Abstract
The angiotensin type 2 (AT2) receptor of angiotensin II has long been thought to be limited to few tissues, with the primary effect of counteracting the angiotensin type 1 (AT1) receptor. Functional studies in neuronal cells have demonstrated AT2 receptor capability to modulate neuronal excitability, neurite elongation, and neuronal migration, suggesting that it may be an important regulator of brain functions. The observation that the AT2 receptor was expressed in brain areas implicated in learning and memory led to the hypothesis that it may also be implicated in cognitive functions. However, linking signaling pathways to physiological effects has always proven challenging since information relative to its physiological functions has mainly emerged from indirect observations, either from the blockade of the AT1 receptor or through the use of transgenic animals. From a mechanistic standpoint, the main intracellular pathways linked to AT2 receptor stimulation include modulation of phosphorylation by activation of kinases and phosphatases or the production of nitric oxide and cGMP, some of which are associated with the Gi-coupling protein. The receptor can also interact with other receptors, either G protein-coupled such as bradykinin, or growth factor receptors such as nerve growth factor or platelet-derived growth factor receptors. More recently, new advances have also led to identification of various partner proteins, thus providing new insights into this receptor’s mechanism of action. This review summarizes the recent advances regarding the signaling pathways induced by the AT2 receptor in neuronal cells, and discussed the potential therapeutic relevance of central actions of this enigmatic receptor. In particular, we highlight the possibility that selective AT2 receptor activation by non-peptide and selective agonists could represent new pharmacological tools that may help to improve impaired cognitive performance in Alzheimer’s disease and other neurological cognitive disorders.
Keywords: AT2 receptor, angiotensin, brain, differentiation, regeneration, neurodegenerative disorders, signaling, cognitive functions
INTRODUCTION
It is now well accepted that the effects of the various components of the renin-angiotensin system (RAS) range in various aspects of peripheral and brain functions well beyond those of regulating blood pressure and hydro-mineral balance. In particular, the existence of a complete RAS in the brain is fully acknowledged. Its activation leads to angiotensin II (Ang II) production, which is usually viewed as the end-product of this system (de Gasparo et al., 2000). Ang II binds two receptors from the G protein-coupled receptor family (GPCR), namely the angiotensin type 1 (AT1) and angiotensin type 2 (AT2) receptor. Although physiological functions of the AT1 receptor are relatively well-established, ranging from vasoconstriction and aldosterone release to cell growth, the effects associated with the AT2 receptor are surrounded by controversy. Both AT1 and AT2 receptors are expressed in various brain areas involved in the regulation of fluid and electrolyte balance and in the regulation of arterial pressure, as well as in structures involved in cognition, behavior, and locomotion (Phillips and de Oliveira, 2008; Horiuchi et al., 2010; Horiuchi and Mogi, 2011; Wright and Harding, 2011, 2012; Mogi and Horiuchi, 2012).
One of the biggest challenges in studying the AT2 receptor is to apply what has been observed using cell lines to in vivo models. Indeed, studies using cell lines expressing the AT2 receptor either endogenously or by transfection, have provided paramount information regarding its intracellular mechanisms of action, although associating these mechanisms with biological functions has proven to be much more difficult. Indeed, most of the relevant information regarding AT2 receptor functions in the brain has emerged from indirect observations, either by use of AT1 receptor blockers (ARB) or via transgenic “knock-down” animals for AT2 receptor expression. The present review summarizes recent advances in AT2 receptor signaling pathways, and discusses how they could be related to the neuroprotective functions of the receptor.
BRAIN EXPRESSION AND ROLE OF THE AT2 RECEPTOR
As summarized in several reviews (de Gasparo et al., 2000; Porrello et al., 2009; Gallo-Payet et al., 2011; Wright and Harding, 2011; Mogi and Horiuchi, 2012), the AT2 receptor is widely expressed during fetal life, which decreases rapidly after birth (Grady et al., 1991; Breault et al., 1996; Schutz et al., 1996; Nuyt et al., 1999), although a recent study has reported opposite results (Yu et al., 2010). This study is indeed in sharp contrast with previous reports using more specific methods, like autoradiography or in situ hybridization. In the adult, AT2 receptor expression is limited to a few tissues and cell types, such as vascular endothelial cells, adrenal gland, kidney, heart, myometrial cells, and ovaries (review in Porrello et al., 2009; Gallo-Payet et al., 2011, 2012; Verdonk et al., 2012). In the adult central nervous system (CNS), the AT2 receptor is observed in certain specific brain areas involved in the control and learning of motor activity, control of autonomous functions, sensory areas, and selected limbic system structures (Lenkei et al., 1996, 1997). In particular, it is the major Ang II receptor in the medulla oblongata (control of autonomous functions), septum and amygdala (associated with anxiety-like behavior), thalamus (sensory perception), superior colliculus (control of eye movements in response to visual information) as well as subthalamic nucleus and cerebellum (areas associated with learning of motor functions). On the other hand, certain areas involved in cardiovascular functions, learning, behavior, and stress reactions (cingulate cortex, molecular layer of the cerebellar cortex, superior colliculus, and paraventricular nuclei) contain both AT1 and AT2 receptors (Millan et al., 1991; Tsutsumi and Saavedra, 1991; Lenkei et al., 1996, 1997). More recently, expression of the AT2 receptor was also detected in the substantia nigra pars compacta, an area involved in dopaminergic signals and associated with Parkinson’s disease (Grammatopoulos et al., 2007), and in the hippocampus (Arganaraz et al., 2008; AbdAlla et al., 2009). At the cellular level, the AT2 receptor is expressed in neurons, but not in astrocytes (Bottari et al., 1992a; Lenkei et al., 1996; Gendron et al., 2003). Evidence also suggests that the AT2 receptor is expressed in the vasculature wall, where it acts on cerebral blood flow (review in Horiuchi and Mogi, 2011; Horiuchi et al., 2012). It should also be noted that existence of a non-AT1/non-AT2 receptor in the CNS has been suggested, which displays high affinity for Ang I, II, and III (Karamyan and Speth, 2007).
ROLE OF THE AT2 RECEPTOR IN NEURONAL EXCITABILITY
One of the first roles of the AT2 receptor to be identified was the modulation of neuronal excitability, which plays a crucial role not only in neuronal differentiation, but also in neuronal functions (review in Gendron et al., 2003; Gao et al., 2011). In particular, in cells of neuronal origin, activation of the AT2 receptor decreases activity of T-type calcium channels (Buisson et al., 1992, 1995). On the other hand, in rat brain neuronal culture, Kang et al. (1994) showed that the AT2 receptor stimulates a delayed rectifier K+ current (IK) and a transient K+ current (IA), an effect dependent on the G-protein Gi and the serine/threonine phosphatase PP2A. Consistent with these observations, a recent study showed that AT2 receptor induces a hyperpolarization and a decrease in firing rate in rostral ventrolateral medulla (RVLM) neurons suggesting that central activation of the AT2 receptor in this region decreases excitability (Matsuura et al., 2005). More recently, another study using C21/M024 demonstrated that selective stimulation of AT2 receptor in the neuronal cell line (called CATH.a neurons) increases the potassium current activity (IKv)(Kv) in a nitric oxide (NO)-dependant pathway (Gao et al., 2011). Moreover, intracerebroventricular infusion of C21/M024 was associated with a decrease in norepinephrine excretion and in blood pressure. Indeed, the modulation of the receptor on neuronal excitability in this region could be one of the mechanism associated with its effect on blood pressure, since RVLM is often considered as the main regulator of vascular tone (review in Dupont and Brouwers, 2010). An inhibitory effect of the AT2 receptor on neuronal excitability has also been observed in the locus coeruleus from brain slice preparations (Xiong and Marshall, 1994) and in the superior colliculus (Merabet et al., 1997). Finally, using the selective agonist C21/M024, Jing et al. (2012) recently demonstrated that direct stimulation of cerebral AT2 receptor increases postsynaptic potential, thus corroborating previous in vitro observations. Interestingly, AT2 receptor-induced neuronal activation of delayed rectifier potassium channels has also been demonstrated to have a neuroprotective effect (Grammatopoulos et al., 2004a). In fact, these AT2 receptor effects on ionic channel activity suggest that it may be implicated in synaptic plasticity, an important process involved in learning and memory.
ROLE OF THE AT2 RECEPTOR IN NEURONAL DIFFERENTIATION
One of the best recognized effects of AT2 receptor stimulation in neuronal cells is the induction of neurite outgrowth (review in Gallo-Payet et al., 2011). In the early 1990s, our group observed that stimulation of the AT2 receptor with its selective agonist CGP42112A induces neurite outgrowth in the neuronal NG108-15 cell line (Laflamme et al., 1996), results that were further confirmed using the recently developed non-peptide selective AT2 receptor agonist C21/M024 (Wan et al., 2004). This effect was associated with an increase in mature neural cell markers, such as βIII-tubulin, and microtubule-associated proteins (MAPs) such as MAP2c (Laflamme et al., 1996), both known to stabilize tubulin in a polymerized state, thus participating actively in differentiation (Sanchez et al., 2000). Similar results have also been reported in the pheochromocytoma-derived cell line PC12W, where Ang II was found to promoted neuronal differentiation characterized by an increase in neurite elongation (Meffert et al., 1996) and enhanced levels of polymerized βIII-tubulin and MAP2 associated with microtubules (Stroth et al., 1998). However, neurite outgrowth in PC12W cells has also been associated with a reduced expression of MAP1B (Stroth et al., 1998) and neurofilament M (Gallinat et al., 1997), two proteins specifically associated with axon elongation (Gordon-Weeks, 1991). These results were further confirmed in primary neuronal cultures, including retinal explants (Lucius et al., 1998), microexplant cultures of the cerebellum (Coté et al., 1999), in neurospheres from mouse fetal brain (Mogi et al., 2006) as well as primary cultures of newborn brain cortex neurons (Li et al., 2007) and hippocampal neurons (Jing et al., 2012). Some studies also showed that this neurite elongation was associated with an increase in the repair of damaged DNA by induction of methyl methanesulfonate sensitive-2 (MMS2), a neural-differentiating factor (Mogi et al., 2006; Jing et al., 2012). Altogether, these results suggest that activation of the AT2 receptor is associated with important rearrangements of the cytoskeleton necessary for induction of neurite elongation.
ROLE OF THE AT2 RECEPTOR IN NEURONAL MIGRATION
In cerebellar microexplants, where both neuronal and glial cells are present, AT2 receptor activation induces not only neurite outgrowth, but cell migration as well (Coté et al., 1999). Indeed, application of Ang II in this model induced cell migration of neurons from the center toward the periphery of the microexplant (Coté et al., 1999). These effects were more pronounced in cells treated with Ang II and DUP 753 (known as the ARB losartan) or in cells treated with 10 nM of CGP42112A an AT2 receptor agonist, and conversely blocked with the AT2 receptor antagonist PD123,319. Similar cell migration has also been observed during AT2 receptor-induced regeneration of post-natal retinal microexplants (Lucius et al., 1998). During migration and neurite outgrowth, cells are characterized by a myriad of advancing, retracting, turning, and branching behavioral patterns. Such dynamics and plasticity are driven by the reorganization of actin and the microtubular cytoskeleton. In particular, during the process of migration, actin filaments play a major role and are putatively considered as the primary target of guidance cues, due to their localization at the cell periphery, and in filopodium in the growth cone, where they are considered to be the driving force for the forward extension of the cell membrane (Gallo and Letourneau, 2004; Kalil and Dent, 2005). Our results on NG108-15 cells have shown that the underlying mechanism involves an Ang II-induced decrease in the amount of F-actin in filopodium and an increase in the pool of unpolymerized actin, through a pertussis toxin (PTX)-sensitive increase in ADF/cofilin activity. These latter effects were found to be AT2 receptor-dependent, since the increase in the rate of migration was abolished by the selective antagonist PD123,319, but not by the selective AT1 receptor antagonist losartan. Interestingly, some co-localization of F-actin with microtubules was also observed in control conditions, but which disappeared during Ang II-induced migration (Kilian et al., 2008). Among the candidate molecules that possibly cross-link actin filaments and microtubules are MAP2c and MAP1B (Dehmelt et al., 2003; Dehmelt and Halpain, 2004), proteins previously shown by our group to be affected during the process of AT2 receptor-stimulated neurite outgrowth, both in NG108-15 cells and in cerebellar granule cells (Laflamme et al., 1996; Coté et al., 1999).
MAIN SIGNALING PATHWAYS OF THE AT2 RECEPTOR
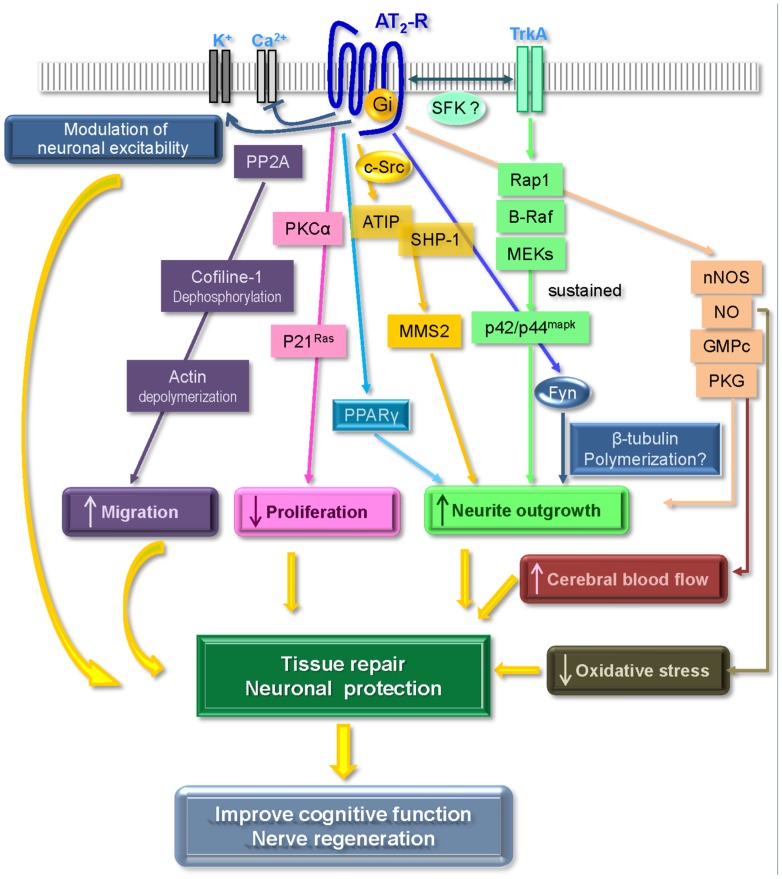
Although the AT2 receptor displays most of the classical features of a GPCR, it is usually considered as an atypical member of this family, since it fails to induce all of the classical signaling pathways such as cAMP, production of inositol triphosphate (IP3) or intracellular calcium release. Signaling pathways associated with the AT2 receptor mainly involve a balance between phosphatase and kinase activities and according to whether the cell is undifferentiated or differentiated and whether it expresses angiotensin AT1 receptors or not. Thus, there is still much controversy surrounding this receptor, and its effects, either protective or deleterious, remain a subject of debate (Widdop et al., 2003; Steckelings et al., 2005, 2010; Porrello et al., 2009; Horiuchi et al., 2012; Verdonk et al., 2012). In our endeavor to elucidate the mechanisms associated with AT2 receptor-induced neurite outgrowth, we and others have investigated signaling pathways activated by this receptor, including G-protein coupling, regulation of kinase activity, interaction with growth factor receptors, and production of NO. Moreover, recent observations have also delineated new partners for the AT2 receptor which play key functions in its regulation (Figure 1).
FIGURE 1.
Main signaling pathways associated with AT2 receptor activation leading to neuroprotective effects (see text for details). Adapted from Gallo-Payet et al. (2011).
G-PROTEIN COUPLING
While coupling of G-protein to AT1 receptors is well described (de Gasparo et al., 2000; Hunyady and Catt, 2006), such coupling is not the rule for the AT2 receptor. Former studies have described a coupling to subunit Gαi2 and Gαi3 in rat fetus (Zhang and Pratt, 1996). In some models (rat hippocampal neurons and other selected cell types), blocking Gαi with PTX or antibodies directed against Gαi inhibited the AT2 receptor effects on actin depolymerization, activation of endothelial NO synthase (NOS), stimulation of neuronal K+ current and on anti-proliferative activity (Kang et al., 1994; Ozawa et al., 1996; Li et al., 2004; Olson et al., 2004; Kilian et al., 2008), indicating that coupling of the AT2 receptor to Gαi is at least implicated in these pathways. However, aside from a few exceptions (Kang et al., 1994), PTX failed to inhibit either p42/p44mapk activation in the neuronal cell line NG108-15 (Gendron et al., 2002) or phosphatase activity in several models (for review see Nouet and Nahmias, 2000; Gendron et al., 2003).
REGULATION OF KINASE ACTIVITY
AT2 Receptor-induced phosphatase activation
Phosphatase activation has been one of the first signals associated with AT2 receptor activation. After the earlier studies in PC12W cells (Bottari et al., 1992b; Brechler et al., 1994), results have been confirmed in other cell lines, including N1E-115 cells (Nahmias et al., 1995), NG108-15 cells (Buisson et al., 1995), and R3T3 fibroblasts (Tsuzuki et al., 1996a,b). This phosphatase activation by the AT2 receptor is essential for its anti-proliferative and pro-apoptotic effects (for reviews, see Nouet and Nahmias, 2000; Steckelings et al., 2005; Porrello et al., 2009; Verdonk et al., 2012). Currently, three main phosphatases have been implicated in AT2 receptor signaling, namely SH2-domain-containing phosphatase 1 (SHP-1), mitogen-activated protein kinase phosphatase 1 (MKP-1), and the serine–threonine phosphatase PP2A.
SHP-1 is a cytosolic phosphatase rapidly activated by the AT2 receptor following Ang II binding. Activation of SHP-1 is associated with AT2-induced growth inhibition in various cells, including neuronal cells (Bedecs et al., 1997; Elbaz et al., 2000; Feng et al., 2002; Li et al., 2007), vascular smooth muscle cells (Cui et al., 2001; Matsubara et al., 2001), CHO, and COS-7 cells transfected with the AT2 receptor (Elbaz et al., 2000; Feng et al., 2002). Activation of SHP-1 is associated with inhibitory effects of the AT2 receptor on the AT1 receptor, including transactivation of the epidermal growth factor (EGF) receptor and activation of c-Jun N-terminal kinase (JNK) (Matsubara et al., 2001; Shibasaki et al., 2001), but also on insulin-induced activation of the phosphatidylinositol 3-kinase (PI3K), its association with the insulin receptor substrate IRS-2 and phosphorylation of Akt (Cui et al., 2001). This inhibition of insulin signaling by AT2 receptor-induced SHP-1 activation has also been associated with an increase in PC12W cell apoptosis (Cui et al., 2002). More recently, Li et al. (2007) have shown that induction of neurite outgrowth in fetal rat neurons by AT2 receptor involves the association of SHP-1 with the newly identified AT2-receptor interacting protein (ATIP; see section AT2 Receptor Interacting Proteins) and an increase in MMS2 protein (Li et al., 2007). Finally, although the mechanisms associated with AT2 receptor-induced activation of SHP-1 have yet to be fully elucidated, implication of G-protein coupling (Bedecs et al., 1997; Feng et al., 2002) as well as activation of Src kinase (Alvarez et al., 2008) have been reported; other studies have also implicated a constitutive association between AT2 receptor and SHP-1 in overexpressing models (Feng et al., 2002; Miura et al., 2005). Another phosphatase associated with AT2 receptor activation is MKP-1, which is a key regulator of p42/p44mapk activity. AT2 receptor-activated MKP-1 has been observed in various cell types, including PC12W cells (Yamada et al., 1996), fibroblasts (Horiuchi et al., 1997; Calo et al., 2010), and cardiac myocytes (Fischer et al., 1998; Hiroi et al., 2001). Activation of MKP-1 by AT2 leads to a decrease in p42/p44mapk activity, and is associated to growth inhibition induced by the AT2 receptor. Moreover, Horiuchi et al. (1997) demonstrated that AT2 receptor-induced MKP-1 activation is implicated in apoptotic effects of the AT2 receptor, leading to Bcl-2 dephosphorylation and an increase in Bax, resulting in cell death. Finally, the serine–threonine phosphatase PP2A is also activated by the AT2 receptor following Ang II binding and may be associated with AT2 receptor regulation of p42/p44mapk. Indeed, in primary neuronal cultures, AT2 receptor-induced activation of PP2A is associated with inhibition of AT1 receptor-induced p42/p44mapk phosphorylation (Huang et al., 1995, 1996a,b) and is implicated in AT2-induced modulation of potassium currents (Huang et al., 1995, 1996a; Caballero et al., 2004). More recently, we have also shown an implication of PP2A activation in actin depolymerization and an increase in neuronal migration (Kilian et al., 2008; Figure 1).
Mitogen-activated protein kinase p42/p44
Among all signaling pathways associated with AT2 receptor activation, regulation of p42/p44mapk is probably the one where variability is the most important. The effect of AT2 receptor stimulation on activation or inhibition of p42/p44mapk activity is dependent on the models studied, on whether they express AT1 receptors or not and whether cells are under physiological or pathological conditions. Thus, AT2 receptor effects on p42/p44mapk remain controversial. Many studies have shown that the AT2 receptor leads to dephosphorylation of p42/p44mapk via one the phosphatases associated with AT2 receptor signaling (see above). This decrease in p42/p44mapk activity is associated with inhibition of growth and pro-apoptotic effects of the AT2 receptor (review in Nouet et al., 2004; Porrello et al., 2009). In addition to activation of phosphatase, AT2 receptor-induced inhibition of p42/p44mapk can be mediated by inhibition of growth factor receptors. Indeed, in vascular smooth muscle cells overexpressing the AT2 receptor, stimulation with Ang II decreases EGF receptor phosphorylation and inhibits p42/p44mapk activation (Shibasaki et al., 2001). Similar observations have also been reported in CHO cells overexpressing the AT2 receptor (Elbaz et al., 2000). Worthy of note is the fact that inhibition of p42/p44mapk induced by the AT2 receptor is observed only in certain conditions, such as in cells overexpressing the AT2 receptor or already exhibiting pathological conditions such as serum-starving (Bedecs et al., 1997; Horiuchi et al., 1997; Elbaz et al., 2000; Cui et al., 2001; Shibasaki et al., 2001).
By contrast, in neuronal cells such as NG108-15 and PC12W cells, the AT2 receptor leads to sustained activation of p42/p44mapk. In these cells, activation of p42/p44mapk is essential to AT2 receptor-induced neurite elongation (Gendron et al., 1999; Stroth et al., 2000). In NG108-15 cells, we observed that this increase in p42/p44mapk activity was associated with the Rap1/B-Raf pathway. However, this Rap1 activation appears to be dependent of nerve growth factor receptor TrkA activation (see latter; Plouffe et al., 2006) rather than through cAMP and protein kinase A (PKA), as usually observed with other GPCR (Figure 1). This activation of p42/p44mapk by the AT2 receptor has also been observed in non-neuronal COS-7 and NIH3T3 cells overexpressing the AT2 receptor (Hansen et al., 2000; De Paolis et al., 2002).
Src family kinase
There are few studies showing an implication of Src family members in AT2 receptor signaling. However, Src family kinases (SFKs) are key regulators in cell growth and differentiation and are implicated in most growth factor signaling pathways. In the CNS, five members of SFK are expressed, namely Src, Fyn, Lyn, Lck, and Yes, where they act as modulators of neurotransmitter receptors as well as in the regulation of excitatory transmission (review in Kalia et al., 2004; Theus et al., 2006; Ohnishi et al., 2011). Recently, we have shown that stimulation of the AT2 receptor in NG108-15 cells leads to rapid but transient activation of SFK and that expression of inactive Fyn abolished AT2 receptor-induced neurite outgrowth in these cells (Guimond et al., 2010). However, inhibition of Fyn had no effect on other signaling pathways induced by the AT2 receptor, including p42/p44mapk and Rap1 activation, suggesting that it may be involved either downstream of these proteins, or in a parallel pathway. Of note, among the five SFKs expressed in the brain, only a deficiency in Fyn-induced neurological deficits, including impairment in spatial learning and in hippocampal development (Grant et al., 1992; Kojima et al., 1997). Interestingly, similar physiological perturbations were also observed in mice lacking the AT2 receptor (Hein et al., 1995; Ichiki et al., 1995; Okuyama et al., 1999; Maul et al., 2008). Therefore, regulation of Fyn activity could be considered as a new player implicated in the protective effect of this receptor in cognitive disorders. Indeed, Fyn has been shown to be involved in tau phosphorylation, thus regulating its affinity for tubulin and stability of microtubules, two parameters implicated in the development of Alzheimer’s disease (AD) and other neurodegenerative diseases (Lee et al., 1998, 2004). Thus, it appears that Fyn is involved in the final steps of induction of elongation, but not in the initial events of AT2 receptor activation. This implication of Fyn in AT2 receptor signaling is further strengthened by the fact that activation of SFKs, as the AT2 receptor, was shown to be important for the induction of long-term potentiation, a key element in learning and memory, in CA1 pyramidal neurons of hippocampal slices (Yu et al., 1997).
To the best of our knowledge, only one other group has demonstrated the implication of a Src family member in AT2 receptor signaling (Alvarez et al., 2008). In this latter study, it was shown that activation of c-Src was present in an immunocomplex including the tyrosine phosphatase SHP-1 and the AT2 receptor following Ang II stimulation in rat fetal membranes. Pre-incubation of membranes with the non-selective inhibitor PP2 inhibited SHP-1 activation and c-Src association. These results indicate that c-Src may represent an important step leading to AT2 receptor-induced SHP-1 activation. More recently, the same group demonstrated that this association also occurred in hindbrain membranes from post-natal day 15 rats, and was associated with focal adhesion kinase (p85FAK) (Seguin et al., 2012). These observations strongly suggest that c-Src may also be implicated in cytoskeleton remodeling associated with neurite elongation and neuronal migration induced by the AT2 receptor.
LINKING THE AT2 RECEPTOR WITH THE GROWTH FACTOR RECEPTORS
Recently, we demonstrated that activation of Rap1/B-Raf/p42/p44mapk pathway by the AT2 receptor was dependent on the nerve growth factor receptor TrkA, although the mechanism involved remains unknown (Plouffe et al., 2006). In addition, we further showed that a SFK member was essential for the initial activation of TrkA by the AT2 receptor, since pre-incubation of NG108-15 cells with the non-selective inhibitor PP1 disrupted this effect (Guimond et al., 2010). However, although Fyn was essential for neurite outgrowth induced by the AT2 receptor, it did not appear to be implicated in TrkA activation, since expression of a dominant negative form did not impede AT2-induced TrkA activation (Guimond et al., 2010). In light of recent data obtained by Ciuffo’s group regarding the involvement of c-Src and other SFK members with AT2 receptors (Alvarez et al., 2008; Seguin et al., 2012), it would be of interest to see whether the association of the AT2 receptor with SHP-1 and c-Src is implicated in this transactivation, and whether TrkA could be involved in FAK activation. Interestingly, transactivation of the TrkA receptor in neurons has also been observed for the pituitary adenylyl cyclase-activating polypeptide receptor (PACAP; Rajagopal et al., 2004), which is also associated with neuronal development in the cerebellum (Basille et al., 2006).
Curiously, although the expression of inactive Fyn is known to disrupt AT2 receptor-induced neurite elongation, non-selective inhibition of SFK in NG108-15 cells with the inhibitor PP1 is sufficient to increase neurite elongation to levels similar to those observed with AT2 receptor stimulation (Guimond et al., 2010), which could be a consequence of a decrease in proliferative signal. Indeed, our group showed that induction of neurite outgrowth was associated with a decrease in cell proliferation through inhibition of PKCα and p21Ras (Gendron et al., 1999; Beaudry et al., 2006). Moreover, as in the case of SFK, inhibition of the platelet-derived growth factor (PDGF) receptor was sufficient to induce neurite outgrowth and to increase microtubule polymerization more extensively than Ang II alone (Plouffe et al., 2006). These findings are in agreement with a previous report demonstrating that expression of an inactive form of the PDGF receptor in PC12 cells was sufficient to increase neurite elongation (Vetter and Bishop, 1995). However, whether AT2 receptor directly inhibits PDGF receptor or inhibits its signaling pathway is still unknown.
NITRIC OXIDE AND CGMP PRODUCTION – A ROLE FOR BRADYKININ
Nitric oxide has been shown to regulate several types of K+ channels, including ATP-dependent K+ channels and Ca2+-activated K+ channels (review in Prast and Philippu, 2001). Indeed, in neuronal cell lines, observations with the selective AT2 receptor agonist C21/M024 revealed that this production of NO induced by AT2 was necessary for AT2-induced hyperpolarization of potassium channel function (Gao and Zucker, 2011). Production of NO following AT2 receptor stimulation has been observed in various cell types, such as neuronal cells (Chaki and Inagami, 1993; Coté et al., 1998; Gendron et al., 2002; Zhao et al., 2003; Muller et al., 2010), vascular endothelial cells (Wiemer et al., 1993; Seyedi et al., 1995; Saito et al., 1996; Thorup et al., 1998; Baranov and Armstead, 2005) as well as in smooth muscle cells (de Godoy et al., 2004). It is already well accepted that AT2 receptor activation plays an important role in the control of renal function particularly in chronic kidney diseases. The AT2 receptor is believed to counterbalance the effects of the AT1 receptor at least by influencing vasodilation through NO production and natriuresis (Carey and Padia, 2008; Siragy, 2010; Siragy and Carey, 2010). This promoter effect of AT2 on natriuresis in pathological conditions (obese Zucker rats) was also recently confirmed using C21/M024 (Ali and Hussain, 2012). Activation of NOS by the AT2 receptor can occur by direct signaling such as in neuronal cells, or indirectly via stimulation of bradykinin production and subsequent activation of its receptor B2. Indeed, heterodimerization between the AT2 receptor and bradykinin has also been described in PC12W cells (Abadir et al., 2006). Moreover, it is already known that bradykinin can modulate AT2 receptor-induced NO production (Siragy and Carey, 1996; Gohlke et al., 1998; Searles and Harrison, 1999). Such involvement of B2 receptors in AT2 receptor-induced production of NO is of prime importance in the modulation of cerebral blood flow. Indeed, an AT2-induced increase in spatial learning was recently observed to be associated with an increase in cerebral blood flow, an effect reduced by co-administration of the B2 receptor antagonist icatibant. This observation strongly suggests that the beneficial effect of the AT2 receptor in cognitive function is partly dependent on bradykinin (Jing et al., 2012). In addition, Abadir et al. (2003) demonstrated in conscious bradykinin B2-null and wild-type mice that the AT2 receptor can induce production of NO in both null and wild-type models, indicating that the B2 receptor may participate in this process, although is not the only means for the AT2 receptor to induce NO production.
AT2 RECEPTOR ASSOCIATED PROTEINS
ATIP
Recently, using a yeast two-hybrid system, the ATIP was cloned and identified as a protein interacting with the C-terminal tail of the AT2 receptor (Nouet et al., 2004). This protein is expressed as five different transcripts, namely ATIP1, ATIP2, ATIP3a, ATIP3b, and ATIP4 (review in Rodrigues-Ferreira and Nahmias, 2010; Horiuchi et al., 2012). While ATIP3 appears to be the major transcript in tissues, ATIP1 and ATIP4 are mainly expressed in the brain, indicating that they may play biological roles in brain functions. ATIP2, on the other hand, is almost undetectable by real-time PCR (Di Benedetto et al., 2006). In CHO cells expressing the AT2 receptor, ATIP is known to decrease growth factor-induced p42/p44mapk activation and DNA synthesis, therefore decreasing cell proliferation, as well as decrease insulin receptor autophosphorylation, similarly to the AT2 receptor. Of particular interest is the fact that, although expression of the AT2 receptor was essential in this instance, stimulation by Ang II was not necessary, and that ATIP was able to exert its effect by its sole expression. Implication of ATIP in AT2 receptor-induced neurite outgrowth has also been reported. In this context, Ang II stimulation of the AT2 receptor induces translocation of ATIP with SHP-1 into the nucleus, resulting in the transactivation of MMS2 (Li et al., 2007). Moreover, ATIP, also known as ATBP50 (AT2 receptor binding protein of 50 kDa), has been reported as a membrane-associated Golgi protein implicated in intracellular localization of the AT2 receptor and necessary for its membrane expression (Wruck et al., 2005). ATIP3, which is also expressed in the CNS, has been shown to strongly interact with stabilized microtubules in a model of breast cancer, suggesting an implication on cell division, where it induces a delayed metaphase, thus decreasing tumor progression (Rodrigues-Ferreira et al., 2009). The brain-specific isoform ATIP4 is highly expressed in the cerebellum and fetal brain, two sites where the AT2 receptor is also highly expressed. Therefore considering (i) the previously described function of the AT2 receptor in preservation of cognitive function, (ii) the role of ATIP protein in AT2 receptor function, and (iii) the link between ATIP protein and microtubule cytoskeleton, it could be suggested that regulation of ATIP expression and regulation of its association with the AT2 receptor could be an important element to consider with regard to the development of neurological disorders, such as AD.
PLZF
Association between the AT2 receptor and the promyelocytic leukemia zinc finger (PLZF) protein has been observed using a yeast two-hybrid system (Senbonmatsu et al., 2003). In CHO cells expressing both PLZF and AT2 receptors, Ang II stimulation induces co-localization of PLZF with the AT2 receptor, followed by internalization of the complex. This observation is in contrast with other studies observing no internalization of the AT2 receptor following Ang II stimulation (Hunyady et al., 1994; Hein et al., 1997). Since internalization of the receptor was observed only in cells expressing PLZF, this could represent a new regulatory pathway of AT2 receptor function, specific only to selected cell types. However, beside internalization of AT2 receptor, a recent study showed that PLZF was implicated in neuroprotection in a stroke model (Seidel et al., 2011). In this study, the authors showed that PLZF exerts neuroprotective effect in a model of in vitro glutamate toxicity. They also showed that overexpression of PLZF in neuronal cells in culture induced a significant increase in AT2 receptor expression, suggesting that PLZF could also be implicated in the regulation of AT2 receptor expression.
PPARγ
A new partner for the AT2 receptor has recently emerged from the study of Zhao et al. (2005) who observed that neurite outgrowth induced by AT2 receptor stimulation in PC12W cells was dependent on the activation of peroxisome proliferator-activated receptor gamma (PPARγ). This observation is in keeping with the implication of PPARγ in NGF-induced neurite outgrowth in the same cell type (Fuenzalida et al., 2005), clearly suggesting a possible crosstalk between the AT2 receptor and NGF pathways. This hypothesis is further reinforced by the observation that inhibition of the NGF receptor TrkA significantly decreases AT2 receptor-induced neurite outgrowth (Plouffe et al., 2006). Moreover, Iwai et al. (2009), using atherosclerotic ApoE-KO mice with an AT2 receptor deficiency (AT2R/ApoE double knockout mice), observed that the lack of AT2 receptor expression decreased the expression of PPARγ in adipocytes cells. These observations strongly suggest a link between the AT2 receptor and PPARγ functions. PPARγ is a transcriptional factor regulating the expression of multiple genes, hence promoting the differentiation and development of various tissues, specifically in adipose tissue, brain, placenta, and skin. Interestingly, neuroprotective effects of PPARγ agonist have also been observed (review in Gillespie et al., 2011). However, a major component of the hypothesis regarding the possible implication of PPARγ in AT2 receptor function is the PPARγ-like activity associated with certain ARBs, including telmisartan, irbesartan, and candesartan (Benson et al., 2004; Schupp et al., 2004; review in Horiuchi et al., 2012). Indeed, there is some evidence suggesting that this PPARγ activation following blockade of the AT1 receptor could be part of its anti-inflammatory and anti-oxidative effects, leading to neuroprotection against ischemia and amyloid β (Aβ) accumulation (Tsukuda et al., 2009; Iwanami et al., 2010; Washida et al., 2010). PPARγ has also been implicated in neural cell differentiation and death, as well as inflammatory and neurodegenerative conditions (review in Gillespie et al., 2011).
LESSONS FROM NEURONAL DIFFERENTIATION: HOW CAN THE AT2 RECEPTOR IMPROVE BRAIN FUNCTION?
ROLE OF THE AT2 RECEPTOR IN NEURONAL REGENERATION
The capacity for nerve regeneration in lower vertebrates has been mostly lost in higher vertebrates and regeneration within the CNS in mammals is essentially inexistent. However, after injury in the peripheral nervous system, regeneration can be achieved successfully. Observations that AT2 receptor stimulation induces neurite elongation associated with modulation of MAP expression strongly suggested that this effect could also be observed following nerve injury. In 1998, two studies demonstrated that the AT2 receptor improved nerve recovery in both optic (Lucius et al., 1998) and sciatic (Gallinat et al., 1998) nerve following nerve crush or in perivascular nerves implicated in vasodilation (Hobara et al., 2007). This effect was accompanied by an increase in AT2 receptor expression, the activation of NFκB and induction of growth-associated protein (GAP-43) leading to a reduction in lesion size. Moreover, Reinecke et al. (2003) demonstrated that activation of NFκB by the AT2 receptor was an essential step to recovery following sciatic nerve crush. This implication of AT2 receptor in neuronal regeneration has even led to the suggestion that Ang II, via the AT2 receptor, could act as a neurotrophic factor.
AT2 RECEPTOR IN COGNITIVE FUNCTION
There is increasing evidence suggesting that the AT2 receptor could be associated with improvement of cognitive function following cerebral ischemia-induced neuronal injury (Iwai et al., 2004; Li et al., 2005; Mogi et al., 2006; McCarthy et al., 2009). Indeed, it has been shown that central administration of CGP42112A increases neuronal survival and minimizes experimental post-stroke injury (McCarthy et al., 2009), indicating that activation of brain AT2 receptors exhibits a neuroprotective effect. More recently, stimulation of the AT2 receptor with the selective agonist C21/M024 was observed to prevent cognitive decline in an AD mouse model with intracerebroventricular injection of Aβ(1-40) (Jing et al., 2012). Indeed, some of the signaling pathways described above may be linked to improvement in impaired signaling functions as observed in AD. One of the major hallmarks of AD is Aβ deposition in senile plaques and the presence of neurofibrillary tangles (NFTs). Formation of NFTs is a consequence of protein tau accumulation, due to its hyperphosphorylation, and the dissociation of microtubules. Thus, regulation of tau phosphorylation is of paramount importance with regard to AD progression. On the other hand, several studies have reported that the AT2 receptor activates PP2A phosphatase (Huang et al., 1995, 1996a; Kilian et al., 2008), which is markedly deficient in AD (Gong et al., 1993, 2000; Wang et al., 2007) and implicated in glycogen synthase kinase-3 (GSK-3) inactivation via a sustained increase in p42/p44mapk. Since tau is a substrate for PP2A phosphatase, GSK-3 and Fyn, the latter of which is also implicated in the AT2 receptor effect on neurite outgrowth (Guimond et al., 2010), AT2 receptor activation could participate in controlling the equilibrium between tau phosphorylation and dephosphorylation (Hernandez and Avila, 2008; Hanger et al., 2009; Hernandez et al., 2009). In addition to acting on tau regulation, the AT2 receptor may also improve neurite architecture, through effects on MAPs, as observed in neuronal cell lines (Laflamme et al., 1996; Meffert et al., 1996; Coté et al., 1999; Li et al., 2007). The observation that central AT2 receptor activation using its selective agonist C21/M024 decreases cognitive loss induced by Aβ intracerebroventricular injection lends further support to this hypothesis (Jing et al., 2012). Although the mechanisms underlying these neuroprotective effects of the AT2 receptor remain to be fully elucidated, they may include PPARγ and the protein MMS2 (Mogi et al., 2006, 2008; for recent reviews see Gallo-Payet et al., 2011, 2012).
Moreover, as indicated earlier, another important feature of AT2 receptor signaling is induction of NO and cGMP production. Recently, Jing et al. (2012) observed that direct stimulation of central AT2 receptors increases NO via a bradykinin-dependent pathway, an effect which leads to an increase in cerebral blood flow and enhanced spatial memory. A further study also showed that administration of C21/M024 reduced early renal inflammatory response with production of NO and cGMP (Matavelli et al., 2011). This increase in NO-cGMP production has also been shown to lead to a decrease in nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) superoxide production (Volpe et al., 2003; Widdop et al., 2003; de la Torre, 2004; Steckelings et al., 2005; Iadecola et al., 2009), thus reducing oxidative stress and potentially associated neuronal apoptosis. This hypothesis is coherent with the observation that the AT2 receptor attenuates chemical hypoxia-induced caspase-3 activation in primary cortical neuronal cultures (Grammatopoulos et al., 2004b). Finally, inflammation is also a common feature of neurodegenerative diseases. In this regard, a recent study conducted in primary cultures of human and murine dermal fibroblasts, has shown that C21/M024 has anti-inflammatory effects, inhibiting tumor necrosis factor (TNF)-α-induced interleukin-6 levels and NFκB activity. This effect was notably initiated through increased activation of protein phosphatases and increased synthesis of epoxyeicosatrienoic acid (Rompe et al., 2010).
CONCLUSION
Since its identification in the early 90s, the AT2 receptor has been and still is shrouded by controversy, its low expression in the adult and its atypical signaling pathways adding to the challenge of studying this receptor. Thanks to the major advances achieved in the past few years, several studies have confirmed that stimulation of the AT2 receptor activates multiple signaling pathways which are linked to beneficial effects on neuronal functions (including excitability, differentiation, and regeneration), inflammation, oxidative stress, and cerebral blood flow (Figure 1). Several neurodegenerative diseases (including cognitive deficits and dementia) are closely associated with these neuronal and synaptic dysfunctions (Iadecola, 2004; Zlokovic, 2005; LaFerla et al., 2007; Boissonneault et al., 2009; Mucke, 2009; Nelson et al., 2009). Moreover, an increasing number of studies suggest that the protective effects of ARBs on brain damage and cognition may result not only from the inhibition of AT1 receptor effects, but also from the beneficial effect due to unopposed activation of the AT2 receptor. Thus, if further research confirms the promising early results obtained with the recently developed selective non-peptide AT2 receptor agonist C21/M024, the latter may represent a new pharmacological tool in the fight against neurological cognitive disorders. In addition, unraveling the underlying effects of the AT2 receptor on neuronal plasticity may lead to the development of even more potent and selective therapies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Pierre Pothier for critical reading of the manuscript and editorial assistance (Les Services PM-SYS Enr., Sherbrooke). This work presented in this review was supported by grants from the Canadian Institutes of Health Research (MOP-82819 to Nicole Gallo-Payet) and from the Alzheimer’s Society of Canada to Nicole Gallo-Payet with Louis Gendron (Université de Sherbrooke) and Thomas Stroh (McGill University) and by the Canada Research Chair program to Nicole Gallo-Payet. Nicole Gallo-Payet is a past holder of the Canada Research Chair in Endocrinology of the Adrenal Gland. Marie-Odile Guimond is a postdoctoral fellowship in the laboratory of Nicole Gallo-Payet. Nicole Gallo-Payet and Marie-Odile Guimond are both members of the FRSQ-funded Centre de recherche clinique étienne-Le Bel.
REFERENCES
- Abadir P. M., Carey R. M., Siragy H. M. (2003). Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension 42 600–604 [DOI] [PubMed] [Google Scholar]
- Abadir P. M., Periasamy A., Carey R. M., Siragy H. M. (2006). Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension 48 316–322 [DOI] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., el Missiry A., Langer A., Sergeev P., el Faramawy Y., et al. (2009). Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J. Biol. Chem. 284 6554–6565 [DOI] [PubMed] [Google Scholar]
- Ali Q., Hussain T. (2012). AT(2) receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens. Res. 35 654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez S. E., Seguin L. R., Villarreal R. S., Nahmias C., Ciuffo G. M. (2008). Involvement of c-Src tyrosine kinase in SHP-1 phosphatase activation by Ang II AT2 receptors in rat fetal tissues. J. Cell. Biochem. 105 703–711 [DOI] [PubMed] [Google Scholar]
- Arganaraz G. A., Konno A. C., Perosa S. R., Santiago J. F., Boim M. A., Vidotti D. B., et al. (2008). The renin-angiotensin system is upregulated in the cortex and hippocampus of patients with temporal lobe epilepsy related to mesial temporal sclerosis. Epilepsia 49 1348–1357 [DOI] [PubMed] [Google Scholar]
- Baranov D., Armstead W. M. (2005). Nitric oxide contributes to AT2 but not AT1 angiotensin II receptor-mediated vasodilatation of porcine pial arteries and arterioles. Eur. J. Pharmacol. 525 112–116 [DOI] [PubMed] [Google Scholar]
- Basille M., Cartier D., Vaudry D., Lihrmann I., Fournier A., Freger P., et al. (2006). Localization and characterization of pituitary adenylate cyclase-activating polypeptide receptors in the human cerebellum during development. J. Comp. Neurol. 496 468–478 [DOI] [PubMed] [Google Scholar]
- Beaudry H., Gendron L., Guimond M. O., Payet M. D., Gallo-Payet N. (2006). Involvement of protein kinase C alpha (PKC alpha) in the early action of angiotensin II type 2 (AT2) effects on neurite outgrowth in NG108-15 cells: AT2-receptor inhibits PKC alpha and p21ras activity. Endocrinology 147 4263–4272 [DOI] [PubMed] [Google Scholar]
- Bedecs K., Elbaz N., Sutren M., Masson M., Susini C., Strosberg A. D., et al. (1997). Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem. J. 325 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. C., Pershadsingh H. A., Ho C. I., Chittiboyina A., Desai P., Pravenec M., et al. (2004). Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 43 993–1002 [DOI] [PubMed] [Google Scholar]
- Boissonneault V., Filali M., Lessard M., Relton J., Wong G., Rivest S. (2009). Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain 132 1078–1092 [DOI] [PubMed] [Google Scholar]
- Bottari S. P., King I. N., Reichlin S., Dahlstroem I., Lydon N, de Gasparo M. (1992a). The angiotensin AT2 receptor stimulates protein tyrosine phosphatase activity and mediates inhibition of particulate guanylate cyclase. Biochem. Biophys. Res. Commun. 183 206–211 [DOI] [PubMed] [Google Scholar]
- Bottari S. P., Obermuller N., Bogdal Y., Zahs K. R., Deschepper C. F. (1992b). Characterization and distribution of angiotensin II binding sites in fetal and neonatal astrocytes from different rat brain regions. Brain Res. 585 372–376 [DOI] [PubMed] [Google Scholar]
- Breault L., Lehoux J. G., Gallo-Payet N. (1996). The angiotensin AT2 receptor is present in the human fetal adrenal gland throughout the second trimester of gestation. J. Clin. Endocrinol. Metab. 81 3914–3922 [DOI] [PubMed] [Google Scholar]
- Brechler V., Reichlin S., De Gasparo M., Bottari S. P. (1994). Angiotensin II stimulates protein tyrosine phosphatase activity through a G-protein independent mechanism. Recept. Channels 2 89–98 [PubMed] [Google Scholar]
- Buisson B., Bottari S. P., de Gasparo M., Gallo-Payet N., Payet M. D. (1992). The angiotensin AT2 receptor modulates T-type calcium current in non-differentiated NG108-15 cells. FEBS Lett. 309 161–164 [DOI] [PubMed] [Google Scholar]
- Buisson B., Laflamme L., Bottari S. P., de Gasparo M., Gallo-Payet N., Payet M. D. (1995). A G protein is involved in the angiotensin AT2 receptor inhibition of the T-type calcium current in non-differentiated NG108-15 cells. J. Biol. Chem. 270 1670–1674 [DOI] [PubMed] [Google Scholar]
- Caballero R., Gomez R., Moreno I., Nunez L., Gonzalez T., Arias C., et al. (2004). Interaction of angiotensin II with the angiotensin type 2 receptor inhibits the cardiac transient outward potassium current. Cardiovasc. Res. 62 86–95 [DOI] [PubMed] [Google Scholar]
- Calo L. A., Schiavo S., Davis P. A., Pagnin E., Mormino P., D’Angelo A., et al. (2010). Angiotensin II signaling via type 2 receptors in a human model of vascular hyporeactivity: implications for hypertension. J. Hypertens. 28 111–118 [DOI] [PubMed] [Google Scholar]
- Carey R. M., Padia S. H. (2008). Angiotensin AT2 receptors: control of renal sodium excretion and blood pressure. Trends Endocrinol. Metab. 19 84–87 [DOI] [PubMed] [Google Scholar]
- Chaki S., Inagami T. (1993). New signaling mechanism of angiotensin II in neuroblastoma neuro-2A cells: activation of soluble guanylyl cyclase via nitric oxide synthesis. Mol. Pharmacol. 43 603–608 [PubMed] [Google Scholar]
- Coté F., Do T. H., Laflamme L., Gallo J. M., Gallo-Payet N. (1999). Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J. Biol. Chem. 274 31686–31692 [DOI] [PubMed] [Google Scholar]
- Coté F., Laflamme L., Payet M. D., Gallo-Payet N. (1998). Nitric oxide, a new second messenger involved in the action of angiotensin II on neuronal differentiation of NG108-15 cells. Endocr. Res. 24 403–407 [DOI] [PubMed] [Google Scholar]
- Cui T., Nakagami H., Iwai M., Takeda Y., Shiuchi T., Daviet L., et al. (2001). Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc. Res. 49 863–871 [DOI] [PubMed] [Google Scholar]
- Cui T. X., Nakagami H., Nahmias C., Shiuchi T., Takeda-Matsubara Y., Li J. M., et al. (2002). Angiotensin II subtype 2 receptor activation inhibits insulin-induced phosphoinositide 3-kinase and Akt and induces apoptosis in PC12W cells. Mol. Endocrinol. 16 2113–2123 [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. (2000). International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 52 415–472 [PubMed] [Google Scholar]
- de Godoy M. A., de Oliveira A. M., Rattan S. (2004). Angiotensin II-induced relaxation of anococcygeus smooth muscle via desensitization of AT1 receptor, and activation of AT2 receptor associated with nitric-oxide synthase pathway. J. Pharmacol. Exp. Ther. 311 394–401 [DOI] [PubMed] [Google Scholar]
- de la Torre J. C. (2004). Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 3 184–190 [DOI] [PubMed] [Google Scholar]
- De Paolis P., Porcellini A., Savoia C., Lombardi A., Gigante B., Frati G., et al. (2002). Functional cross-talk between angiotensin II and epidermal growth factor receptors in NIH3T3 fibroblasts. J. Hypertens. 20 693–699 [DOI] [PubMed] [Google Scholar]
- Dehmelt L., Halpain S. (2004). Actin and microtubules in neurite initiation: are MAPs the missing link? J. Neurobiol. 58 18–33 [DOI] [PubMed] [Google Scholar]
- Dehmelt L., Smart F. M., Ozer R. S., Halpain S. (2003). The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J. Neurosci. 23 9479–94790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto M., Bieche I., Deshayes F., Vacher S., Nouet S., Collura V., et al. (2006). Structural organization and expression of human MTUS1, a candidate 8p22 tumor suppressor gene encoding a family of angiotensin II AT2 receptor-interacting proteins, ATIP. Gene 380 127–136 [DOI] [PubMed] [Google Scholar]
- Dupont A. G., Brouwers S. (2010). Brain angiotensin peptides regulate sympathetic tone and blood pressure. J. Hypertens. 28 1599–1610 [DOI] [PubMed] [Google Scholar]
- Elbaz N., Bedecs K., Masson M., Sutren M., Strosberg A. D., Nahmias C. (2000). Functional trans-inactivation of insulin receptor kinase by growth-inhibitory angiotensin II AT2 receptor. Mol. Endocrinol. 14 795–804 [DOI] [PubMed] [Google Scholar]
- Feng Y. H., Sun Y., Douglas J. G. (2002). Gbeta gamma -independent constitutive association of Galpha s with SHP-1 and angiotensin II receptor AT2 is essential in AT2-mediated ITIM-independent activation of SHP-1. Proc. Natl. Acad. Sci. U.S.A. 99 12049–12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. A., Singh K., O’Hara D. S., Kaye D. M., Kelly R. A. (1998). Role of AT1 and AT2 receptors in regulation of MAPKs and MKP-1 by ANG II in adult cardiac myocytes. Am. J. Physiol. 275 H906–H916 [DOI] [PubMed] [Google Scholar]
- Fuenzalida K. M., Aguilera M. C., Piderit D. G., Ramos P. C., Contador D., Quinones V., et al. (2005). Peroxisome proliferator-activated receptor gamma is a novel target of the nerve growth factor signaling pathway in PC12 cells. J. Biol. Chem. 280 9604–9609 [DOI] [PubMed] [Google Scholar]
- Gallinat S., Csikos T., Meffert S., Herdegen T., Stoll M., Unger T. (1997). The angiotensin AT2 receptor down-regulates neurofilament M in PC12W cells. Neurosci. Lett. 227 29–32 [DOI] [PubMed] [Google Scholar]
- Gallinat S., Yu M., Dorst A., Unger T., Herdegen T. (1998). Sciatic nerve transection evokes lasting up-regulation of angiotensin AT2 and AT1 receptor mRNA in adult rat dorsal root ganglia and sciatic nerves. Brain Res. Mol. Brain Res. 57 111–122 [DOI] [PubMed] [Google Scholar]
- Gallo G., Letourneau P. C. (2004). Regulation of growth cone actin filaments by guidance cues. J. Neurobiol. 58 92–102 [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N., Guimond M.-O., Bilodeau L., Wallinder C., Alterman M., Hallberg A. (2011). Angiotensin II, a neuropeptide at the frontier between endocrinology and neuroscience: is there a link between the angiotensin II type 2 receptor (AT2R) and Alzheimer’s disease? Front. Endocrinol. 2:17. 10.3389/fendo.2011.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo-Payet N., Shum M., Baillargeon J.-P., Langlois M.-F., Alterman M., Hallberg A., et al. (2012). AT2 receptor agonists: exploiting the beneficial arm of Ang II signaling. Curr. Hypertens. Rev. 8 47–59 [Google Scholar]
- Gao J., Zhang H., Le K. D., Chao J., Gao L. (2011). Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am. J. Hypertens. 24 724–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Zucker I. H. (2011). AT2 receptor signaling and sympathetic regulation. Curr. Opin. Pharmacol. 11 124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L., Coté F., Payet M. D., Gallo-Payet N. (2002). Nitric oxide and cyclic GMP are involved in angiotensin II AT(2) receptor effects on neurite outgrowth in NG108-15 cells. Neuroendocrinology 75 70–81 [DOI] [PubMed] [Google Scholar]
- Gendron L., Laflamme L., Rivard N., Asselin C., Payet M. D., Gallo-Payet N. (1999). Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol. Endocrinol. 13 1615–1626 [DOI] [PubMed] [Google Scholar]
- Gendron L., Payet M. D., Gallo-Payet N. (2003). The angiotensin type 2 receptor of angiotensin II and neuronal differentiation: from observations to mechanisms. J. Mol. Endocrinol. 31 359–372 [DOI] [PubMed] [Google Scholar]
- Gillespie W., Tyagi N., Tyagi S. C. (2011). Role of PPARgamma, a nuclear hormone receptor in neuroprotection. Indian J. Biochem. Biophys. 48 73–81 [PubMed] [Google Scholar]
- Gohlke P., Pees C., Unger T. (1998). AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension 31 349–355 [DOI] [PubMed] [Google Scholar]
- Gong C. X., Lidsky T., Wegiel J., Zuck L., Grundke-Iqbal I., Iqbal K. (2000). Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J. Biol. Chem. 275 5535–5544 [DOI] [PubMed] [Google Scholar]
- Gong C. X., Singh T. J., Grundke-Iqbal I., Iqbal K. (1993). Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 61 921–927 [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. R. (1991). Control of microtubule assembly in growth cones. J. Cell Sci. Suppl. 15 45–49 [DOI] [PubMed] [Google Scholar]
- Grady E. F., Sechi L. A., Griffin C. A., Schambelan M., Kalinyak J. E. (1991). Expression of AT2 receptors in the developing rat fetus. J. Clin. Invest. 88 921–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos T. N., Johnson V., Moore S. A., Andres R., Weyhenmeyer J. A. (2004a). Angiotensin type 2 receptor neuroprotection against chemical hypoxia is dependent on the delayed rectifier K+ channel, Na+/Ca2+ exchanger and Na+/K+ ATPase in primary cortical cultures. Neurosci. Res. 50 299–306 [DOI] [PubMed] [Google Scholar]
- Grammatopoulos T. N., Morris K., Bachar C., Moore S., Andres R., Weyhenmeyer J. A. (2004b). Angiotensin II attenuates chemical hypoxia-induced caspase-3 activation in primary cortical neuronal cultures. Brain Res. Bull. 62 297–303 [DOI] [PubMed] [Google Scholar]
- Grammatopoulos T. N., Jones S. M., Ahmadi F. A., Hoover B. R., Snell L. D., Skoch J., et al. (2007). Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol. Neurodegener. 2 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. G., O’Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. (1992). Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258 1903–1910 [DOI] [PubMed] [Google Scholar]
- Guimond M. O., Roberge C., Gallo-Payet N. (2010). Fyn is involved in angiotensin II type 2 receptor-induced neurite outgrowth, but not in p42/p44mapk in NG108-15 cells. Mol. Cell. Neurosci. 45 201–212 [DOI] [PubMed] [Google Scholar]
- Hanger D. P., Anderton B. H., Noble W. (2009). Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15 112–119 [DOI] [PubMed] [Google Scholar]
- Hansen J., Servant G., Baranski T., Fujita T., Iiri T., Sheikh S. (2000). Functional reconstitution of the angiotensin II type 2 receptor and G(i) activation. Circ. Res. 87 753–759 [DOI] [PubMed] [Google Scholar]
- Hein L., Barsh G. S., Pratt R. E., Dzau V. J., Kobilka B. K. (1995). Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 377 744–747 [DOI] [PubMed] [Google Scholar]
- Hein L., Meinel L., Pratt R. E., Dzau V. J., Kobilka B. K. (1997). Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol. Endocrinol. 11 1266–1277 [DOI] [PubMed] [Google Scholar]
- Hernandez F., Avila J. (2008). The role of glycogen synthase kinase 3 in the early stages of Alzheimers’ disease. FEBS Lett. 582 3848–3854 [DOI] [PubMed] [Google Scholar]
- Hernandez F., Gomez de Barreda E., Fuster-Matanzo A., Lucas J. J., Avila J. (2009). GSK3: a possible link between beta amyloid peptide and tau protein. Exp. Neurol. 223 322–325 [DOI] [PubMed] [Google Scholar]
- Hiroi Y., Hiroi J., Kudoh S., Yazaki Y., Nagai R., Komuro I. (2001). Two distinct mechanisms of angiotensin II-induced negative regulation of the mitogen-activated protein kinases in cultured cardiac myocytes. Hypertens. Res. 24 385–394 [DOI] [PubMed] [Google Scholar]
- Hobara N., Goda M., Yoshida N., Takatori S., Kitamura Y., Mio M., Kawasaki H. (2007). Angiotensin II type 2 receptors facilitate reinnervation of phenol-lesioned vascular calcitonin gene-related peptide-containing nerves in rat mesenteric arteries. Neuroscience 150 730–741 [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Hayashida W., Kambe T., Yamada T., Dzau V. J. (1997). Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J. Biol. Chem. 272 19022–19026 [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Iwanami J., Mogi M. (2012). Regulation of angiotensin II receptors beyond the classical pathway. Clin. Sci. (Lond) 123 193–203 [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Mogi M. (2011). Role of angiotensin II receptor subtype activation in cognitive function and ischaemic brain damage. Br. J. Pharmacol. 163 1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M., Mogi M., Iwai M. (2010). The angiotensin II type 2 receptor in the brain. J. Renin Angiotensin Aldosterone Syst. 11 1–6 [DOI] [PubMed] [Google Scholar]
- Huang X. C., Richards E. M., Sumners C. (1995). Angiotensin II type 2 receptor-mediated stimulation of protein phosphatase 2A in rat hypothalamic/brainstem neuronal cocultures. J. Neurochem. 65 2131–2137 [DOI] [PubMed] [Google Scholar]
- Huang X. C., Richards E. M., Sumners C. (1996a). Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J. Biol. Chem. 271 15635–15641 [DOI] [PubMed] [Google Scholar]
- Huang X. C., Sumners C., Richards E. M. (1996b). Angiotensin II stimulates protein phosphatase 2A activity in cultured neuronal cells via type 2 receptors in a pertussis toxin sensitive fashion. Adv. Exp. Med. Biol. 396 209–215 [DOI] [PubMed] [Google Scholar]
- Hunyady L., Bor M., Balla T., Catt K. J. (1994). Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J. Biol. Chem. 269 31378–37382 [PubMed] [Google Scholar]
- Hunyady L., Catt K. J. (2006). Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 20 953–970 [DOI] [PubMed] [Google Scholar]
- Iadecola C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5 347–360 [DOI] [PubMed] [Google Scholar]
- Iadecola C., Park L., Capone C. (2009). Threats to the mind: aging, amyloid, and hypertension. Stroke 40 S40–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki T., Labosky P. A., Shiota C., Okuyama S., Imagawa Y., Fogo A., et al. (1995). Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 377 748–750 [DOI] [PubMed] [Google Scholar]
- Iwai M., Liu H. W., Chen R., Ide A., Okamoto S., Hata R., et al. (2004). Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 110 843–848 [DOI] [PubMed] [Google Scholar]
- Iwai M., Tomono Y., Inaba S., Kanno H., Senba I., Mogi M., Horiuchi M. (2009). AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am. J. Hypertens. 22 784–791 [DOI] [PubMed] [Google Scholar]
- Iwanami J., Mogi M., Tsukuda K., Min L. J., Sakata A., Jing F., et al. (2010). Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 28 1730–1737 [DOI] [PubMed] [Google Scholar]
- Jing F., Mogi M., Sakata A., Iwanami J., Tsukuda K., Ohshima K., et al. (2012). Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J. Cereb. Blood Flow Metab. 32 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia L. V., Gingrich J. R., Salter M. W. (2004). Src in synaptic transmission and plasticity. Oncogene 23 8007–8016 [DOI] [PubMed] [Google Scholar]
- Kalil K., Dent E. W. (2005). Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr. Opin. Neurobiol. 15 521–526 [DOI] [PubMed] [Google Scholar]
- Kang J., Posner P., Sumners C. (1994). Angiotensin II type 2 receptor stimulation of neuronal K+ currents involves an inhibitory GTP binding protein. Am. J. Physiol. 267 C1389–C1397 [DOI] [PubMed] [Google Scholar]
- Karamyan V. T., Speth R. C. (2007). Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res. 1143 83–91 [DOI] [PubMed] [Google Scholar]
- Kilian P., Campbell S., Bilodeau L., Guimond M. O., Roberge C., Gallo-Payet N., et al. (2008). Angiotensin II type 2 receptor stimulation increases the rate of NG108-15 cell migration via actin depolymerization. Endocrinology 149 2923–2933 [DOI] [PubMed] [Google Scholar]
- Kojima N., Wang J., Mansuy I. M., Grant S. G., Mayford M., Kandel E. R. (1997). Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc. Natl. Acad. Sci. U.S.A. 94 4761–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla F. M., Green K. N., Oddo S. (2007). Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 8 499–509 [DOI] [PubMed] [Google Scholar]
- Laflamme L., Gasparo M., Gallo J. M., Payet M. D., Gallo-Payet N. (1996). Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. Effect counteracted by the AT1 receptors. J. Biol. Chem. 271 22729–22735 [DOI] [PubMed] [Google Scholar]
- Lee G., Newman S. T., Gard D. L., Band H., Panchamoorthy G. (1998). Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 111(Pt. 21) 3167–3177 [DOI] [PubMed] [Google Scholar]
- Lee G., Thangavel R., Sharma V. M., Litersky J. M., Bhaskar K., Fang S. M., et al. (2004). Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J. Neurosci. 24 2304–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkei Z., Palkovits M., Corvol P., Llorens-Cortes C. (1996). Distribution of angiotensin II type-2 receptor (AT2) mRNA expression in the adult rat brain. J Comp. Neurol. 373 322–339 [DOI] [PubMed] [Google Scholar]
- Lenkei Z., Palkovits M., Corvol P., Llorens-Cortes C. (1997). Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front. Neuroendocrinol. 18:383–439 10.1006/frne.1997.0155 [DOI] [PubMed] [Google Scholar]
- Li J., Culman J., Hortnagl H., Zhao Y., Gerova N., Timm M., et al. (2005). Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 19 617–619 [DOI] [PubMed] [Google Scholar]
- Li J. M., Mogi M., Tsukuda K., Tomochika H., Iwanami J., Min L. J., et al. (2007). Angiotensin II-induced neural differentiation via angiotensin II type 2 (AT2) receptor-MMS2 cascade involving interaction between AT2 receptor-interacting protein and Src homology 2 domain-containing protein-tyrosine phosphatase 1. Mol. Endocrinol. 21 499–511 [DOI] [PubMed] [Google Scholar]
- Li X., Lerea K. M., Li J., Olson S. C. (2004). Src kinase mediates angiotensin II-dependent increase in pulmonary endothelial nitric oxide synthase. Am. J. Respir. Cell Mol. Biol. 31 365–372 [DOI] [PubMed] [Google Scholar]
- Lucius R., Gallinat S., Rosenstiel P., Herdegen T., Sievers J., Unger T. (1998). The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J. Exp. Med. 188 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matavelli L. C., Huang J., Siragy H. M. (2011). Angiotensin AT receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 57 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H., Shibasaki Y., Okigaki M., Mori Y., Masaki H., Kosaki A., et al. (2001). Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c-Jun NH2-terminal kinase via SHP-1 tyrosine phosphatase activity: evidence from vascular-targeted transgenic mice of AT2 receptor. Biochem. Biophys. Res. Commun. 282 1085–1091 [DOI] [PubMed] [Google Scholar]
- Matsuura T., Kumagai H., Onimaru H., Kawai A., Iigaya K., Onami T., et al. (2005). Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension 46 349–354 [DOI] [PubMed] [Google Scholar]
- Maul B., von Bohlen und Halbach O., Becker A., Sterner-Kock A., Voigt J. P., Siems W. E., et al. (2008). Impaired spatial memory and altered dendritic spine morphology in angiotensin II type 2 receptor-deficient mice. J. Mol. Med. 86 563–571 [DOI] [PubMed] [Google Scholar]
- McCarthy C. A., Vinh A., Callaway J. K., Widdop R. E. (2009). Angiotensin AT2 Receptor Stimulation Causes Neuroprotection in a Conscious Rat Model of Stroke. Stroke 40 1482–1489 [DOI] [PubMed] [Google Scholar]
- Meffert S., Stoll M., Steckelings U. M., Bottari S. P., Unger T. (1996). The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol. Cell. Endocrinol. 122 59–67 [DOI] [PubMed] [Google Scholar]
- Merabet L., de Gasparo M., Casanova C. (1997). Dose-dependent inhibitory effects of angiotensin II on visual responses of the rat superior colliculus: AT1 and AT2 receptor contributions. Neuropeptides 31 469–481 [DOI] [PubMed] [Google Scholar]
- Millan M. A., Jacobowitz D. M., Aguilera G., Catt K. J. (1991). Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc. Natl. Acad. Sci.U.S.A. 88 11440–11444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Karnik S. S., Saku K. (2005). Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signaling independent of receptor conformation and ligand stimulation. J. Biol. Chem. 280 18237–18244 [DOI] [PubMed] [Google Scholar]
- Mogi M., Horiuchi M. (2012). Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr. Gerontol. Int. 10.1111/j.1447 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Mogi M., Li J. M., Iwanami J., Min L. J., Tsukuda K., Iwai M., et al. (2006). Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension 48 141–148 [DOI] [PubMed] [Google Scholar]
- Mogi M., Li J. M., Tsukuda K., Iwanami J., Min L. J., Sakata A., et al. (2008). Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem. Biophys. Res. Commun. 375 446–449 [DOI] [PubMed] [Google Scholar]
- Mucke L. (2009). Neuroscience: Alzheimer’s disease. Nature 461 895–897 [DOI] [PubMed] [Google Scholar]
- Muller D., Greenland K. J., Speth R. C., Middendorff R. (2010). Neuronal differentiation of NG108-15 cells has impact on nitric oxide- and membrane (natriuretic peptide receptor-A) cyclic GMP-generating proteins. Mol. Cell. Endocrinol. 320 118–127 [DOI] [PubMed] [Google Scholar]
- Nahmias C., Cazaubon S. M., Briend-Sutren M. M., Lazard D., Villageois P., Strosberg A. D. (1995). Angiotensin II AT2 receptors are functionally coupled to protein tyrosine dephosphorylation in N1E-115 neuroblastoma cells. Biochem. J. 306(Pt. 1) 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. T., Braak H., Markesbery W. R. (2009). Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol. 68 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouet S., Amzallag N., Li J. M., Louis S., Seitz I., Cui T. X., et al. (2004). Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J. Biol. Chem. 279 28989–28997 [DOI] [PubMed] [Google Scholar]
- Nouet S., Nahmias C. (2000). Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol. Metab. 11 1–6 [DOI] [PubMed] [Google Scholar]
- Nuyt A. M., Lenkei Z., Palkovits M., Corvol P., Llorens-Cortes C. (1999). Ontogeny of angiotensin II type 2 receptor mRNA expression in fetal and neonatal rat brain. J. Comp. Neurol. 407 193–206 [PubMed] [Google Scholar]
- Ohnishi H., Murata Y., Okazawa H., Matozaki T. (2011). Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 34 629–637 [DOI] [PubMed] [Google Scholar]
- Okuyama S., Sakagawa T., Chaki S., Imagawa Y., Ichiki T., Inagami T. (1999). Anxiety-like behavior in mice lacking the angiotensin II type-2 receptor. Brain Res. 821 150–159 [DOI] [PubMed] [Google Scholar]
- Olson S., Oeckler R., Li X., Du L., Traganos F., Zhao X., et al. (2004). Angiotensin II stimulates nitric oxide production in pulmonary artery endothelium via the type 2 receptor. Am. J. Physiol. Lung Cell. Mol. Physiol. 287 L559–L568 [DOI] [PubMed] [Google Scholar]
- Ozawa Y., Suzuki Y., Murakami K., Miyazaki H. (1996). The angiotensin II type 2 receptor primarily inhibits cell growth via pertussis toxin-sensitive G proteins. Biochem. Biophys. Res. Commun. 228 328–333 [DOI] [PubMed] [Google Scholar]
- Phillips M. I, de Oliveira E. M. (2008). Brain renin angiotensin in disease. J. Mol. Med. 86 715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe B., Guimond M. O., Beaudry H., Gallo-Payet N. (2006). Role of tyrosine kinase receptors in angiotensin II AT2 receptor signaling: involvement in neurite outgrowth and in p42/p44mapk activation in NG108-15 cells. Endocrinology 147 4646–4654 [DOI] [PubMed] [Google Scholar]
- Porrello E. R., Delbridge L. M., Thomas W. G. (2009). The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front. Biosci. 14:958–972 10.2741/3289 [DOI] [PubMed] [Google Scholar]
- Prast H., Philippu A. (2001). Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 64 51–68 [DOI] [PubMed] [Google Scholar]
- Rajagopal R., Chen Z. Y., Lee F. S., Chao M. V. (2004). Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J. Neurosci. 24 6650–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke K., Lucius R., Reinecke A., Rickert U., Herdegen T., Unger T. (2003). Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. FASEB J. 17 2094–2096 [DOI] [PubMed] [Google Scholar]
- Rodrigues-Ferreira S., Di Tommaso A., Dimitrov A., Cazaubon S., Gruel N., Colasson H., et al. (2009). 8p22 MTUS1 gene product ATIP3 is a novel anti-mitotic protein underexpressed in invasive breast carcinoma of poor prognosis. PLoS ONE 4:e7239 10.1371/journal.pone.0007239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Ferreira S., Nahmias C. (2010). An ATIPical family of angiotensin II AT2 receptor-interacting proteins. Trends Endocrinol. Metab. 21 684–690 [DOI] [PubMed] [Google Scholar]
- Rompe F., Artuc M., Hallberg A., Alterman M., Stroder K., Thone-Reineke C., et al. (2010). Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55 924–931 [DOI] [PubMed] [Google Scholar]
- Saito S., Hirata Y., Emori T., Imai T., Marumo F. (1996). Angiotensin II activates endothelial constitutive nitric oxide synthase via AT1 receptors. Hypertens. Res. 19 201–206 [DOI] [PubMed] [Google Scholar]
- Sanchez C., Diaz-Nido J., Avila J. (2000). Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog. Neurobiol. 61 133–168 [DOI] [PubMed] [Google Scholar]
- Schupp M., Janke J., Clasen R., Unger T., Kintscher U. (2004). Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation 109 2054–2057 [DOI] [PubMed] [Google Scholar]
- Schutz S., Le Moullec J. M., Corvol P., Gasc J. M. (1996). Early expression of all the components of the renin-angiotensin-system in human development. Am. J. Pathol. 149 2067–2079 [PMC free article] [PubMed] [Google Scholar]
- Searles C. D., Harrison D. G. (1999). The interaction of nitric oxide, bradykinin, and the angiotensin II type 2 receptor: lessons learned from transgenic mice. J. Clin. Invest. 104 1013–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L. R., Villarreal R. S., Ciuffo G. M. (2012). AT2 receptors recruit c-Src, SHP-1 and FAK upon activation by Ang II in PND15 rat hindbrain. Neurochem. Int. 60 199–207 [DOI] [PubMed] [Google Scholar]
- Seidel K., Kirsch S., Lucht K., Zaade D., Reinemund J., Schmitz J., et al. (2011). The promyelocytic leukemia zinc finger (PLZF) protein exerts neuroprotective effects in neuronal cells and is dysregulated in experimental stroke. Brain Pathol. 21 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbonmatsu T., Saito T., Landon E. J., Watanabe O., Price E., Jr., Roberts R. L., et al. (2003). A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J. 22 6471–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedi N., Xu X., Nasjletti A., Hintze T. H. (1995). Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension 26 164–170 [DOI] [PubMed] [Google Scholar]
- Shibasaki Y., Matsubara H., Nozawa Y., Mori Y., Masaki H., Kosaki A., Tsutsumi Y., et al. (2001). Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension 38 367–372 [DOI] [PubMed] [Google Scholar]
- Siragy H. M. (2010). The angiotensin II type 2 receptor and the kidney. J. Renin Angiotensin Aldosterone Syst. 11 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragy H. M., Carey R. M. (1996). The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3’, 5’-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J. Clin. Invest. 97 1978–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragy H. M., Carey R. M. (2010). Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 31 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelings U. M., Kaschina E., Unger T. (2005). The AT2 receptor-a matter of love and hate. Peptides 26 1401–1409 [DOI] [PubMed] [Google Scholar]
- Steckelings U. M., Rompe F., Kaschina E., Namsolleck P., Grzesiak A., Funke-Kaiser H., et al. (2010). The past, present and future of angiotensin II type 2 receptor stimulation. J. Renin. Angiotensin. Aldosterone. Syst. 11 67–73 [DOI] [PubMed] [Google Scholar]
- Stroth U., Blume A., Mielke K., Unger T. (2000). Angiotensin AT(2) receptor stimulates ERK1 and ERK2 in quiescent but inhibits ERK in NGF-stimulated PC12W cells. Brain Res. Mol. Brain Res. 78 175–180 [DOI] [PubMed] [Google Scholar]
- Stroth U., Meffert S., Gallinat S., Unger T. (1998). Angiotensin II and NGF differentially influence microtubule proteins in PC12W cells: role of the AT2 receptor. Brain Res. Mol. Brain Res. 53 187–195 [DOI] [PubMed] [Google Scholar]
- Theus M., Wei L., Francis K, Ping Yu S. (2006). Critical roles of Src family tyrosine kinases in excitatory neuronal differentiation of cultured embryonic stem cells. Exp. Cell Res. 312 3096–3107 [DOI] [PubMed] [Google Scholar]
- Thorup C., Kornfeld M., Winaver J. M., Goligorsky M. S., Moore L. C. (1998). Angiotensin-II stimulates nitric oxide release in isolated perfused renal resistance arteries. Pflugers Arch. 435 432–434 [DOI] [PubMed] [Google Scholar]
- Tsukuda K., Mogi M., Iwanami J., Min L. J., Sakata A., Jing F., et al. (2009). Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension 54 782–787 [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Saavedra J. M. (1991). Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 261 R209–R216 [DOI] [PubMed] [Google Scholar]
- Tsuzuki S., Eguchi S., Inagami T. (1996a). Inhibition of cell proliferation and activation of protein tyrosine phosphatase mediated by angiotensin II type 2 (AT2) receptor in R3T3 cells. Biochem. Biophys. Res. Commun. 228 825–830 [DOI] [PubMed] [Google Scholar]
- Tsuzuki S., Matoba T., Eguchi S., Inagami T. (1996b). Angiotensin II type 2 receptor inhibits cell proliferation and activates tyrosine phosphatase. Hypertension 28 916–918 [DOI] [PubMed] [Google Scholar]
- Verdonk K., Danser A. H, van Esch J. H. (2012). Angiotensin II type 2 receptor agonists: where should they be applied? Expert Opin. Investig. Drugs 21 501–513 [DOI] [PubMed] [Google Scholar]
- Vetter M. L., Bishop J. M. (1995). Beta PDGF receptor mutants defective for mitogenesis promote neurite outgrowth in PC12 cells. Curr. Biol. 5 168–178 [DOI] [PubMed] [Google Scholar]
- Volpe M., Musumeci B., De Paolis P., Savoia C., Morganti A. (2003). Angiotensin II AT2 receptor subtype: an uprising frontier in cardiovascular disease? J. Hypertens. 21 1429–1443 [DOI] [PubMed] [Google Scholar]
- Wan Y., Wallinder C., Plouffe B., Beaudry H., Mahalingam A. K., Wu X., et al. (2004). Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 47 5995–6008 [DOI] [PubMed] [Google Scholar]
- Wang J. Z., Grundke-Iqbal I., Iqbal K. (2007). Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washida K., Ihara M., Nishio K., Fujita Y., Maki T., Yamada M., et al. (2010). Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator-activated receptor-gamma activation in mice with chronic cerebral hypoperfusion. Stroke 41 1798–1806 [DOI] [PubMed] [Google Scholar]
- Widdop R. E., Jones E. S., Hannan R. E., Gaspari T. A. (2003). Angiotensin AT2 receptors: cardiovascular hope or hype? Br. J. Pharmacol. 140 809–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer G., Scholkens B. A., Wagner A., Heitsch H., Linz W. (1993). The possible role of angiotensin II subtype AT2 receptors in endothelial cells and isolated ischemic rat hearts. J. Hypertens. Suppl. 11 S234–S235 [PubMed] [Google Scholar]
- Wright J. W., Harding J. W. (2011). Brain renin-angiotensin–a new look at an old system. Prog. Neurobiol. 95 49–67 [DOI] [PubMed] [Google Scholar]
- Wright J. W., Harding J. W. (2012). The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch. 10.1234/12345678 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wruck C. J., Funke-Kaiser H., Pufe T., Kusserow H., Menk M., Schefe J. H., et al. (2005). Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler. Thromb. Vasc. Biol. 25 57–64 [DOI] [PubMed] [Google Scholar]
- Xiong H., Marshall K. C. (1994). Angiotensin II depresses glutamate depolarizations and excitatory postsynaptic potentials in locus coeruleus through angiotensin II subtype 2 receptors. Neuroscience 62 163–175 [DOI] [PubMed] [Google Scholar]
- Yamada T., Horiuchi M., Dzau V. J. (1996). Angiotensin II type 2 receptor mediates programmed cell death. Proc. Natl. Acad. Sci. U.S.A. 93 156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Zheng M., Wang W., Rozanski G. J., Zucker I. H., Gao L. (2010). Developmental changes in AT1 and AT2 receptor-protein expression in rats. J. Renin Angiotensin Aldosterone Syst. 11 214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M., Askalan R., Keil G. J., II, Salter M. W. (1997). NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275 674–678 [DOI] [PubMed] [Google Scholar]
- Zhang J., Pratt R. E. (1996). The AT2 receptor selectively associates with Gialpha2 and Gialpha3 in the rat fetus. J. Biol. Chem. 271 15026–15033 [PubMed] [Google Scholar]
- Zhao Y., Biermann T., Luther C., Unger T., Culman J., Gohlke P. (2003). Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells. J. Neurochem. 85 759–767 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Foryst-Ludwig A., Bruemmer D., Culman J., Bader M., Unger T., et al. (2005). Angiotensin II induces peroxisome proliferator-activated receptor gamma in PC12W cells via angiotensin type 2 receptor activation. J. Neurochem. 94 1395–1401 [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. (2005). Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 28 202–208 [DOI] [PubMed] [Google Scholar]