Abstract
The goal of the present study was to examine the neuroprotective and functional significance of targeting both N-methyl-d-aspartate (NMDA) receptor–mediated excitotoxicity and oxidative stress using a dual-acting compound, Neu2000, in rat model of moderate spinal cord injury (SCI). An initial set of experiments was conducted in uninjured rats to study the pharmacokinetic profile of Neu2000 following intraperitoneal and intravenous administration. A second experiment measured free radical production in mitochondria isolated from sham or injured spinal cords of animals receiving vehicle or Neu2000 treatment. A third set of animals was divided into three treatment groups consisting of vehicle treatment, a single dose of Neu2000 (50 mg/kg) administered at 10 min following injury, or a repeated treatment paradigm consisting of a single bolus of Neu2000 at 10 min following injury (50 mg/kg) plus a maintenance dose (25 mg/kg) administered every 24 h for an additional 6 days. Animals were tested once a week for a period of 6 weeks for evidence of locomotor recovery in an open field and kinematic analysis of fine motor control using the DigiGait Image Analysis System. At the end of the testing period, spinal cord reconstruction was performed to obtain nonbiased stereological measures of tissue sparing. The results of this study demonstrate that Neu2000 treatment significantly reduced the production of mitochondrial free radicals and improved locomotor outcomes that were associated with a significant increase in the volume of spared spinal cord tissue.
Key words: excitotoxicity, functional recovery, oxidative stress, spinal cord contusion, tissue sparing
Introduction
The temporal pattern of secondary injury following acute spinal cord injury (SCI) is highly complex and encompasses a number of pathophysiological events including glutamate-mediated excitotoxicity, mitochondrial dysfunction, oxidative damage, ischemia, edema, inflammatory responses, and necrotic and apoptotic cell death (Hall and Springer, 2004). Many of these secondary events are interrelated and there is clear evidence that a relationship exists between glutamate-mediated excitotoxicity (Agrawal and Fehlings, 1997; Liu et al., 1999; McAdoo et al., 1999; WratHall et al., 1992, 1996), mitochondrial dysfunction (Azbill et al., 1997; Jin et al., 2004; McEwen et al., 2007; Ravikumar et al., 2007; Sullivan et al., 2005, 2007), and oxidative damage (Azbill et al., 1997; Blight and Zimber, 2001; Hall and Springer, 2004; Hall et al., 1992; Mu et al., 2000b; Springer et al., 1997).
SCI results in a rapid increase in extracellular glutamate, leading to calcium influx through voltage-dependent channels as well as glutamate receptor subtypes. The mitochondrial uptake of excessive levels of Ca2+ can lead to a failure of aerobic energy metabolism, inhibition of ATP synthesis, disruption of the mitochondrial membrane potential, increased reactive oxygen species (ROS) production, and onset of mitochondrial permeability transition, which is thought to occur in response to formation of the transition pore (Brown et al., 2004; Brustovetsky et al., 2003; Friberg and Wieloch, 2002; Hansson et al., 2003; Sullivan et al., 2005). Importantly, glutamate-mediated excitotoxicity, increased intracellular Ca2+, and oxidative stress have all been shown to promote the onset of the permeability transition. Recent observations from this and other laboratories have demonstrated that mitochondrial function and bioenergetics are severely compromised at acute times following SCI (Azbill et al., 1997; Jin et al., 2004; McEwen et al., 2007; Ravikumar et al., 2007; Sullivan et al., 2005, 2007). As a consequence, cellular damage and death can occur through necrosis-related events due to excitotoxicity, a loss of energy production, and oxidative damage (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005), as well as apoptosis-related events, including the mitochondrial release of pro-apoptotic proteins (Brustovetsky et al., 2002; Cande et al., 2002; Chai et al., 2000; Petronilli et al., 2001).
Given the central role of glutamate-related excitotoxicity, oxidative damage, and mitochondrial dysfunction in secondary injury following SCI, strategies that target these pathophysiological events, either directly or indirectly, have potential clinical significance in the treatment of acute SCI. Neu2000 [2-hydroxy-5-(2,3,5,6-tetrafluoro-4-trifluoromethyl-benzylamino)-benzoic acid] is a recently developed derivative of acetylsalicylic acid and sulfasalazine. Acetylsalicylic acid and salicylic acid have been shown to indirectly prevent NMDA receptor–mediated excitotoxicity by blocking nuclear factor κB and c-Jun N-terminal kinase (Grilli et al., 1996; Ko et al., 1998). Although of limited potency, acetylsalicylic acid can also protect against ischemic neuronal death by inhibiting voltage-gated Ca2+ channels, p44/42 mitogen-activated protein kinase, and glutamate release (De Cristobal et al., 2002; Kim et al., 2001; Vartiainen et al., 2003). Sulfasalazine, another anti-inflammatory drug, is a conjugate of 5-aminosalicylic acid and sulfapyridine that inhibits NMDA neurotoxicity more potently than acetylsalicylic acid. Sulfasalazine offers additional neuroprotection as a scavenger of free radicals (Gionchetti et al., 1990; Ryu et al., 2003). Therefore, by combining the actions of these two compounds, Neu2000 exerts dual neuroprotective actions by serving both as an uncompetitive NMDA receptor antagonist and a free radical scavenger. However, as indicated above, the precise molecular mechanism(s) by which Neu2000 exerts its neuroprotective properties may involve several interrelated signaling pathways (De Cristobal et al., 2002; Grilli et al., 1996; Kim et al., 2001; Ko et al., 1998; Vartiainen et al., 2003).
In a recent study, Gwag et al. (2007) showed that Neu2000 more potently protected cultured cortical neurons against NMDA-induced neurotoxicity than either acetylsalicylic acid or sulfasalazine alone. Neu2000 was also more potent in protecting glial cells as well as neurons from iron-induced free radical injury than acetylsalicylic acid, sulfasalazine, vitamin E, trolox, or the free radical scavenger edaravone. It is important to note that this study also reported that Neu2000 treatment did not exhibit neurotoxicity in the retrosplenial cortex, which has been shown to occur in animals treated with potent NMDA receptor antagonists such as MK-801 (Olney et al., 1991). Additional in vivo studies revealed that infarct volumes at 24 h following middle cerebral artery occlusion were significantly reduced when Neu2000 was administered 5 min following reperfusion. The level of tissue sparing observed with Neu2000 was significantly greater than the neuroprotective effects of trolox or MK-801. Importantly, Neu2000 was also found to have a clinically relevant therapeutic time window. Infarct volumes were significantly reduced when Neu2000 was administered as late as 8 h following reperfusion, although not to the extent observed when the drug was administered only 5 min after reperfusion (Gwag et al., 2007). These findings are very encouraging and suggest that Neu2000 has potent neuroprotective properties in the reduction of cell death following central nervous system injury.
While clinical trials are currently underway to test Neu2000 for human use in cerebral stroke, the efficacy of Neu2000 as a neuroprotective agent in SCI has not been tested. We present here a series of experiments in an animal model of SCI demonstrating that post-injury treatment with Neu2000 reduces mitochondrial free radical production and promotes significant tissue sparing and functional recovery of open field and fine locomotor behavior. The positive outcomes of these studies and the relative safety profile of Neu2000 provide additional justification to examine the therapeutic potential of this dual-acting compound in the treatment of acute SCI.
Methods
Subjects
Adult female Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 225 to 250 g at the time of surgery were used in all experiments. The rats were housed in the vivarium of the University of Kentucky College of Medicine and maintained on a 12 h:12 h light/dark cycle with food and water available ad libitum. Rats were acclimated to the facility for 7 days prior to the start of the experiments. The animal unit of the College of Medicine at the University of Kentucky Medical Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee, were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, and conformed to guidelines of the Society for Neurotrauma and the Society for Neuroscience.
Neu2000 pharmacokinetic study
The pharmacokinetic profile of Neu2000 in normal rats was examined using intraperitoneal (ip) route of administration. Rats were anesthetized by ether inhalation and the femoral artery cannulated. Rats were placed into Bollman cages for 1 h and then received injections (10, 25, or 50 mg/kg ip, n = 6 per dose) of Neu2000 dissolved in sterile saline. Blood was collected from the femoral artery at 15, 30, 60, 120, 240, 480, and 1440 min following injection. Plasma aliquots of 50 μL were spiked with 100 μL of internal standard solution (Neu2000IS, 10 μg/mL in acetonitrile). After vortex mixing for 1 min, the samples were centrifuged at 12,000 g for 5 min. A total of 50 μL of the supernatant phase was separated and diluted with 50 μL of distilled water. Plasma concentrations relative to time were obtained using liquid chromatography–mass spectrometry and fitted with the WinNonlin software (Pharsight, Inc., St. Louis, MO).
Spinal cord contusion model
All surgical techniques were performed under aseptic conditions. Using a randomized, blinded study design, rats were anesthetized with 40 mg/kg (ip) sodium pentobarbital (Nembutal, Abbott Laboratories, North Chicago, IL), dorsal incisions were made in the skin and underlying muscles, and the muscles were retracted. A partial laminectomy was performed at thoracic segment 10 (T10), and the vertebral column was stabilized by clamping the vertebrae at T9 and T11 so that the spinal cord remained in a horizontal position. The spinal cord was then injured with the Infinite Horizons impactor (Precision Systems and Instrumentation, Lexington, KY), which has been described in detail (Scheff et al., 2003). As in a previous report (McEwen et al., 2007), a stainless steel-tipped probe (2.5 mm diameter) was rapidly lowered onto the dorsal surface of the spinal cord until an impact force of at least 150 kdynes was achieved. This injury parameter produced a mild to moderate SCI. A data acquisition program subsequently displayed the actual force applied to the spinal cord, the maximum spinal cord displacement, and the velocity of the probe at the time of peak force and peak displacement. The incision site was closed in layers with 5–0 absorbable braided suture (Ethicon, Somerville, NJ), and the skin was then closed with 9 mm wound clips (Becton Dickinson, Sparks, MD). Following surgery, animals were placed in an Isolette C100 Infant Incubator (Draeger Medical, Telford, PA) to maintain body temperature and were closely monitored until they completely recovered from the anesthesia. The animals were then returned to the animal care facility and monitored daily including expressing the bladder twice daily, checking for bladder infections and providing antibiotic treatment if necessary. Sham animals received the same surgical procedure but did not sustain any contusion injury.
Effect of Neu2000 on mitochondrial reactive oxygen species production
Animals received spinal cord contusions as described above and were treated 10 min later with either 50 mg/kg Neu2000 dissolved in sterile 0.9% saline (n = 6) or vehicle (0.9% saline) alone (n = 6). Sham operated animals (n = 6) were also included. At 24 h post-injury, rats were deeply anesthetized with 100 mg/kg (ip) sodium pentobarbital, decapitated, and 10 mm of thoracic spinal cord (centered on the lesion/laminectomy site) was rapidly removed. The tissue segment was immediately minced with scissors and homogenized in 2.0 mL of ice-cold isolation buffer (pH 7.2) containing 215 mM mannitol, 75 mM sucrose, 0.1% bovine serum albumin, 1 mM ethylene glycol-bis(2-aminoethyl-ether)-N,N,N′,N′-tetraacetic acid (EGTA), and 20 mM N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES). Homogenates were centrifuged at 1300 g at 4°C for 5 min to pellet blood and nuclei. The supernatant was carefully removed and centrifuged at 13,000 g at 4°C for 10 min to pellet myelin, synaptosomes, and nonsynaptic mitochondria. The supernatant was discarded and the crude mitochondrial pellet was immediately resuspended in 500 μL of isolation buffer. The suspension was carefully applied to a discontinuous Ficoll gradient (Sigma, St. Louis, MO), and centrifuged at 100,000 g at 4°C for 30 min. The synaptosomal layer was carefully removed and incubated for 10 min on ice in 500 μL isolation buffer with 0.1% digitonin (Sigma) to disrupt the synaptosomal membranes and release the synaptic mitochondria. The digitonin in the samples was diluted with additional isolation buffer (2 mL final volume) and the samples were recentrifuged at 10,000 g at 4°C for 10 min. The supernatant was discarded, and the mitochondrial pellet was resuspended in 100 μL of isolation buffer without EGTA and kept on ice. The protein content of the final mitochondrial fraction was determined with the BCA assay (Pierce, Rockford, IL) by measuring absorbance at 560 nm with a Synergy HT plate reader (Biotek, Winooski, VT).
The effect of Neu2000 on basal and oligomycin-induced mitochondrial ROS levels was assayed according to a previously published study (McEwen et al., 2007). Synaptic mitochondria isolated from sham or injured spinal cords were placed into a sealed, thermoregulated chamber (37°C with continuous stirring) in respiration buffer (215 mM mannitol, 75 mM sucrose, 0.1% bovine serum albumin, 2 mM magnesium chloride, 2.5 mM potassium phosphate monobasic anhydrous, and 20 mM HEPES, pH 7.2) to yield a final protein concentration of 1 mg/mL. The respiratory chamber was equipped with fluorescence/absorbance probes to allow measurement of ROS production in real-time. Production of basal mitochondrial ROS was assessed by adding the hydrogen peroxide indicator, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes, Eugene, OR) to the chamber. Specifically, 10 μM H2DCFDA was added to the chamber with the mitochondria, and fluorescence was measured with a fluorometric plate reader for 15 min (excitation 490 nm, emission 526 nm). An increase in fluorescence units indicated an increase in ROS production. Oligomycin (2.0 μmol/L) was added to maximize mitochondrial membrane potential and induce maximum ROS production.
Behavioral studies
For all of the behavioral studies, animals received either vehicle treatment (saline), a single dose (ip) of Neu2000 (50 mg/kg) administered 10 min following injury, or a repeated treatment paradigm consisting of a single bolus of Neu2000 (50 mg/kg) at 10 min following injury plus a maintenance dose (25 mg/kg) administered every 24 h for an additional 6 days. Rats were assessed in an open field for overground locomotion using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (Basso et al., 1995). The open field test was conducted in a circular, molded plastic swimming pool with a diameter of 100 cm and a wall height of 23 cm. Specific parameters of locomotion were quantified using the DigiGait Image Analysis System (Amende et al., 2005; Hampton et al., 2004; Kale et al., 2004; Li et al., 2005; Sharma et al., 2005; Wooley et al., 2005). The DigiGait Image Analysis System (Mouse Specifics, Quincy, MA) is an automated system that enables quantification of several spatial and temporal indices of gait while rats walk on a motorized treadmill held at a constant speed (35 cm/s). Data collection for DigiGait analysis occurred on post-operative day 42 when rats demonstrated consistent weight-supported hindlimb stepping on the treadmill at a speed of 35 cm/s.
Tissue sparing
At the end of the behavioral testing period (6 weeks post SCI), rats were deeply anesthetized with 100 mg/kg (ip) sodium pentobarbital (Abbott Laboratories) and perfused transcardially with 100 mL of ice-cold 0.1 M phosphate-buffered saline (PBS) followed by 400 ml of 4% paraformaldehyde in PBS (pH = 7.4). The laminectomy site was re-opened, and the T10 segment of spinal cord was demarcated with 0.4% bromophenol blue (Sigma). The laminectomy was then extended in the rostral and caudal directions, and the spinal cords were transected rostrally at the T7 rootlet and caudally at the T13 rootlet. The spinal cord segment (approximately 20 mm) was removed from the vertebral column, post-fixed in 4% paraformaldehyde for 1 h at 4°C, and then cryoprotected in 20% sucrose-PBS for 48 h at 4°C. Subsequently, four spinal cords (from different experimental groups) were aligned at their rostral ends and co-embedded in plastic cryomolds that contained tissue freezing medium (Triangle Biomedical Sciences, Durham, NC). The molds were subsequently snap-frozen in chilled acetone (−60°C) and the blocks were stored at −70°C until cut into 20 μm thick sections on a Microm HM560 cryostat (Richard-Allan Scientific, Kalamazoo, MI). Based on the Cavalieri principle (Mouton et al., 2002; Reed and Howard, 1998), every fifth section was collected on a series of 10 electrostatically charged glass slides (Fisher Scientific) such that adjacent sections mounted on each slide represented regions spaced 1 mm apart in the spinal cord. After the sections air-dried, the slides were placed at 4°C overnight and then stored at −20°C.
Slides were randomly chosen and the gray matter was subsequently differentiated from the white matter by a modified staining protocol for Eriochrome cyanine-R (Sigma), as previously published (Rabchevsky et al., 2001; Ravikumar et al., 2005). Briefly, sections were dehydrated at room temperature in a graded ethanol series (5 min each), cleared in Hemo-De (Fisher Scientific) for 5 min, and subsequently rehydrated in a reverse graded ethanol series followed by distilled water (5 min each). To stain myelinated fibers, the tissue sections were placed for 10 min into a solution that contained 0.16% Eriochrome cyanine-R, 0.5% sulfuric acid, and 0.4% iron chloride. Sections were rinsed under running tap water for 10 min and then differentiated in 0.5% aqueous ammonium hydroxide for 2 min. The reaction was terminated by subsequently rinsing the sections in distilled water for 10 min. Sections were then dehydrated in a graded ethanol series (2 min each), cleared in Hemo-De (twice for 2 min), and cover-slipped with Permount (Fisher Scientific, Pittsburgh, PA). Using unbiased stereological techniques, stained tissue sections were examined using a Zeiss AxioPlan microscope (Oberkochen, Germany). The lesion epicenter was designated as the section containing the largest central core lesion with the least myelin-stained tissue. Myelin staining was examined under brightfield and images were captured with a Zeiss AxioCam color digital camera ( × 50). Each section was viewed with SCION Image software (National Institutes of Health), and the outer border of the entire section was traced (tissue + lesion). Next, the lesion was outlined, followed by any areas of intact gray matter. The area of white matter spared in each section was calculated by subtracting the area of the lesion plus remaining gray matter from the area of the entire section. Based on the thickness of each section (20 μm) and the distance between each analyzed section (1 mm), the area measurements were converted to volume measurements. The stereological analyses were conducted by an individual blinded to the treatment conditions.
Statistical analyses
A two-factor ANOVA with one repeated measure was used to assess the effects of drug and injury on open field locomotor scores using the BBB scale. One-factor ANOVAs were used to analyze changes in ROS levels and indices of locomotor function on DigiGait and histological measures of tissue sparing. Post hoc comparisons were made using the Bonferroni test with corrections for multiple comparisons. A p value of <0.05 is considered statistically significant.
Results
Neu2000 pharmacokinetic study
The results of the Neu2000 pharmacokinetic studies are presented in Table 1. Pharmacokinetic analysis revealed that the half-life of Neu2000 is 1.42, 2.14, and 1.79 h following intraperitoneal administration of 10, 25, and 50 mg/kg, respectively. In addition, the Cmax (maximum plasma concentration) was calculated as 3.86, 18.73, and 52.83 μg/mL and the AUC (area under the curve) was determined to be 7.37, 55.15, and 96.77 μg/h/mL at the same respective doses.
Table 1.
Pharmacokinetic Profile of Neu2000 in Normal Rats following Intraperitoneal Route of Administration
| |
IP injectionsb |
||
|---|---|---|---|
| Pharmacokinetic parametersa | 10 mg/kg (n = 6) | 25 mg/kg (n = 6) | 50 mg/kg (n = 6) |
| Cmax (μg/mL) | 3.86 ± 0.65 | 18.73 ± 2.45 | 52.83 ± 5.89 |
| AUClast (μg/h/mL) | 7.37 ± 1.59 | 55.15 ± 12.56 | 96.77 ± 11.51 |
| t1/2 (h) | 1.42 ± 0.27 | 2.14 ± 0.45 | 1.79 ± 0.34 |
| CL/F (mL/h/kg) | 1337.53 ± 174.13 | 554.66 ± 97.03 | 550.15 ± 65.72 |
| MRTlast (h) | 1.37 ± 0.25 | 1.89 ± 0.33 | 1.46 ± 0.18 |
Pharmacokinetic parameters were determined using liquid chromatography–mass spectrometry analysis from plasma concentrations versus time (see text for methodology) using a non-compartment method (mean ± SEM).
In saline.
IP, intraperitoneal; Cmax, maximum plasma concentration; AUClast, area under the curve versus time up to the last measurable concentration; t1/2, elimination half-life; CL/F, total clearance; MRT, mean residence time.
Effects of Neu2000 on mitochondrial ROS production
Neu2000 has previously been shown to function as a noncompetitive NMDA receptor antagonist and block ROS-mediated toxicity in cultures of cortical neurons (Gwag et al., 2007). Therefore, we examined the effect of Neu2000 treatment (50 mg/kg) on basal and induced ROS production by mitochondria isolated from SCI animals. The levels of basal mitochondrial ROS were significantly elevated at 24 h post-surgery in both the vehicle-treated (4.1-fold, p < 0.01) and Neu2000-treated (2.9-fold, p < 0.01) groups compared to sham controls (Fig. 1). The basal levels of ROS did not significantly differ between mitochondria isolated from injured rats treated with Neu2000 or vehicle (p > 0.05). When oligomycin was injected into the respiratory chamber to maximize the mitochondrial membrane potential, induced mitochondrial ROS levels significantly increased in both the vehicle-treated (5.7-fold, p < 0.01) and Neu2000-treated (3.4-fold, p < 0.01) groups compared to sham controls. However, oligomycin-induced ROS production by mitochondria from injured rats treated with Neu2000 was significantly less (p < 0.05) than that measured in mitochondria from vehicle-treated rats.
FIG. 1.
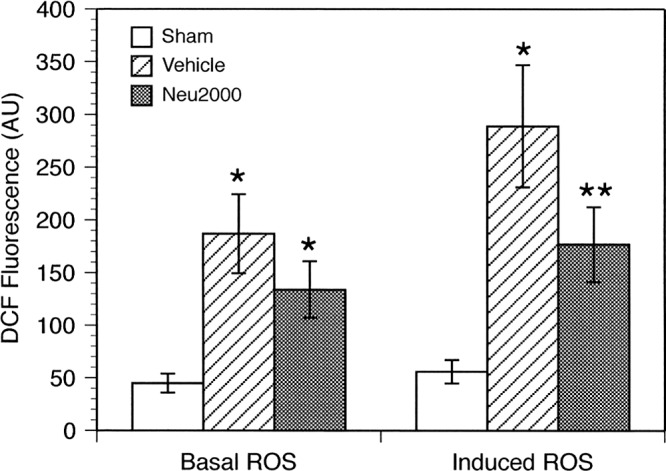
Basal and oligomycin-induced reactive oxygen species (ROS) production in mitochondria isolated from sham, spinal cord injury (SCI) + vehicle, and SCI + Neu2000 animals. Animals received 50 mg/kg Neu2000 at 10 min after SCI, and spinal cord mitochondria were obtained 24 h later to examine ROS production. The relative levels of ROS produced under basal conditions were unaffected by Neu2000, while ROS production induced by oligomycin was significantly reduced compared to vehicle. *p < 0.01 compared to sham, **p < 0.05 compared to vehicle and sham. DCF, dichlorodihydrofluorescein.
Behavioral analyses
A total of four spinal cord–injured animals were removed from the behavioral study due to mortality within 24 h after injury. This resulted in the following group sizes that were maintained throughout the remainder of the study; Sham control, n = 8; Group A (vehicle), n = 11; Group B (repeated Neu2000 treatment), n = 11; and Group C (single Neu2000 treatment), n = 10. The biomechanical parameters (peak force, displacement, and impact velocity) of the injury produced by the Infinite Horizons impactor were not different between the three treatment groups (data not shown).
BBB Scoring
All animals received testing using the BBB scoring system on days 0, 3, 7, 14, 21, 28, 35, and 42 following surgery. As shown in Fig. 2, all sham animals received a score of 21 across the entire 42-day testing period. All injured animals showed a significant decrease (p < 0.05) in overall BBB scores across the 42-day testing period compared to the sham group. The results of the BBB test also revealed significant changes in open field locomotion in spinal cord–injured animals treated with Neu2000 compared to vehicle-treated animals. Specifically, post hoc analysis showed that animals treated with repeated doses of Neu2000 had significantly higher BBB scores (p < 0.05) compared to vehicle-treated animals on days 21, 28, 35, and 42 after injury. Repeated Neu2000 treatment also resulted in a significant improvement on BBB scores (p < 0.05) on days 35 and 42 compared to the injured group receiving a single dose of Neu2000. There were no significant differences between the single treatment group and vehicle-treated animals at any of the time points examined.
FIG. 2.

Group mean (±SEM) Basso, Beattie, and Bresnahan (BBB) scores in sham and spinal cord–injured animals treated with vehicle or Neu2000. All injured animals showed an initial deficit in locomotor scores that improved over time in a pattern consistent with the level of injury. However, there was a significant improvement (*p < 0.05) in the group mean BBB scores in animals receiving repeated (but not single) treatment with Neu2000 compared to vehicle treatment.
DigiGait analysis
The DigiGait Image Analysis System generates data for numerous (over 25) spatial and temporal indices of gait (see Fig. 3A for representative traces of paw print patterns from sham and injured animals). Analysis of the DigiGait data revealed significant changes on certain indices across the different treatment groups. The percentage of the stride duration spent in the swing phase was significantly decreased (p < 0.01) in vehicle-treated animals (Fig. 3B), and this measure significantly improved (p < 0.05) in both the single and repeated Neu2000 treatment groups compared to vehicle. As could be predicted, the percentage of the stride duration spent in the stance phase was significantly increased (p < 0.01) in the injured vehicle group compared to sham animals, and this was significantly reduced (p < 0.05) following Neu2000 treatment (single or repeated) compared to vehicle-treated animals (data not shown). In addition, the swing to stance ratio was significantly increased (p < 0.01) in the vehicle-treated animals compared to shams (Fig. 3C). Single (p < 0.05) or repeated (p < 0.01) Neu2000 treatment resulted in a decreased swing to stance ratio compared to vehicle-treated animals.
FIG. 3.
(A) Representative DigiGait traces of sham and injured rats treated with vehicle. Effects of Neu2000 treatment was observed on several indices of locomotor function at 42 days following injury including (B) percentage of stride in swing, (C) swing to stance ratio, (D) degree of hindpaw external rotation, and (E) hindpaw area. *p < 0.05 compared to sham and Neu2000 treatment, **p < 0.01 compared to sham and Neu2000 treatment.
The hindlimb paw angle during peak stance was significantly (p < 0.01) rotated outwards in vehicle-treated SCI animals compared to shams (Fig. 3D). This is shown in the representative trace image shown in Fig. 3A. In addition, the paw area at peak stance was significantly decreased (p < 0.05) in the vehicle-treated animals (Fig. 3E). Both of these hindlimb measures were “normalized” in animals treated with either single or repeated Neu2000 treatment. Finally, stance width (the width between the centroids of homologous set of paws during peak stance) was significantly reduced (p < 0.05) in vehicle-treated animals and animals receiving a single treatment with Neu2000, but not in injured animals receiving repeated Neu2000 treatment (data not shown).
Stereological assessment of lesion volume and tissue sparing
Representative transverse spinal cord sections at the epicenter show the typical morphological changes observed at 42 days post-injury (Fig. 4). Histological examination revealed that the lesion epicenter of all injured rats was characterized by a peripheral, and in some cases incomplete, rim of residual tissue. In addition, cavitation and vacuolization in gray and white matter was observed as far as 5 mm rostral and caudal to the epicenter. Post-injury treatment with a single (Group C) or repeated (Group B) dose of Neu2000 was found to significantly increase tissue sparing compared to vehicle treatment (Group A), as reflected by a decrease in overall lesion volume (Fig. 4). Repeated treatment with Neu2000 resulted in a 45.6% decrease (p < 0.01) in overall lesion volume compared to vehicle treatment, while a single administration of Neu2000 resulted in a 36.8% decrease (p < 0.05) in overall lesion volume. While there was an apparent trend towards greater tissue sparing following repeated treatment compared to single administration, the differences were not statistically significant (Fig. 4B). However, repeated treatments with Neu2000 significantly increased the total volume of white matter sparing compared to vehicle-treated animals and animals receiving a single treatment with Neu2000 (Fig. 4C, 4D). This is clearly apparent in the series of photomicrographs presented in Fig. 4, especially in spinal cord sections around the injury epicenter (also see graphs in Fig. 4A, 4C).
FIG. 4.
Representative photomicrographs of spinal cord sections demonstrating the effects of vehicle or repeated or single Neu2000 treatment on tissue sparing (upper set of panels). Note the pronounced effect of repeated Neu2000 treatment (Group B) on tissue sparing compared to vehicle (Group A) and single Neu2000 treatment (Group C). The images across the treatment groups correspond to spinal cord sections equidistant rostral and caudal to the injury epicenter. Panels A–D demonstrate the results of the stereological analysis of total lesion volume and spared white matter. A 20 mm segment from each animal was used for the stereological studies. The X axis refers to the spinal cord section being analyzed relatively to the injury epicenter, with 1 referring to the most rostral section and 20 the most caudal section. (A) The lesion area and (C) spared white matter area at each millimeter along the 20 mm segment demonstrate the pattern of tissue sparing due to vehicle (Group A), repeated (Group B), or single (Group C) Neu2000 treatment. Calculation of (B) total lesion volume and (D) spared white matter volume demonstrate a significant decrease (*p < 0.05) in lesion volume in both Neu2000 treatment groups, while only the repeated Neu2000 treatment showed a significant increase (*p < 0.05) in white matter sparing.
Discussion
The complex nature of secondary injury after SCI requires the use of pharmacological strategies targeting multiple pathophysiological events (Baptiste and Fehlings, 2006; Baptiste et al., 2009; Blight and Zimber, 2001; Hall and Springer, 2004; Mu et al., 2000a). Several properties of Neu2000 make it an attractive candidate, including its dual action, safety, small molecular weight, high solubility, and stability. The pharmacology of Neu2000 is based on its actions as a free radical scavenger and an uncompetitive NMDA receptor antagonist. In animal models of cerebral ischemia, Neu2000 has been shown to exhibit a wide therapeutic time window and was comparable to the combined effects of the NMDA receptor antagonist MK-801 plus the antioxidant trolox (Gwag et al., 2007). The overall results of the current study provide evidence that treatment with a small molecule targeting both oxidative damage and excitotoxicity has therapeutic potential in the treatment of acute SCI. As a first step in this direction, Phase I trials conducted in the United States have resulted in promising safety and pharmacokinetic profiles.
The precise mechanism(s) by which Neu2000 exerts its neuroprotective actions was not the major focus on the current study, and several possible signaling pathways may contribute to its efficacy (De Cristobal et al., 2002; Grilli et al., 1996; Kim et al., 2001; Ko et al., 1998; Vartiainen et al., 2003). Regardless, our results show that a single treatment with 50 mg/kg Neu2000 reduced ROS produced by mitochondria isolated from the injured spinal cord. This dose of Neu2000 was used as the single bolus in the behavioral studies and chosen based on the results of our pharmacokinetic studies. Mitochondria isolated from the injured spinal cord exhibit a significant increase in ROS production when membrane potential is maximized by the presence of oligomycin (Jin et al., 2004; McEwen et al., 2007; Sullivan et al., 2005, 2007). The induction of mitochondrial ROS by oligomycin provides a method for studying mitochondria under conditions of maximum membrane potential similar to what would be expected to occur at acute times following SCI (McEwen et al., 2007; Sullivan et al., 2007). It is interesting to note that Neu2000 treatment had no significant effect on ex vivo mitochondrial ROS production under basal or resting conditions. This suggests that Neu2000 treatment is effective in limiting mitochondrial ROS production only under conditions that mimic cellular stress associated with ongoing secondary injury events. Future studies will be required to test whether Neu2000 treatment also improves mitochondrial integrity and bioenergetics and whether the actions observed ex vivo reflect what is occurring in vivo.
In a recently published study, Neu2000 was found to reversibly suppress NMDA currents with fast binding kinetics (Noh et al., 2009). This study reported that Neu2000 acts as an NMDA receptor 2B (NR2B)-specific low-affinity gating modifier in an uncompetitive manner that enhances NMDA desensitization and stabilizes the closed state of NMDA receptor. In contrast to psychosis and neurotoxicity induced by competitive and noncompetitive NMDA antagonists including phencyclidine, ketamine, and MK-801, such adverse effects are not observed with low-affinity uncompetitive and NR2B-specific NMDA antagonists such as memantine (Chen et al., 1998). Thus, these observations suggest that Neu2000 prevents NMDA receptor–mediated neuronal death in the absence of adverse side effects.
The major focus of the present study was to ascertain the functional and neuroprotective potential of using a single versus repeated Neu2000 treatment paradigm. The doses and treatment windows were based on our pharmacokinetic studies and previous experiments demonstrating a positive outcome for Neu2000 in rat models of cerebral ischemia (Gwag et al., 2007). Using these dosing parameters, we were able to observe significant effects of Neu2000 on several measures of functional recovery. Compared to injured animals receiving vehicle, animals treated with repeated doses of Neu2000 exhibited a relatively rapid and significant improvement in measures of locomotion as assessed using the BBB open field rating scale. The improvement in BBB scores was first observed at 21 days post-injury and persisted for at least 42 days, the experimental end-point of this study. Based on the BBB scores, these animals exhibited consistent weight-supported plantar stepping, hindlimb–forelimb coordination, and appropriate paw position during the stance phase. Appropriate paw position refers to the paw being nearly parallel to the body during stance versus rotated outward or inward, both of which are typically observed in spinal cord–injured animals. At no time following injury was there a significant improvement in BBB scores in animals receiving a single dose of Neu2000 compared to vehicle.
Several key measures of gait were significantly altered in injured animals treated with vehicle compared to sham. Saline was used as vehicle and, therefore, we do not attribute any injury-related gait differences to be related to saline treatment. In our evaluation of the DigiGait data, many of these key gait indices were significantly improved in animals receiving either single or repeated treatments with Neu2000. We found that the percentage of the stride spent in the swing phase (percentage of the stride duration that the paw is in the air) was significantly decreased with injury and that Neu2000 treatment significantly increased this measure. As can be predicted from this result, the converse was also found to occur. Specifically, the percentage of stride spent in the stance phase (percentage of the stride duration that the paw is in contact with the treadmill) was significantly improved in injured animals treated with Neu2000, but not with vehicle. The ratio of swing phase time to stance phase time is a temporal measure of gait configuration at a specific stride frequency. We found that the swing to stance ratio increased by approximately 25% following injury and that the ratio was normalized by Neu2000 treatment.
Other DigiGait parameters affected by injury and Neu2000 treatment related to hindlimb measures including the degree of external rotation, hindpaw area, and stance width. Some of these results are consistent with a previous study utilizing footprint analysis to examine spontaneous recovery following experimental SCI (McEwen and Springer, 2006). However, as pointed out in the previous study, normal rats will use different gaits depending upon the speed at which they traverse the runway. This makes it difficult to compare results across groups and across studies and was the incentive for utilizing the DigiGait, which provides a constant treadmill speed. The SCI model used in this study typically results in the outward rotation of the hindpaws, in this case approximately 12°. This can be observed in the traces shown in Fig. 3 in which the right hindpaw of the SCI animal (in red) shows a high degree of outward rotation. In addition, hindpaw area, defined as the maximum paw area at peak stance, is significantly reduced following SCI (green paw print in Fig. 3). While the BBB and DigiGait have the capacity to measure different locomotor outcomes, these results support the use of multiple testing paradigms when examining functional changes following SCI. However, these two scoring systems are not mutually exclusive and, on some measures, are complementary. In particular, both scoring systems showed that paw position during stance was “normalized” in animals receiving repeated treatments with Neu2000. Therefore, using these complementary measures of locomotor and gait activity provides a stronger argument for the therapeutic potential of Neu2000.
The ability of Neu2000 to improve tissue sparing over the 42-day recovery period was clearly evident. Stereological assessment of the histological sections revealed that Neu2000 treatment resulted in a significant reduction in overall lesion volume compared to vehicle-treated animals (Rabchevsky et al., 2001; Ravikumar et al., 2007). While there was a trend toward a reduction in lesion volume in the repeated Neu2000 treatment group compared to single treatment, it did not reach statistical significance. Subsequent examination of the histological staining revealed that the significant decrease in lesion volume in the repeated Neu2000 treatment group was due primarily to an increase in white matter sparing. This was more pronounced near the injury epicenter, which is typically the most affected region in this animal model of SCI. An increase in white matter sparing has been associated with improved scoring on the BBB scale and this is consistent with our current findings (Basso et al., 1996). However, there was no clear correlation between white matter sparing and the DigiGait data. Specifically both the single and repeated Neu2000 treatment groups showed significant improvement on several gait parameters; however, only the repeated dosing with Neu2000 resulted in significant white matter sparing. These observations support the need for using multiple dosing and testing paradigms to examine treatment efficacy on recovery of function and tissue sparing following SCI.
In conclusion, Neu2000 has previously been shown to significantly reduce secondary injury associated with glutamate excitotoxicity and oxidative damage and to improve functional outcomes associated with cerebral ischemia (Gwag et al., 2007). Secondary pathophysiological events involving glutamate excitotoxicity and oxidative damage also occur following SCI (Azbill et al., 1997; Grossman et al., 2000; Hall and Springer, 2004; Liu et al., 1999; McAdoo et al., 1999; Springer et al., 1997; WratHall et al., 1992). The results of this study demonstrate the efficacy of Neu2000 on limiting mitochondrial ROS production, promoting tissue sparing, and improving locomotor outcomes in a well-defined experimental model of SCI. Given that Neu2000 is moving towards Phase II trials in stroke, there is clear therapeutic potential for the use of Neu2000 in SCI and future studies need to be performed to identify the optimal therapeutic time window, cell types affected, and the level of efficacy at different injury severities.
Acknowledgments
We are indebted to Melanie L. McEwen for insightful discussions related to the DigiGait analysis. This work supported by a grant from the Kentucky Spinal Cord and Head Injury Research Trust, the Driving Force Project for the Next Generation of Gyeonggi Provincial Government in Republic of Korea, and an endowment from Cardinal Hill Rehabilitation Hospital.
Author Disclosure Statement
The following authors have competing financial interests: Hyang Ran Lim, Sung Ig Cho, Gyoeng Joon Moon, Hee Young Lee, Eui Jin Park, and Byoung Joo Gwag.
References
- Agrawal S.K. Fehlings M.G. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J. Neurosci. 1997;17:1055–1063. doi: 10.1523/JNEUROSCI.17-03-01055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amende I. Kale A. McCue S. Glazier S. Morgan J.P. Hampton T.G. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J. Neuroengineering Rehabil. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azbill R.D. Mu X. Bruce-Keller A.J. Mattson M.P. Springer J.E. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- Baines C.P. Kaiser R.A. Purcell N.H. Blair N.S. Osinska H. Hambleton M.A. Brunskill E.W. Sayen M.R. Gottlieb R.A. Dorn G.W. Robbins J. Molkentin J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baptiste D.C. Fehlings M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- Baptiste D.C. Tighe A. Fehlings M.G. Spinal cord injury and neural repair: focus on neuroregenerative approaches for spinal cord injury. Expert Opin. Investig. Drugs. 2009;18:663–673. doi: 10.1517/13543780902897623. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D. Beattie M. Breshnahan J. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso E. Fante L. Fowlkes J. Petronilli V. Forte M.A. Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Blight A.R. Zimber M.P. Acute spinal cord injury: pharmacotherapy and drug development perspectives. Curr. Opin. Investig. Drugs. 2001;2:801–808. [PubMed] [Google Scholar]
- Brown M.R. Geddes J.W. Sullivan P.G. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 2004;36:401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N. Brustovetsky T. Jemmerson R. Dubinsky J.M. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N. Brustovetsky T. Purl K.J. Capano M. Crompton M. Dubinsky J.M. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J. Neurosci. 2003;23:4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande C. Cohen I. Daugas E. Ravagnan L. Larochette N. Zamzami N. Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- Chai J. Du C. Wu J.W. Kyin S. Wang X. Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- Chen H.S. Wang Y.F. Rayudu P.V. Edgecomb P. Neill J.C. Segal M.M. Lipton S.A. Jensen F.E. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- De Cristobal J. Madrigal J.L. Lizasoain I. Lorenzo P. Leza J.C. Moro M.A. Aspirin inhibits stress-induced increase in plasma glutamate, brain oxidative damage and ATP fall in rats. Neuroreport. 2002;13:217–221. doi: 10.1097/00001756-200202110-00009. [DOI] [PubMed] [Google Scholar]
- Friberg H. Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- Gionchetti P. Guarnieri C. Campieri M. Belluzzi A. Brignola C. Iannone P. Miglioli M. Barbara L. Scavenger effect of sulphasalazine (SASP), 5-aminosalicylic acid (5-ASA), and olsalazine (OAZ) Gut. 1990;31:730–731. doi: 10.1136/gut.31.6.730-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M. Pizzi M. Memo M. Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Grossman S.D. Wolfe B.B. Yasuda R.P. Wrathall J.R. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J. Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- Gwag B.J. Lee Y.A. Ko S.Y. Lee M.J. Im D.S. Yun B.S. Lim H.R. Park S.M. Byun H.Y. Son S.J. Kwon H.J. Lee J.Y. Cho J.Y. Won S.J. Kim K.W. Ahn Y.M. Moon H.S. Lee H.U. Yoon S.H. Noh J.H. Chung J.M. Cho S.I. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J. Cereb. Blood Flow Metab. 2007;27:1142–1151. doi: 10.1038/sj.jcbfm.9600418. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Braughler J.M. McCall J.M. Antioxidant effects in brain and spinal cord injury. J. Neurotrauma. 1992;9(Suppl. 1):S165–S172. [PubMed] [Google Scholar]
- Hall E.D. Springer J.E. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T.G. Stasko M.R. Kale A. Amende I. Costa A.C. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol. Behav. 2004;82:381–389. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Persson T. Friberg H. Keep M.F. Rees A. Wieloch T. Elmer E. Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res. 2003;960:99–111. doi: 10.1016/s0006-8993(02)03798-8. [DOI] [PubMed] [Google Scholar]
- Jin Y. McEwen M.L. Nottingham S.A. Maragos W.F. Dragicevic N.B. Sullivan P.G. Springer J.E. The mitochondrial uncoupling agent 2,4-dinitrophenol improves mitochondrial function, attenuates oxidative damage, and increases white matter sparing in the contused spinal cord. J. Neurotrauma. 2004;21:1396–1404. doi: 10.1089/neu.2004.21.1396. [DOI] [PubMed] [Google Scholar]
- Kale A. Amende I. Meyer G.P. Crabbe J.C. Hampton T.G. Ethanol's effects on gait dynamics in mice investigated by ventral plane videography. Alcohol Clin. Exp. Res. 2004;28:1839–1848. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- Kim E.Y. Chang S.Y. Chung J.M. Ryu B.R. Joo C.K. Moon H.S. Kang K. Yoon S.H. Han P.L. Gwag B.J. Attenuation of Zn2+ neurotoxicity by aspirin: role of N-type Ca2+ channel and the carboxyl acid group. Neurobiol. Dis. 2001;8:774–783. doi: 10.1006/nbdi.2001.0421. [DOI] [PubMed] [Google Scholar]
- Ko H.W. Park K.Y. Kim H. Han P.L. Kim Y.U. Gwag B.J. Choi E.J. Ca2+−mediated activation of c-Jun N-terminal kinase and nuclear factor kappa B by NMDA in cortical cell cultures. J. Neurochem. 1998;71:1390–1395. doi: 10.1046/j.1471-4159.1998.71041390.x. [DOI] [PubMed] [Google Scholar]
- Li S. Kim J.E. Budel S. Hampton T.G. Strittmatter S.M. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. Xu G.Y. Pan E. McAdoo D.J. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience. 1999;93:1383–1389. doi: 10.1016/s0306-4522(99)00278-x. [DOI] [PubMed] [Google Scholar]
- McAdoo D.J. Xu G.Y. Robak G. Hughes M.G. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp. Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- McEwen M.L. Springer J.E. Quantification of locomotor recovery following spinal cord contusion in adult rats. J. Neurotrauma. 2006;23:1632–1653. doi: 10.1089/neu.2006.23.1632. [DOI] [PubMed] [Google Scholar]
- McEwen M.L. Sullivan P.G. Springer J.E. Pretreatment with the cyclopsorin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. J. Neurotrauma. 2007;24:613–624. doi: 10.1089/neu.2006.9969. [DOI] [PubMed] [Google Scholar]
- Mouton P.R. Gokhale A.M. Ward N.L. West M.J. Stereological length estimation using spherical probes. J. Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Mu X. Azbill R.D. Springer J.E. Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J. Neurotrauma. 2000a;17:773–780. doi: 10.1089/neu.2000.17.773. [DOI] [PubMed] [Google Scholar]
- Mu X. Azbill R.D. Springer J.E. Riluzole improves measures of oxidative stress in traumatic spinal cord injury. Brain Res. 2000b;870:66–72. doi: 10.1016/s0006-8993(00)02402-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Shimizu S. Watanabe T. Yamaguchi O. Otsu K. Yamagata H. Inohara H. Kubo T. Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Noh J. Lee E.S. Chung J.M. The novel NMDA receptor antagonist, 2-hydroxy-5-(2,3,5,6-tetrafluoro-4-trifluoromethyl-benzylamino)-benzoic acid, is a gating modifier in cultured mouse cortical neurons. J. Neurochem. 2009;109:1261–1271. doi: 10.1111/j.1471-4159.2009.06044.x. [DOI] [PubMed] [Google Scholar]
- Olney J.W. Labruyere J. Wang G. Wozniak D.F. Price M.T. Sesma M.A. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- Petronilli V. Penzo D. Scorrano L. Bernardi P. Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Rabchevsky A.G. Fugaccia I. Sullivan P.G. Scheff S.W. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- Ravikumar R. Fugaccia I. Scheff S.W. Geddes J.W. Srinivasan C. Toborek M. Nicotine attenuates morphological deficits in a contusion model of spinal cord injury. J. Neurotrauma. 2005;22:240–251. doi: 10.1089/neu.2005.22.240. [DOI] [PubMed] [Google Scholar]
- Ravikumar R. McEwen M.L. Springer J.E. Post-treatment with the cyclosporin derivative, NIM811, reduced indices of cell death and increased the volume of spared tissue in the acute period following spinal cord contusion. J. Neurotrauma. 2007;24:1618–1630. doi: 10.1089/neu.2007.0329. [DOI] [PubMed] [Google Scholar]
- Reed M.G. Howard C.V. Surface-weighted star volume: concept and estimation. J. Microsc. 1998;190:350–356. doi: 10.1046/j.1365-2818.1998.00331.x. [DOI] [PubMed] [Google Scholar]
- Ryu B.R. Lee Y.A. Won S.J. Noh J.H. Chang S.Y. Chung J.M. Choi J.S. Joo C.K. Yoon S.H. Gwag B.J. The novel neuroprotective action of sulfasalazine through blockade of NMDA receptors. J. Pharmacol. Exp. Ther. 2003;305:48–56. doi: 10.1124/jpet.102.042606. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schinzel A.C. Takeuchi O. Huang Z. Fisher J.K. Zhou Z. Rubens J. Hetz C. Danial N.N. Moskowitz M.A. Korsmeyer S.J. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N. Baxter M.G. Petravicz J. Bragg D.C. Schienda A. Standaert D.G. Breakefield X.O. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J. Neurosci. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer J.E. Azbill R.D. Mark R.J. Begley J.G. Waeg G. Mattson M.P. 4-Hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J. Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Krishnamurthy S. Patel S.P. Pandya J.D. Rabchevsky A.G. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J. Neurotrauma. 2007;24:991–999. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Waldmeier P.C. Springer J.E. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Vartiainen N. Goldsteins G. Keksa-Goldsteine V. Chan P.H. Koistinaho J. Aspirin inhibits p44/42 mitogen-activated protein kinase and is protective against hypoxia/reoxygenation neuronal damage. Stroke. 2003;34:752–757. doi: 10.1161/01.STR.0000057813.31798.1F. [DOI] [PubMed] [Google Scholar]
- Wooley C.M. Sher R.B. Kale A. Frankel W.N. Cox G.A. Seburn K.L. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall J.R. Teng Y.D. Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D- aspartate receptors. Exp. Neurol. 1996;137:119–126. doi: 10.1006/exnr.1996.0012. [DOI] [PubMed] [Google Scholar]
- Wrathall J.R. Teng Y.D. Choiniere D. Mundt D.J. Evidence that local non-NMDA receptors contribute to functional deficits in contusive spinal cord injury. Brain Res. 1992;586:140–143. doi: 10.1016/0006-8993(92)91384-q. [DOI] [PubMed] [Google Scholar]




