Abstract
In vitro anti-genotoxic properties of bile pigments have been explored and confirmed recently. Despite these reports mechanisms to explain DNA protection by endogenous bile pigments remain unclear. Surprisingly, the quantification of cellular pigment absorption which could represent a fundamental prerequisite for intracellular (e.g., anti-mutagenic) effects, has not been explored. Therefore, we aimed to measure the amounts of un-/conjugated bilirubin as well as biliverdin absorbed into colonies of Salmonella typhimurium, utilising HPLC analyses, and to observe whether intracellular compound concentrations could predict anti-genotoxic effects. HPLC analyses confirmed that bacterial bile pigment absorption was concentration-dependent. Plate bile pigment concentrations were inversely associated with genotoxicity of all tested mutagens, irrespective of strain and test conditions. However, protection against frame-shift mutation in strain TA98 most strongly depended on the bacterial absorption of bilirubin and biliverdin, which indicates that bile pigments can protect by intercepting mutations extracellularly and specifically inhibit frame-shift mutations intracellularly.
Abbreviations: AfB1, aflatoxin B1; BP(s), bile pigment(s); BR, unconjugated bilirubin; BRDT, bilirubin ditaurate; BV, biliverdin; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; S. typhimurium/Salmonella, Salmonella typhimurium; t-BOOH, tertiary butyl hydroperoxide; TNFone, 2,4,7-trinitro-9H-fluoren-9-one
Keywords: Bilirubin, Biliverdin, Frame-shift, TA102, TA98, Cancer
1. Introduction
Bile pigments (BPs) such as bilirubin (BR) and biliverdin (BV) are tetrapyrrolic, dicarboxylic compounds derived from the enzymatic heme degradation. They are distributed throughout the body and thus could play an essential role in systemic and tissue-specific health promotion. Numerous studies have identified anti-mutagenic and anti-oxidative activity of specific tetrapyrroles (TPs) in vitro (Asad et al., 2001; Bulmer et al., 2007). In vivo data also demonstrate disease prevention through vasoprotection, inhibition of inflammation and anti-oxidant activity (Bulmer et al., 2008b; McCarty, 2007). Multiple underlying mechanisms of anti-genotoxic action have been hypothesised but remain to be confirmed. Surprisingly, no publication has focused on quantifying cellular BP absorption which could form a fundamental basis for intracellular action. Therefore, we aimed to quantify the absorption of BR, BV and conjugated BR (ditaurate; BRDT) into two distinct strains of Salmonella typhimurium (S. typhimurium) via HPLC analyses. It was hypothesised that BPs would be absorbed in a dose-dependent manner into bacteria, and that extracellular (plate) and intracellular (absorbed) BP concentrations would broadly protect against genotoxicity mediated by various mutagens.
2. Materials and methods
2.1. Salmonella reverse mutation assay
The Salmonella reverse mutation assay is an in vitro test assessing the mutagenic potential of chemicals. Bacterial wild-type reversion in the presence of mutagens, allowing growth and colony formation represents its fundamental, technical basis. Experiments were conducted as previously published (Maron and Ames, 1983), and included 48 h of BP incubation at 37 °C. In some assays, S9 liver homogenate (S9 microsomal fraction from Aroclor-treated rats) was used as an enzymatic activation system. Bile pigment concentrations were tested in triplicate, negative/positive controls were tested in each assay (n = 6). Experiments were repeated again on a different day and results were then pooled (n = 6 minimum).
2.1.1. Bacterial strains
Two strains of S. typhimurium were tested: TA102, susceptible to oxidative damage, reverts by cross-linking agents, TA98 detects frame-shift mutations and base-pair deletions (Mortelmans and Zeiger, 2000). Strains were kindly provided by Dr. Bruce N. Ames and were attested to their genetic integrity and spontaneous mutation rate (Mortelmans and Zeiger, 2000) in our laboratory.
2.1.2. Chemicals
Unconjugated bilirubin 1Xα (CAS# 635-65-4), conjugated bilirubin (ditaurate; CAS# 635-65-4) and biliverdin 1Xα (CAS# 55482-27-4) were purchased from Frontier Scientific Europe, UK. Chemical structures can be found online (Supplementary material 1). Pigment purity (>98%) and solubility were measured using HPLC and spectrophotometry. The S9 liver homogenate was obtained from MP Biomedicals, France. All other reagents and mutagens were purchased from Sigma Aldrich, Austria (unless otherwise noted), were of the highest analytical grade available, and stored according to instructions. Bile pigment solutions were prepared in DMSO, protected from light, and used immediately. Composition and preparation of all necessary solutions can be found elsewhere (Bulmer et al., 2007). To assess different possibilities of anti-genotoxic action (e.g., structural interactions, radical scavenging, complex formation), four different mutagens were applied at their respective appropriate concentrations (Table 1): 2,4,7-trinitro-9H-fluoren-9-one (J & K Ltd., China; TNFone), tertiary-butyl hydroperoxide (Merck; t-BOOH), aflatoxin B1 (AfB1) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (Toronto Research Chemicals, Canada; PhIP).
Table 1.
Correlations between BP plate concentrations and bacterial pigment absorption in Salmonella strains TA98 and TA102 (“availability-based absorption”).
| Compound | Strain | Mutagen conc. [mol/plate] | S9 | Correlation (r) BP absorption × BP plate conc. |
|---|---|---|---|---|
| BR | TA98 | TNFone, 0.3 × 10−6 | − | 0.693⁎⁎ |
| BR | TA102 | TNFone, 0.2 × 10−7 | − | 0.972⁎⁎ |
| BR | TA98 | PhIP, 0.1 × 10−7 | + | 0.687⁎⁎ |
| BR | TA98 | AfB1, 0.8 × 10−7 | + | 0.982⁎⁎ |
| BR | TA102 | t-BOOH, 0.75 × 10−6 | − | 0.691⁎⁎ |
| BV | TA98 | PhIP, 0.1 × 10−7 | + | 0.949⁎⁎ |
| BV | TA98 | AfB1, 0.8 × 10−7 | + | 0.972⁎⁎ |
| BV | TA102 | AfB1, 0.24 × 10−6 | + | 0.949⁎⁎ |
| BV | TA102 | t-BOOH, 0.75 × 10−6 | − | 0.949⁎⁎ |
| BV | TA102 | t-BOOH, 0.75 × 10−6 | + | 0.949⁎⁎ |
| BRDT | TA98 | PhIP, 0.1 × 10−7 | + | 0.885⁎⁎ |
| BRDT | TA102 | AfB1, 0.24 × 10−6 | + | 0.972⁎⁎ |
| BRDT | TA102 | t-BOOH, 0.75 × 10−6 | − | 0.878⁎ |
| BRDT | TA102 | t-BOOH, 0.75 × 10−6 | + | 0.814⁎⁎ |
S9: metabolic activation system (microsomal fraction from Aroclor-treated rats); BP: bile pigment; BR: unconjugated bilirubin; BV: biliverdin; BRDT: bilirubin ditaurate; TNFone: 2,4,7-trinitro-9H-fluoren-9-one; PhIP: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; AfB1: aflatoxin B1; t-BOOH: tertiary butyl hydroperoxide.
Significant on p ⩽ 0.01.
Significant on p ⩽ 0.05.
2.1.3. Bile pigment sample preparation for the Salmonella reverse mutation assay
Based on preceding investigations (Bulmer et al., 2007), BRDT and BV were tested at concentrations of 0.01, 0.05, 0.1, 0.5, 1 and 2 μmol/plate (equals 3.4, 17.2, 34.5, 172.4, 349 and 689.6 μM). Unconjugated BR was tested over a range of five doses including: 0.01, 0.05, 0.1, 0.5 and 0.75 μmol/plate (equals 258.6 μM). Maximum BP plate concentrations had been ascertained by (1) testing the maximum amount of DMSO that did not result in bacterial cytotoxicity (200 μl/plate) and (2) by the respective maximum solubility of each test compound (spectrophotometric supernatant analysis: BR, BRDT: 455 nm; BV: 380 nm), read on a Perkin Elmer Lambda 2 UV/VIS spectrophotometer after high-speed centrifugation.
2.2. Sample preparation for HPLC analyses
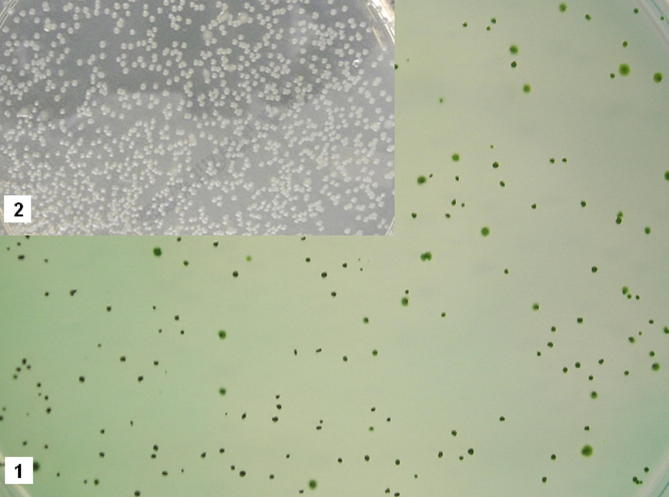
Briefly, S. typhimurium colonies (⩾1 mm in diameter) were collected from agar plates, and were lysed for 30 min in 40 μl of isocratic mobile phase (950 ml HPLC-grade methanol, 50 ml HPLC-grade water, 24.2 g n-dioctylamine and 6.01 g glacial acetic acid per litre). Supernatants were diluted at 1:4, and injected (50 μl) into a Hitachi HPLC, equipped with a Shimadzu SPD-M20A detector, and a C18 reverse phase column (5micron, 250 × 4.6 m) (Brower et al., 2001; Bulmer et al., 2008b). Oven temperature was set at 35 °C, column pressure at 140 bar. Sixteen BP standards were run, ranging from 500 to 0.01 μM. The method’s detection limit (LOD) was calculated at 18 nM. Photographs of bacterial colonies can be found online (Supplementary material 2).
2.3. Measurement of total protein content
As a reference parameter for bacterial BP absorption, the total protein content in each diluted sample was measured photometrically (Bradford, 1976). Bile pigment concentrations were then expressed as nmol/mg total protein.
2.4. Statistical analyses
Data were analysed using SPSS 17.0. A p-value ⩽0.05 was considered significant. Data were tested for normal distribution using the Kolmogorov–Smirnov test. Parametric statistical analysis (one-way ANOVA) and the post hoc Scheffé test were performed on normally distributed, and corresponding non-parametric tests (Kruskal–Wallis H-test, Dunn’s post hoc test) on skewed data. Relationships between (1) BP bacterial absorption and plate concentrations; (2) BP bacterial absorption and anti-genotoxic effects; and (3) between BP plate concentrations and anti-mutagenicity were determined by performing bivariate correlations (Pearson or Spearman for parametric and non-parametric data, respectively).
3. Results
HPLC analyses showed significant concentration-dependent BP absorption from agar plates, which was independent of strain, test condition (± S9) and the applied mutagen (Table 1). Furthermore, anti-mutagenic effects of all BPs against all tested mutagens were observed (Table 2).
Table 2.
Correlations between BP plate concentrations or bacterial BP absorption, respectively, and anti-mutagenic effects in Salmonella strains TA98 and TA102 (“availability-based anti-mutagenic effects” and “absorption-based anti-mutagenic effects”).
| Compound | Strain | Mutagen conc. [mol/plate] | S9 | Correlation (r) BP plate conc. x anti-mut. effects | Correlation (r) BP absorption x anti-mut. effects |
|---|---|---|---|---|---|
| BR | TA98 | TNFone, 0.3 × 10−6 | − | −0.794⁎⁎ | −0.938⁎⁎ |
| BR | TA102 | TNFone, 0.2 × 10−7 | − | −0.682⁎⁎ | −0.639⁎⁎ |
| BV | TA102 | TNFone, 0.2 × 10−7 | − | −0.493⁎⁎ | −0.124 |
| BV | TA98 | PhIP, 0.1 × 10−7 | + | −0.927⁎⁎ | −0.917⁎⁎ |
| BV | TA98 | AfB1, 0.8 × 10−7 | + | −0.473⁎⁎ | −0.827⁎⁎ |
| BV | TA102 | AfB1, 0.24 × 10−6 | + | −0.601⁎⁎ | −0.216 |
| BV | TA102 | t-BOOH, 0.75 × 10−6 | − | −0.607⁎⁎ | −0.570 |
| BRDT | TA98 | TNFone, 0.3 × 10−6 | − | −0.475⁎⁎ | −0.198 |
| BRDT | TA102 | TNFone, 0.2 × 10−7 | − | −0.378⁎ | −0.203 |
| BRDT | TA98 | AfB1, 0.8 × 10−7 | + | −0.548⁎⁎ | −0.665 |
| BRDT | TA102 | t-BOOH, 0.75 × 10−6 | − | −0.754⁎⁎ | −0.481 |
S9: metabolic activation system (microsomal fraction from Aroclor-treated rats); BP: bile pigment; BR: unconjugated bilirubin; BV: biliverdin; BRDT: bilirubin ditaurate; TNFone: 2,4,7-trinitro-9H-fluoren-9-one; PhIP: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; AfB1: aflatoxin B1; t-BOOH: tertiary butyl hydroperoxide.
Significant on p ⩽ 0.01.
Significant on p ⩽ 0.05.
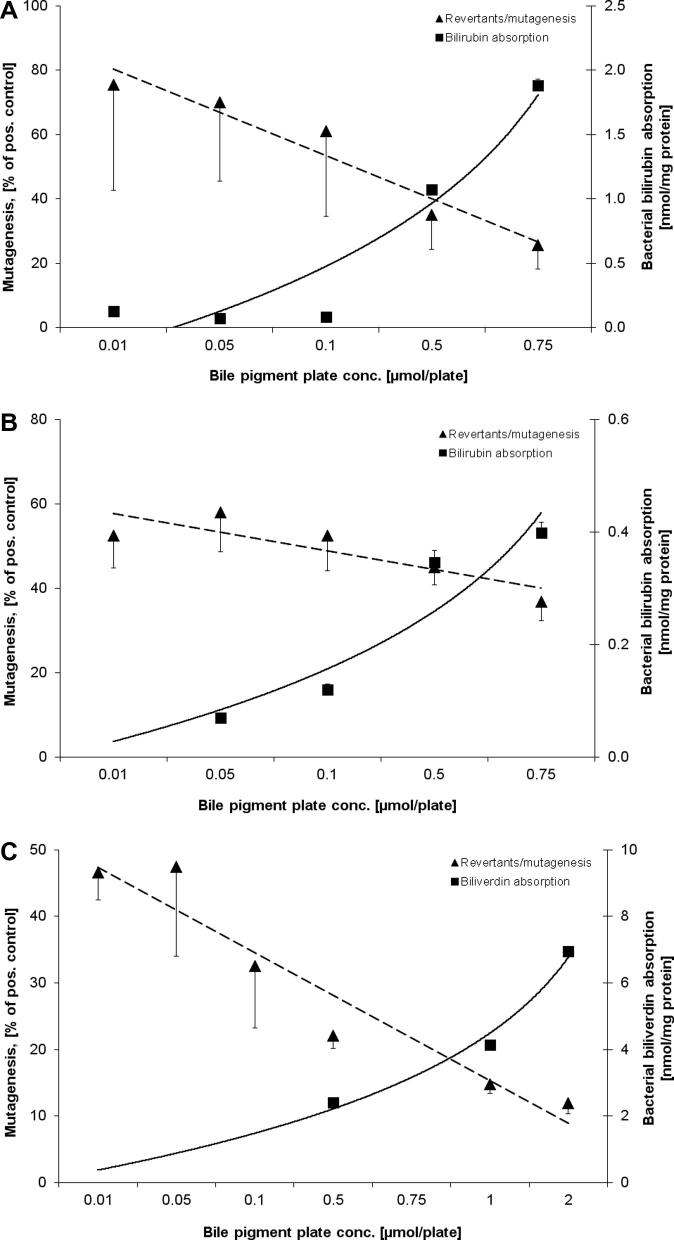
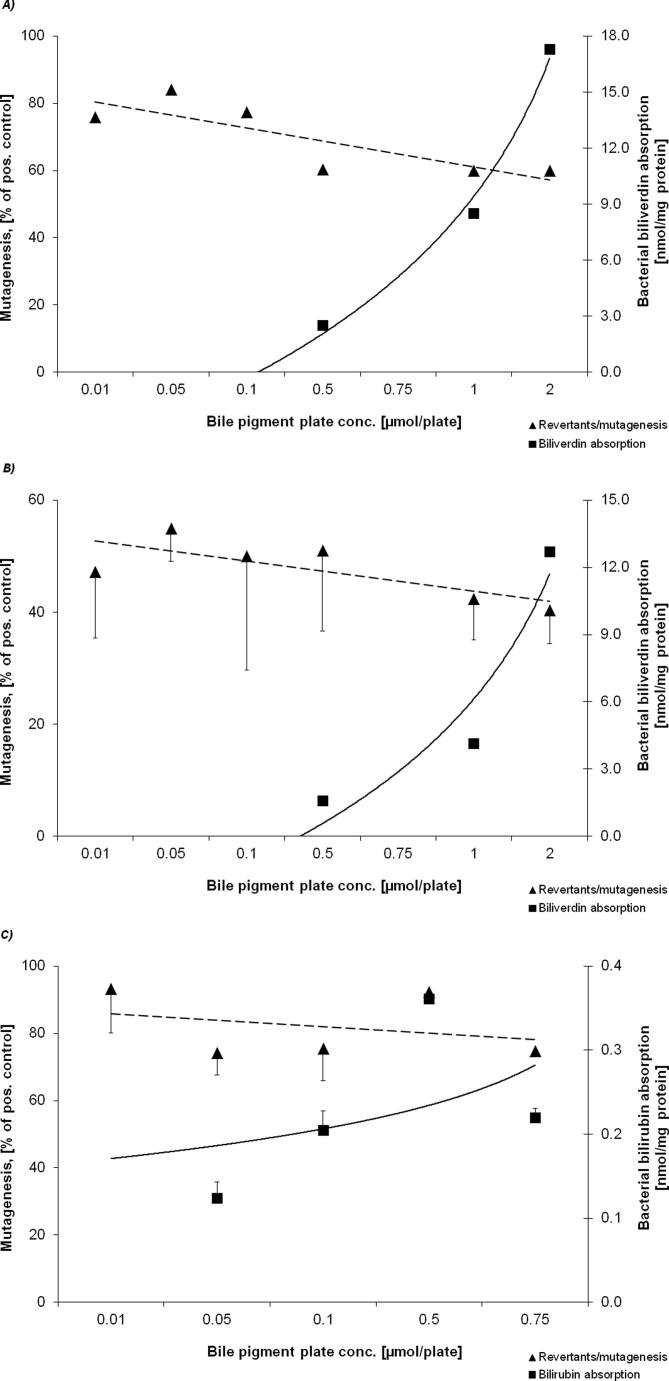
The relationships between BP plate concentrations, bacterial BP absorption and observed anti-mutagenic effects are shown in Fig. 1A–C and in Supplementary material 3. Significant inverse relationships were found between BR plate concentrations in strains TA98 and TA102 and anti-mutagenic action against TNFone, as well as between BV plate concentration and PhIP mutagenesis in TA98 (Fig. 1A–C). A typical chromatogram showing BR absorption into strain TA98 is shown in Fig. 2. Furthermore, significant correlations between pigment plate concentrations and anti-genotoxicity were found for all test conditions (Table 2), indicating a stronger concentration-dependent anti-mutagenic effect based on extracellular (vs. intracellular) BP concentration. Interestingly, the anti-mutagenic effects of BR and BV were most strongly dependent on the bacterial BP absorption exclusively in strain TA98 (Table 2).
Fig. 1.
(A–C) Relationship between bacterial BP absorption and observed anti-mutagenic effects against different mutagens; (A) BR in strain TA98 with TNFone, (B) BR in strain TA102 with TNFone, (C) BV in strain TA98 with PhIP and with S9.
Fig. 2.
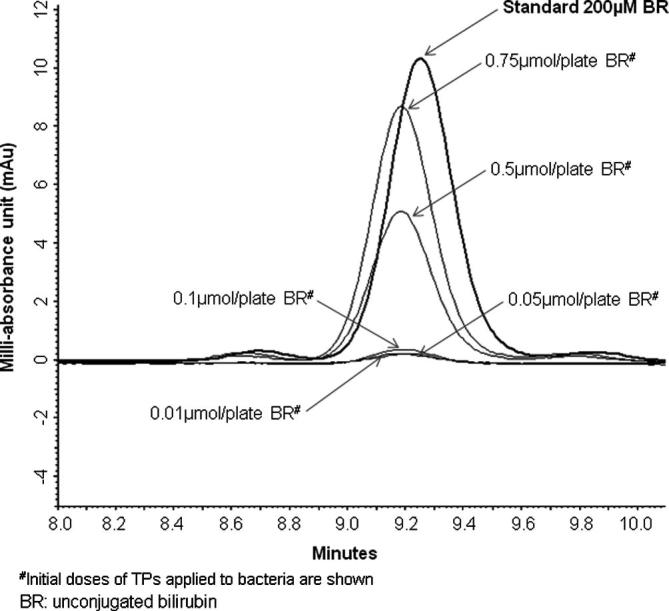
Chromatogram of BR (with TNFone) in Salmonella TA98 colonies.
An entirely novel observation was also made in that the obtained HPLC spectra (not shown) suggest appearance of BR in plates supplemented with BV, which could imply biliverdin reductase activity in S. typhimurium. The ratio of BV to BR (BV:BR) bacterial concentrations calculated from HPLC chromatograms (at 1 μmol/plate BV) approximated 4.4:1 in TA98 and 9.6:1 in TA102.
4. Discussion
This study is the first to report on bacterial BP absorption and its relationship with observed anti-mutagenic effects. When exposed to mutagens, extracellular (plate) BP concentrations negatively correlated with genotoxicity. Furthermore, testing in TA98 revealed that BV and BR absorption was more strongly related with anti-mutagenesis, when compared to the anti-mutagenic effect relative to plate concentrations.
Previous reports refer to the ability of BPs to act in an anti-oxidant and anti-genotoxic manner in vitro (Asad et al., 2001; Bulmer et al., 2007) and in vivo (Boon et al., 2012; Horsfall et al., 2011). Vastly unclear to date however, are the underlying mechanisms of anti-genoxic action. In this context mainly electron scavenging or hydrogen donating capacities (MacLean et al., 2008) and structural interactions between BPs and mutagens (Hayatsu, 1995) are discussed. However, data on cellular compound absorption are lacking and so far only one recent report on enzymatic BRDT reduction in bacteria (Konickova et al., 2012) exists. Therefore, we explored whether bacterial BP absorption was more closely related to anti-mutagenesis compared to extracellular BP concentrations around S. typhimurium experiencing genotoxic stress.
In this study, physiologically relevant concentrations of BPs were tested. Un-/conjugated BR is found in the blood, the liver, the intestine (where about 70% are recycled via the enterohepatic cycle), and the urinary tract. In these compartments BR is further metabolised, recycled and/or excreted (Klatskin, 1961). The liver and gut, which are sites of BP accumulation, are at particular risk of genotoxicity due to the absorption, metabolism (Guengerich, 2000; Turesky et al., 2002) and excretion of mutagens. The abundance of BPs within these organs suggests BPs could exert physiological protection against DNA damage specifically at these sites.
Interestingly, BR and BV absorption strongly protected against frame-shift mutation in the TA98 strain. This mutation represents an important mechanism of pathogenesis in gastric and colorectal cancers (Kim et al., 2010). We speculate that the affinity of BPs to protect against frame-shift mutation, might partly explain the protective relationship between serum BR levels and colorectal cancer in vivo (Zucker et al., 2004). It should be emphasised, however, that BPs do protect against oxidative and frame-shift mutation when present extracellularly, indicating a clear role for BPs in neutralising mutagens before entering cells. Furthermore, it should be noted that BR causes apoptosis in cancer cells in vitro (Keshavan et al., 2004), providing an additional mechanism for chemoprevention. These data further emphasise the importance of therapeutically elevating BR concentrations for the prevention of cardiovascular disease and cancer (McCarty, 2007). Reports to indicate that BV and BRDT are readily absorbed across cultured enterocytes (Bulmer et al., 2008a) support this theory. These data confirm that potential anti-mutagenic BP effects in vivo could be induced by increasing concentrations in the gut lumen (Bulmer et al., 2011) where food-borne mutagens are found, or by increasing blood BP content in vivo to impart protection from DNA damage (Wallner et al., 2012). Although the results of these in vitro experiments cannot be directly extrapolated to in vivo settings, the results suggest BPs in the extracellular milieu (e.g., in the gut lumen/blood) could play a key role in cellular protection, by intercepting mutagens before they arrive at their site of action (e.g., DNA).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was funded by the Austrian Science Fund (FWF), Grant number P21162-B11.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tiv.2012.08.004.
Appendix A. Supplementary data
Supplementary Figure 1.
Chemical structures of test compounds.
Supplementary Figure 2.
Salmonella typhimurium TA102 colonies with (1) and without (2) 2 μmol/plate BV.
Supplementary Figure 3.
Relationship between bacterial BP absorption and observed anti-mutagenic effects against different mutagens; (A) BV in strain TA102 with t-BOOH, (B) BV in strain TA102 with AfB1 + S9, (C) BR in strain TA102 with t-BOOH.
References
- Asad S.F., Singh S., Ahmad A., Khan N.U., Hadi S.M. Prooxidant and antioxidant activities of bilirubin and its metabolic precursor biliverdin: a structure-activity study. Chem. Biol. Interact. 2001;137:59–74. doi: 10.1016/s0009-2797(01)00209-5. [DOI] [PubMed] [Google Scholar]
- Boon A.C., Hawkins C.L., Bisht K., Coombes J.S., Bakrania B., Wagner K.H., Bulmer A.C. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic. Biol. Med. 2012;52:2120–2127. doi: 10.1016/j.freeradbiomed.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brower J.O., Lightner D.A., McDonagh A.F. Aromatic congeners of bilirubin: synthesis, stereochemistry, glucuronidation and hepatic transport. Tetrahedron. 2001;57:7813–7827. [Google Scholar]
- Bulmer A.C., Blanchfield J.T., Coombes J.S., Toth I. In vitro permeability and metabolic stability of bile pigments and the effects of hydrophilic and lipophilic modification of biliverdin. Bioorg. Med. Chem. 2008;16:3616–3625. doi: 10.1016/j.bmc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Bulmer A.C., Blanchfield J.T., Toth I., Fassett R.G., Coombes J.S. Improved resistance to serum oxidation in Gilbert’s syndrome: a mechanism for cardiovascular protection. Atherosclerosis. 2008;199:390–396. doi: 10.1016/j.atherosclerosis.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Bulmer A.C., Coombes J.S., Blanchfield J.T., Toth I., Fassett R.G., Taylor S.M. Bile pigment pharmacokinetics and absorption in the rat: therapeutic potential for enteral administration. Br. J. Pharmacol. 2011;164:1857–1870. doi: 10.1111/j.1476-5381.2011.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer A.C., Ried K., Coombes J.S., Blanchfield J.T., Toth I., Wagner K.H. The anti-mutagenic and antioxidant effects of bile pigments in the Ames Salmonella test. Mutat. Res. 2007;629:122–132. doi: 10.1016/j.mrgentox.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Guengerich F.P. Metabolism of chemical carcinogens. Carcinogenesis. 2000;21:345–351. doi: 10.1093/carcin/21.3.345. [DOI] [PubMed] [Google Scholar]
- Hayatsu, H., 1995. Complex formation of heterocyclic amines with porphyrins: its use in detection and prevention, Princess Takamatsu Symposium, 1995/01/01 ed, pp. 172–180. [PubMed]
- Horsfall L.J., Rait G., Walters K., Swallow D.M., Pereira S.P., Nazareth I., Petersen I. Serum bilirubin and risk of respiratory disease and death. JAMA. 2011;305:691–697. doi: 10.1001/jama.2011.124. [DOI] [PubMed] [Google Scholar]
- Keshavan P., Schwemberger S.J., Smith D.L., Babcock G.F., Zucker S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer. 2004;112:433–445. doi: 10.1002/ijc.20418. [DOI] [PubMed] [Google Scholar]
- Kim Y.R., Chung N.G., Kang M.R., Yoo N.J., Lee S.H. Novel somatic frameshift mutations of genes related to cell cycle and DNA damage response in gastric and colorectal cancers with microsatellite instability. Tumori. 2010;96:1004–1009. [PubMed] [Google Scholar]
- Klatskin G. Bile pigment metabolism. Annu. Rev. Med. 1961;12:211–250. doi: 10.1146/annurev.me.12.020161.001235. [DOI] [PubMed] [Google Scholar]
- Konickova R., Jiraskova A., Zelenka J., Leseticky L., Sticha M., Vitek L. Reduction of bilirubin ditaurate by the intestinal bacterium Clostridium perfringens. Acta. Biochim. Pol. 2012;59:289–292. [PubMed] [Google Scholar]
- MacLean P.D., Chapman E.E., Dobrowolski S.L., Thompson A., Barclay L.R. Pyrroles as antioxidants: solvent effects and the nature of the attacking radical on antioxidant activities and mechanisms of pyrroles, dipyrrinones, and bile pigments. J. Org. Chem. 2008;73:6623–6635. doi: 10.1021/jo8005073. [DOI] [PubMed] [Google Scholar]
- Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- McCarty M.F. “’Iatrogenic Gilbert syndrome”–a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med. Hypotheses. 2007;69:974–994. doi: 10.1016/j.mehy.2006.12.069. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Turesky R.J., Guengerich F.P., Guillouzo A., Langouet S. Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P4501A2. Mutat. Res. 2002;506–507:187–195. doi: 10.1016/s0027-5107(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Wallner, M., Blassnigg, S.M., Marisch, K., Pappenheim, M.T., Müllner, E., Mölzer, C., Nersesyan, A., Marculescu, R., Doberer, D., Knasmüller, S., Bulmer, A.C., Wagner, K.-H., 2012. Effects of unconjugated bilirubin on chromosomal damage in individuals with Gilbert‘s syndrome measured with the micronucleus cytome assay. Mutagenesis, in press. http://dx.doi.org/10.1093/mutage/ges039. [DOI] [PubMed]
- Zucker S.D., Horn P.S., Sherman K.E. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827–835. doi: 10.1002/hep.20407. [DOI] [PubMed] [Google Scholar]