Abstract
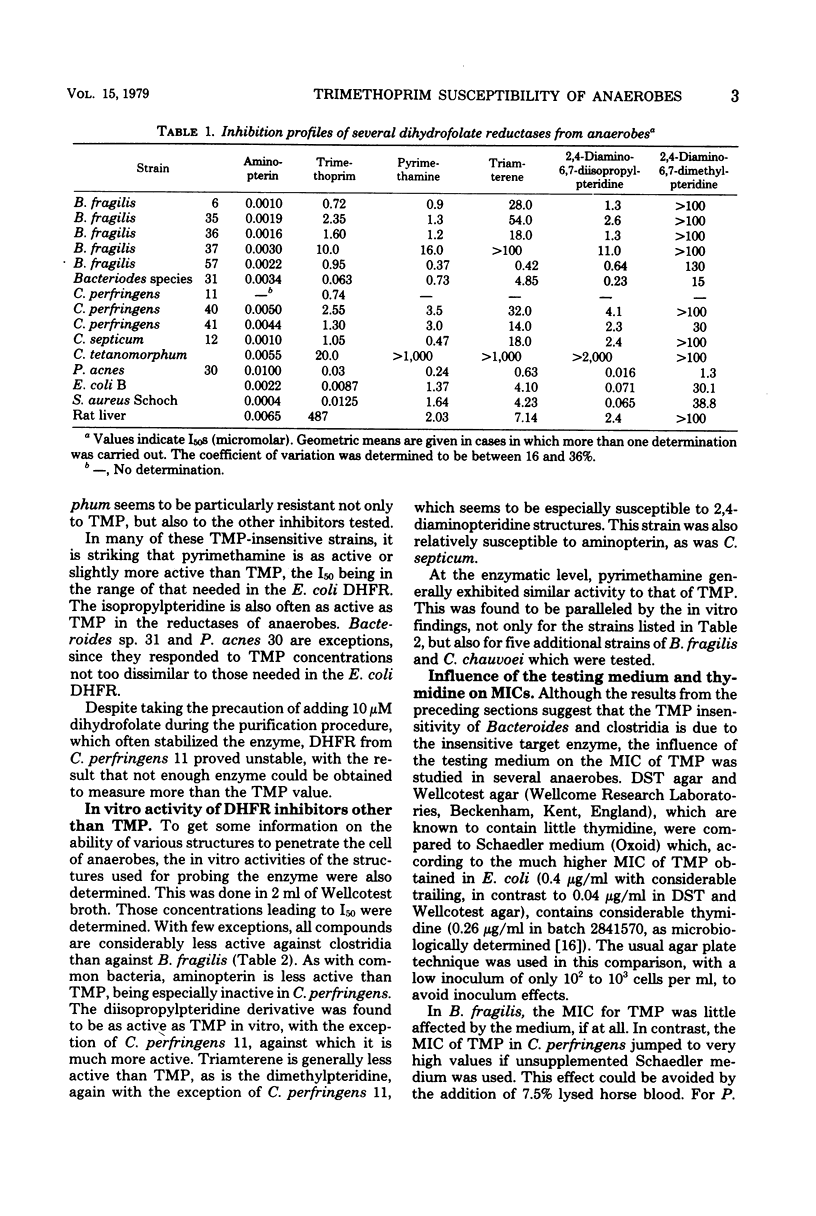
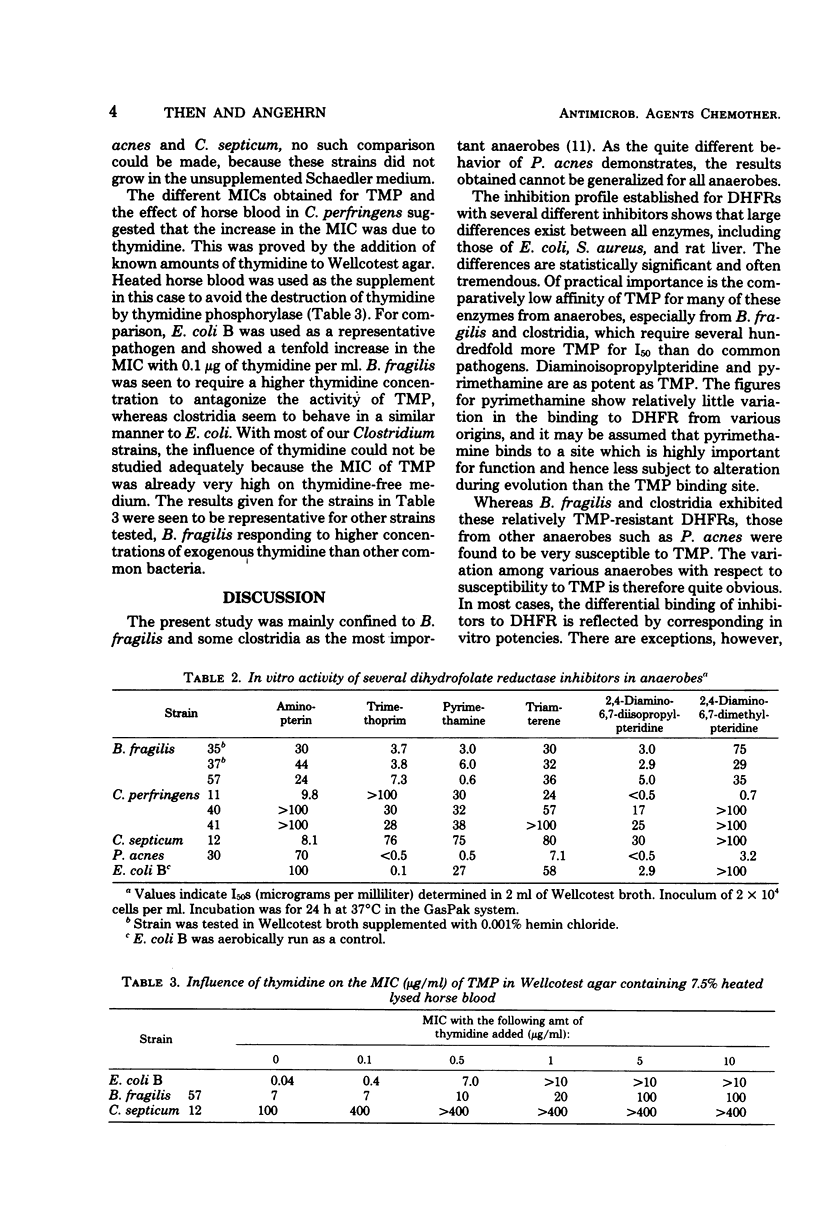
All the 28 Bacteroides fragilis strains investigated were susceptible to sulfamethoxazole (minimal inhibitory concentration < 16 μg/ml) and resistant to trimethoprim (TMP; minimal inhibitory concentration > 4 μg/ml). Synergism between sulfamethoxazole and TMP was present in all strains at a ratio of 1:1. The few clostridia investigated proved more resistant to both compounds. Dihydrofolate reductases from B. fragilis, C. perfringens, and some other anaerobic species were isolated. Inhibition profiles with six structurally different inhibitors revealed major differences in all enzymes. For 50% inhibition, the enzyme from B. fragilis and all clostridia required concentrations of TMP which were between several hundredfold and 1,000-fold higher than those required for the enzyme of Escherichia coli, whereas the enzyme from Propionibacterium acnes only needed a threefold higher concentration. In vitro activities of TMP were seen to correspond to the activity at the enzymatic level in B. fragilis and P. acnes, but correspond to a much lesser extent to the activity at the enzymatic level in clostridia, where a poor penetration is assumed to be involved. Dihydrofolate reductase inhibitors other than TMP were found to be as active as TMP both at the enzyme and in vitro. In B. fragilis, higher concentrations of exogenous thymidine were required for increasing the minimal inhibitory concentration of TMP than in E. coli and probably also in C. perfringens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Smith J. T. The purification and properties of the trimethoprim-resistant dihydrofolate reductase mediated by the R-factor, R388. Eur J Biochem. 1976 Jan 15;61(2):597–603. doi: 10.1111/j.1432-1033.1976.tb10055.x. [DOI] [PubMed] [Google Scholar]
- Amyes S. G., Smith J. T. Trimethoprim action and its analogy with thymine starvation. Antimicrob Agents Chemother. 1974 Feb;5(2):169–178. doi: 10.1128/aac.5.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby S. R., Hitchings G. H. Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother. 1968 May;33(1):72–90. doi: 10.1111/j.1476-5381.1968.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A., Kunin C. M. Distribution of trimethoprim-sulfamethoxazole in tissues of rhesus monkeys. J Infect Dis. 1973 Nov;128(Suppl):575–p. doi: 10.1093/infdis/128.supplement_3.s575. [DOI] [PubMed] [Google Scholar]
- Darrell J. H., Garrod L. P., Waterworth P. M. Trimethoprim: laboratory and clinical studies. J Clin Pathol. 1968 Mar;21(2):202–209. doi: 10.1136/jcp.21.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Nord C. E., Dahlbäck A. Antibiotic susceptibility of Clostridium species isolated from human infections. Scand J Infect Dis. 1975;7(2):127–134. doi: 10.3109/inf.1975.7.issue-2.09. [DOI] [PubMed] [Google Scholar]
- Dornbusch K., Nord C. E., Wadström T. Biochemical characterization and in vitro determination of antibiotic susceptibility of clinical isolates of Bacteroides fragilis. Scand J Infect Dis. 1974;6(3):253–258. doi: 10.3109/inf.1974.6.issue-3.08. [DOI] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Hillcoat B. L., Nixon P. F., Blakley R. L. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal Biochem. 1967 Nov;21(2):178–189. doi: 10.1016/0003-2697(67)90179-0. [DOI] [PubMed] [Google Scholar]
- Holland J. W., Hill E. O., Altemeier W. A. Numbers and types of anaerobic bacteria isolated from clinical specimens since 1960. J Clin Microbiol. 1977 Jan;5(1):20–25. doi: 10.1128/jcm.5.1.20-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzenmeier G., Neussel H. Die Kombination Sulfamethoxazol-Trimethoprim in vitro. Zentralbl Bakteriol Orig A. 1972 Sep;221(4):511–526. [PubMed] [Google Scholar]
- Okubadejo O. A. Letter: Susceptibility of Bacteroides fragilis to co-trimoxazole. Lancet. 1974 May 25;1(7865):1061–1061. doi: 10.1016/s0140-6736(74)90471-1. [DOI] [PubMed] [Google Scholar]
- Phillips I., Warren C. Activity of sulfamethoxazole and trimethoprim against Bacteroides fragilis. Antimicrob Agents Chemother. 1976 May;9(5):736–740. doi: 10.1128/aac.9.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz E., Michel M. F., Zwart H. G. Potentiation of sulphamethoxazole by trimethoprim in Neisseria gonorrhoeae strains. Chemotherapy. 1977;23(2):65–72. doi: 10.1159/000221973. [DOI] [PubMed] [Google Scholar]
- Then R. L., Riggenbach H. Dihydrofolate reductases in some folate-requiring bacteria with low trimethoprim susceptibility. Antimicrob Agents Chemother. 1978 Jul;14(1):112–117. doi: 10.1128/aac.14.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst J., Wilkins T. D. Susceptibility of anaerobic bacteria to sulfamethoxazole/trimethoprim and routine susceptibility testing. Antimicrob Agents Chemother. 1978 Sep;14(3):384–390. doi: 10.1128/aac.14.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]



