. At each step, the least significant variable was removed from the model until all remaining variables were significant at p<0.05. All analyses were performed using SPSS 14.0 software.
Results
The animals tested (56 males and 66 females), 1–12 years old (mean age 4.15 years), were grouped as hunting dogs, watchdogs, stray dogs, service dogs, and pets. Considering their shelter, nutrition, care, training, working conditions, health protection, and sanitation, 38 were dogs bred in partially controlled life conditions (PCLC), and the remaining 84 lived in uncontrolled life conditions (ULC).
Parasitological results are summarized in Table 1. Microfilariae were detected in 21/122 dogs (17.2%), which harbored D. repens (n=21) and D. immitis (n=2), and co-infection was seen in 2 cases. Mean body length and width of 2719 measured microfilariae were: 360.9±8.5×7.48±0.35 μm for D. repens (n=2400), and 305.38±12.51×6.43±0.42 μm for D. immitis (n=319). Histochemical staining showed the expected acid phosphatase distribution: one red-stained spot at the excretory pore and one at the anal pore in D. immitis, and only one red spot at the anal pore in D. repens.
Table 1.
Results of Microscopic and Immunological Analyses Carried Out on the Tested Dogs by Area
| Dogs examined | Pančevo (n=59) | Veliko Gradište (n=63) | Total (n=122) |
|---|---|---|---|
| Detection of: | |||
| Microfilariae | 6 (10.2%) | 15 (23.8%) | 21 (17.2%) |
| D. immitis antigen | 8 (13.6%) | 7 (11.1%) | 15 (12.3%) |
| Antibodies to D. immitis | 11 (18.6%) | 17 (27.0%) | 28 (22.9%) |
| Antibodies to D. repens | 33 (55.9%) | 19 (30.2%) | 52 (42.6%) |
| Overall prevalence | 47 (79.7%) | 36 (57.1%) | 83 (68.0%) |
D. immitis antigens were detected in the blood of 15 (12.3%) dogs (two of them with a confirmed presence of circulating microfilariae, and the remaining 13 as occult infection); moreover, antibodies to this parasite were found in all 15 animals and in another 13 subjects, indicating an overall heartworm seroprevalence of 22.9%.
Reactivity to D. repens polyproteins was found in 52/122 (42.6%) dogs (17 microfilaremic and 35 amicrofilaremic subjects); however, it was absent in 4 microfilaremic dogs.
Therefore, the overall dirofilariae seroprevalence was 63.1% (77/122), and it indicated that contact with D. repens occurs markedly more frequently (p=0.001) than contact with D. immitis, particularly in Pančevo (≈56% versus≈19%, respectively).
Considering the results of all the analyses performed, the overall prevalence found in the study area was 68%. In detail, it was 79.7% in Pančevo and 57.1% in Veliko Gradište, suggesting no significant epidemiological differences (p=0.103). In fact, even if the prevalence of proven dirofilariosis (microfilariae and/or D. immitis antigens) was higher (but not significantly so; p=0.234) in Veliko Gradište (33.3%) than in Pančevo (22%), serology showed that most dogs living in Pančevo (72.9%) were reactive, more than those living in Veliko Gradište (57.1%).
As for the risk of infection, no significant differences were found between infected and uninfected dogs by any considered factor. The presence of ectoparasites proved significant at the 10% level in the univariate analysis, but when included in the multivariate regression model, turned out not to be a significant risk factor (Tables 2 and 3).
Table 2.
Univariate Logistic Regression Analysis of Risk Factors for Infection
| Factor | B | SE | Wald | p | OR | (95% CI) |
|---|---|---|---|---|---|---|
| Age | 0.034 | 0.071 | 0.229 | 0.632 | 1.035 | (0.900,1.189) |
| Female | 0.137 | 0.391 | 0.123 | 0.725 | 1.147 | (0.553,2.467) |
| Race | 15.065 | 0.447 | ||||
| Purpose | 9.669 | 0.041 | ||||
| ULC | −0.390 | 0.434 | 0.806 | 0.369 | 0.677 | (0.289,1.586) |
| Presence of ectoparasites | 0.839 | 0.441 | 3.619 | 0.057 | 2.313 | (0.975,5.487) |
B, B coefficient; SE, standard error; Wald, Wald chi-square test; OR, odds ratio; 95% CI, 95% confidence interval; ULC, uncontrolled life conditions.
Table 3.
Multivariate Logistic Regression Analysis of Risk Factors for Infection (Stepwise Forward Method)
| Factor | B | SE | Wald | p | OR | (95% CI) |
|---|---|---|---|---|---|---|
| Purpose | 6.870 | 0.143 | ||||
| Presence of ectoparasites | 0.317 | 0.640 | 0.245 | 0.621 | 1.372 | (0.392,4.808) |
| Constant | 0.486 | 1.295 | 0.141 | 0.708 | 1.625 |
B, B coefficient; SE, standard error; Wald, Wald chi-square test; OR, odds ratio; 95% CI, 85% confidence interval.
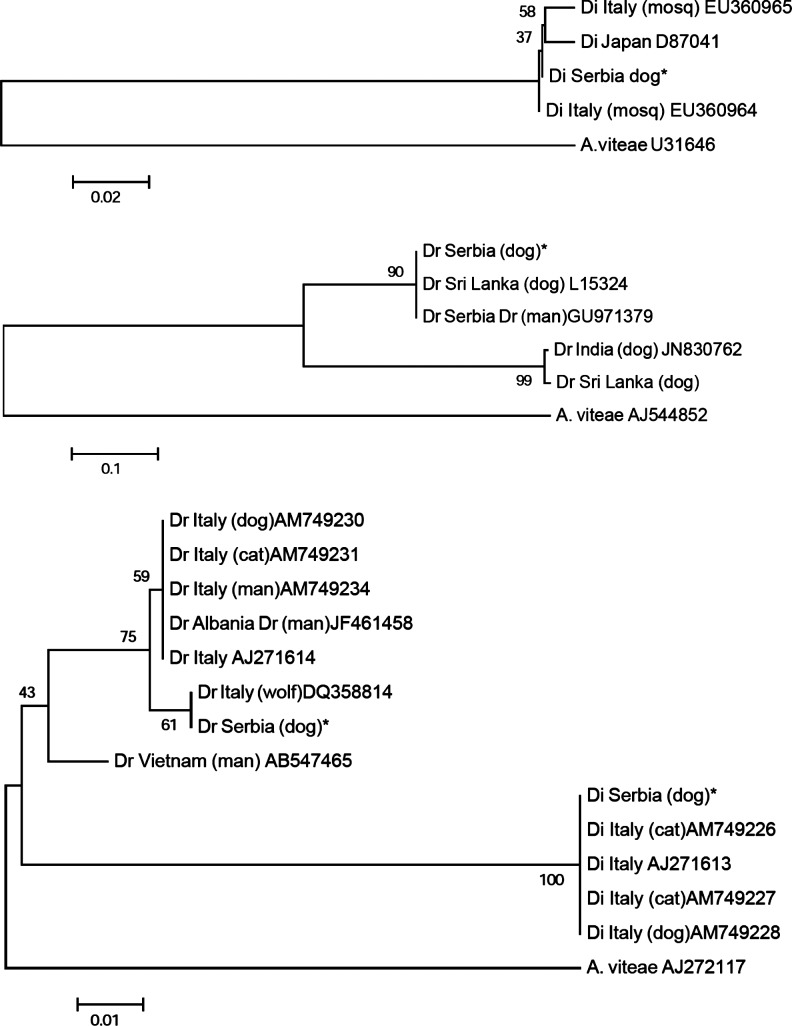
BLAST analysis of dirofilarial-specific sequences proved that the D. immitis was closely related to isolates from Italy and Japan, whereas D. repens exactly matched that from Serbia and Sri Lanka, but it was less closely related to other strains described in Asian countries. Cox1 sequences of D. immitis showed 100% identity to available Italian isolates; as for D. repens, it showed a close relationship with an Italian isolate, and it was less closely related to other Italian strains and to that reported in Europe and Asia (Fig. 2).
FIG. 2.
Phylogenetic relationships of Serbian isolates obtained in this study (marked with asterisks) with those retrieved from GenBank, and based both on dirofilarial-specific and cox1 gene sequences. The trees were obtained using the neighbor-joining method after 2000 bootstrap replicates, and rooted against Acanthocheilonema viteae. The scale bar indicates the distance in substitutions per nucleotide (Di, D. immitis; Dr, D. repens).
Discussion
The present study aimed to extend research on canine Dirofilaria infections in an area of Vojvodina (a region proven hyperendemic for D. repens and endemic for D. immitis in 2008), and in the territory between Vojvodina and Central Serbia. Both areas proved interesting to veterinarians, and though in Pančevo a higher prevalence was found, no significant epidemiological differences were noted.
In both areas D. immitis and D. repens were identified, whereas A. reconditum, previously reported in Serbia, was not found. D. repens was more prevalent (17.2%) than D. immitis (1.6%), even though the detection of its antigens in another 13 animals with occult infections sent the D. immitis prevalence up to 12.3%. Serology confirmed this above trend; contacts with D. repens occur markedly more frequently than with D. immitis (42.6% versus 22.9%). Differences in the spread of the two dirofilarial species could be ascribed to the mosquito population present in the studied areas, which could include vectors more efficient for the spread of D. repens than for D. immitis (Cancrini et al. 1992), or to chemoprophylaxis that only prevents heartworm infection, as suggested by laboratory data (Cancrini et al. 1989).
Concentration techniques and the search for antigens found that infection rates were high (22–33.3%), but were lower (p=0.001) than those observed in other regions of Vojvodina, such as the cities of Kikinda and Zrenjanin (>60%; Tasić et al. 2008). However, the very high seroprevalence of antibodies against dirofilarial nematodes found here suggests that in the study area many dogs became infected, even if the infection remained undetectable by circulating microfilariae and by antigens. Since the serological tests we used should be largely unaffected by cross-reactions (specificity 90%, positive-predictive value 75%), and are sensitive (can detect 2-month-old infections), we purport that the age of the tested animals may have affected the microscopy results. In fact, young animals have only been exposed to the infection for a short time, and thus the worms could not have completed their slow development to mature, mating adults producing circulating microfilariae. Furthermore, occult/abortive infections could have been induced by chemoprophylaxis, or by the use of antibiotics such as tetracyclines that reduce the numbers of the obligate endosymbionts wolbachiae, which indirectly inhibits the development and reproduction of filarial nematodes (Casiraghi et al. 2001). On the other hand, the failures in sensitivity shown by serology could also be due to the immunological condition of the 4 negative animals.
The unexpected lack of the ability of life-controlled conditions (which affords protection from mosquitos at night) to prevent infection leads us to suspect that by day, sunrise- or sunset-biting mosquitoes may actively take part in transmission, and opens up many questions about them. Thus further entomological research utilizing dog-baited traps and molecular diagnostics, which can recognize mosquito vector species for each dirofilaria species and their relative importance, are advisable, as proven by the results obtained in other countries (Cancrini and Gabrielli, 2007).
The phylogenetic analyses performed showed low variability among the D. immitis strains isolated to date, whereas the intra-species variability found in D. repens, which fit with biological features like the suitability of cats as hosts, requires further investigation.
As for the diagnostic tools applied: (1) the results of our biochemical methods closely matched those of the morphometric methods, and allow simple identification of microfilariae; (2) the Knott technique allows the microscopic study of concentrated microfilariae more readily than the Difil concentration test; (3) searching for the parasite antigens is advisable, even if the results are incomplete (it recognizes only the D. immitis female antigens), as demonstrated by a further 10.6% of infections detected by serology; (4) notwithstanding their failures in sensitivity, the home-made ELISAs proved essential to assess the impact of Dirofilaria infections; and (5) molecular diagnostics add important data on the strains present in the area, and on their possible origins, and therefore should be implemented.
Conclusions
Canine Dirofilaria infections are endemic in this area of Serbia, though the prevalence is decreased in Central Serbia. Luckily for the dogs, the infections are mainly due to D. repens, a less pathogenic filarial species. However, home-made serological tests detected active transmission of both species, an alarming result in accord with the human infections (about 30) detected in recent years, in a country where this disease is under-diagnosed because it is difficult to recognize, or it may simply be under-reported since it has been considered a minor pathogen, and has only recently been considered a major public health problem.
Therefore, further entomological investigations are needed to identify the mosquito species involved in dirofilarial transmission and the vector efficiency for each of them. These data are essential to the design of appropriate control programs. The intra-species genetic heterogeneity evidenced by D. repens DNA also merits further investigation.
Acknowledgments
This work was partially financially supported by Serbian Ministry of Education and Science grant 175034. The authors are grateful to Mrs. G. Croce for her excellent technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Bolio-Gonzalez ME. Rodriguez-Vivas RI. Sauri-Arceo CH, et al. Prevalence of the Dirofilaria immitis infection in dogs from Merida, Yucatan, Mexico. Vet Parasitol. 2007;148:166–169. doi: 10.1016/j.vetpar.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Cancrini G. Della Torre A. Coluzzi M. Different probabilities of transmission of Dirofilaria immitis and D. repens by Culex pipiens. Proc VIth EMOP. 1992:90. [Google Scholar]
- Cancrini G. Gabrielli S. Vectors of Dirofilaria nematodes: biology, behaviour and host/parasite relationships. In: Genchi C, editor; Rinaldi L, editor; Cringoli G, editor. Mappe Parassitologiche. Vol. 8. 2007. pp. 47–58. [Google Scholar]
- Cancrini G. Montoya MN. Prieto G, et al. Current advances in experimental diagnosis of human and animal dirofilariosis. In: Simon F, editor; Genchi C, editor. Heartworm Infection in Humans and Animals. Salamanca: Ediciones Universidad Salamanca; 2001. pp. 103–120. [Google Scholar]
- Cancrini G. Tassi P. Coluzzi M. Ivermectin against larval stages of Dirofilaria repens in dogs. Parassitologia. 1989;31:177–182. [PubMed] [Google Scholar]
- Casiraghi M. Bain O. Guerrero R, et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Casiraghi M. Bazzocchi C. Sacchi L, et al. The Wolbachia endosymbionts of Dirofilaria immitis. In: Simon F, editor; Genchi C, editor. Heartworm Infection in Humans and Animals. Salamanca: Ediciones Universidad Salamanca; 2001. pp. 203–210. [Google Scholar]
- Chalifoux L. Hunt RD. Histochemical differentiation of D. immitis and D. reconditum. JAVMA. 1971;158:601–605. [PubMed] [Google Scholar]
- Cringoli G. Rinaldi L. Veneziano V, et al. A prevalence survey and risk analysis of filariosis in dogs from the Mt. Vesuvius area of southern Italy. Vet Parasitol. 2001;102:243–252. doi: 10.1016/s0304-4017(01)00529-5. [DOI] [PubMed] [Google Scholar]
- Duran-Struuck JC. Hernandez HA. Dirofilaria immitis prevalence in a canine population in Samana Peninsula (Dominican Republic) Vet Parasitol. 2001;133:323–327. doi: 10.1016/j.vetpar.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Džamić AM. Arsić-Arsenijević V. Radonjić I, et al. Subcutaneous Dirofilaria repens infection of the eye in Serbia. J Helminth. 2009;83:129–137. doi: 10.1017/S0022149X09341346. [DOI] [PubMed] [Google Scholar]
- Favia G. Lanfrancotti A. Della Torre A, et al. Polymerase chain reaction-identification of Dirofilaria repens and Dirofilaria immitis. Parasitology. 1996;113:567–571. doi: 10.1017/s0031182000067615. [DOI] [PubMed] [Google Scholar]
- Genchi C. Kramer LH. Rivasi F. Dirofilarial infections in Europe. Vector Borne Zoonotic Dis. 2011;11:1307–1317. doi: 10.1089/vbz.2010.0247. [DOI] [PubMed] [Google Scholar]
- Marcos-Atxutegi C. Gabrielli S. Kramer LH, et al. Antibody response against Dirofilaria immitis antigens and the Wolbachia endosymbiont in naturally infected canine and human hosts. Parassitologia. 2004;46(Suppl):116. [Google Scholar]
- Pampiglione S Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: an update of world literature from 1995 to 2000. Parassitologia. 2000;42:235–254. [PubMed] [Google Scholar]
- Tasić A. Rossi L. Tasić S, et al. Survey of canine dirofilariasis in Vojvodina, Serbia. Parasitol Res. 2008;103:1297–1302. doi: 10.1007/s00436-008-1132-z. [DOI] [PubMed] [Google Scholar]
- Tasić S. Stoiljković N. Miladinović-Tasić N, et al. Subcutaneous dirofilariosis in South-East Serbia—case report. Zoonoses Public Health. 2011;58:318–322. doi: 10.1111/j.1863-2378.2010.01379.x. [DOI] [PubMed] [Google Scholar]