Abstract
Purpose
The use of radiotherapy in pediatric low-grade glioma (LGG) is controversial, especially for young patients. We conducted a phase II trial of conformal radiation therapy (CRT) to estimate disease control by using a 10-mm clinical target volume (CTV) margin.
Materials and Methods
Between August 1997 and August 2006, 78 pediatric patients with LGG and a median age of 8.9 years (range, 2.2 to 19.8 years) received 54 Gy CRT by using a 10-mm CTV and by targeting with systematic magnetic resonance imaging (MRI) registration. Tumor locations were diencephalon (n = 58), cerebral hemisphere (n = 3), and cerebellum (n = 17). Sixty-seven patients had documented or presumed WHO grade 1 tumors, 25 patients had prior chemotherapy, and 13 patients had neurofibromatosis type 1.
Results
During a median follow-up of 89 months, 13 patients experienced disease progression. One patient experienced marginal treatment failure, eight experienced local failures, and four experienced metastatic failure. The mean and standard error 5- and 10-year event-free (87.4% ± 4.4% and 74.3% ± 15.4%, respectively) and overall (98.5% ± 1.6% and 95.9% ± 5.8%, respectively) survival rates were determined. The mean and standard error cumulative incidences of local failure at 5 and 10 years were 8.7% ± 3.5% and 16.4% ± 5.4%, respectively. The mean and standard error cumulative incidence of vasculopathy was 4.79% ± 2.73% at 6 years, and it was higher for those younger than 5 years of age (P = .0105) at the time of CRT.
Conclusion
This large, prospective series of irradiated children with LGG demonstrates that CRT with a 10-mm CTV does not compromise disease control. The results suggest that CRT should be delayed in young patients to reduce the risk of vasculopathy.
INTRODUCTION
Pediatric low-grade glioma (LGG) represents a spectrum of conditions for which radiation therapy (RT) is indicated at the time of diagnosis or of progression after prior therapy. The recommended age varies by institution, and the current threshold for primary RT in multi-institutional protocols is set at 8 years of age for European trials1 and at 10 years of age for North American studies.2 Fear of radiation-related adverse effects3–5 has been used to justify this threshold despite the paucity of data,6 questions about the relative effectiveness of RT,7 and questions about the appropriate dose and target volume.8–10 The unclear role of RT in malignant transformation,11,12 the modifying effect of neurofibromatosis type 1 (NF-1),13–15 and the observance that early RT does not confer a survival benefit16 have contributed to chemotherapy trials or observation for selected patients.
A number of chemotherapy regimens have been used to delay the need for RT, including those regimens that deploy carboplatin and vincristine17; procarbazine, thioguanine, lomustine, and vincristine18; cisplatin and etoposide19; and carboplatin, vincristine, and etoposide (Table 1).20 Early studies suggested 2- to 3-year progression-free survival (PFS) rates of 50% to 78%, and one series reported a 5-year PFS rate of 61% for the combination of carboplatin and vincristine, depending on patient age and histologic subtype.20 Results from a recent, randomized trial that compared the regimen of carboplatin and vincristine to the regimen of procarbazine, thioguanine, lomustine, and vincristine showed no difference in 5-year event-free survival rates, which were 35% and 48%, respectively.21 Although second-line chemotherapy may be considered, RT is often required, which introduces the possibility that some patients would benefit from RT earlier in their disease management if the benefits and risks were more clearly understood. The long-term functional outcome for patients initially managed with chemotherapy has not been reported.
Table 1.
Target Volumes for Pediatric Patients With Low-Grade Glioma
| Tumor Location* | Target Volumes (mL) |
|||||
|---|---|---|---|---|---|---|
| GTV |
CTV |
PTV |
||||
| Mean | SD | Mean | SD | Mean | SD | |
| Infratentorial | 11.82 | 12.53 | 46.78 | 73.82 | 82.10 | 26.87 |
| Supratentorial | 21.03 | 32.97 | 73.82 | 81.27 | 119.00 | 114.33 |
Abbreviations: GTV, gross tumor volume; CTV, clinical target volume; PTV, planning target volume; SD, standard deviation.
Infratentorial, No. of patients = 17; supratentorial, No. of patients = 61.
Observation after surgery also has been pursued as an alternative. Observation was first considered for completely resected tumors,22 and consideration was expanded later to include incompletely resected tumors23; during this time, much has been learned about the natural history of LGG. In a prospective study of more than 726 patients, extent of resection, tumor location, and histologic subtype predicted early progression: midline and optic pathway tumors had the highest progression rates. Those patients treated with less than gross total resection had 5-year PFS rates of only 50% to 60%.23 A subset of patients from the same trial were tested and were found at risk for cognitive and adaptive impairment regardless of tumor location.24 These findings suggested that factors other than RT impact long-term functional outcomes. Recent assessment of 76 survivors who underwent surgery only at a single institution revealed normal intelligence quotients and adaptive skills for most patients; however, when patients were assessed individually, 30% were functioning in the clinically deficient range. Executive functioning accounted for 32% of the difference.25 On the basis of these findings, there is firm evidence to support the concept that deficits observed in long-term survivors are not entirely attributable to RT.
Modern RT methods have been used in pediatric patients to target tumor by using three-dimensional imaging. One series that included 14 patients reported local PFS rates of 87% at 3 years.26 Another series, which included 50 patients with target volumes that measured less than 60 mm in greatest dimension, reported PFS rates of 82.5% at 5 years and 65% at 8 years.27 These data compare favorably to historic series that have PFS rates of 82% when measured at 5 years and of 69% to 77% when measured at 10 years.28,29 Because of the high rate of disease control with RT, the relatively limited duration of chemotherapy response, and emerging data that suggest that patients with LGG are at risk for adverse effects independent of RT, the role of RT and the timing for pediatric patients should be reappraised to maximize disease control and functional outcome.
We initiated a phase II trial in 1997 of conformal RT (CRT) for pediatric patients with localized primary brain tumors. Our goal was to prospectively study a wide range of adverse effects in pediatric LGG, the rate of disease control, and the patterns of failure with CRT. We adopted the International Commission on Radiation Units and Measurements report-50 definitions30 for targeting, and we selected our clinical target volume (CTV) margin on the basis of our intention to treat patients regardless of tumor size, including patients with large or infiltrative tumors.31 This report includes disease control and acute effects of CRT in this patient population.
MATERIALS AND METHODS
Patients
Between August 1997 and August 2006, 78 pediatric patients diagnosed with LGG were enrolled on a phase II study of CRT at St Jude Children's Research Hospital. The median age was 8.9 years (range, 2.2 to 19.8 years). There were 39 female and 39 male patients. Patients were additionally characterized before CRT according to specific clinical and treatment-related factors, including tumor location (diencephalic, n = 58; cerebral hemisphere, n = 3; cerebellum, n = 17), prior chemotherapy (n = 25), number of surgical procedures (none, n = 13; one, n = 42; two, n = 18; three, n = 5), and extent of resection before CRT (no biopsy, n = 13; biopsy, n = 30; subtotal resection, n = 35). Histologic diagnosis was reviewed for each patient on the basis of WHO tumor grade classification, as follows32: WHO grade 1 (juvenile pilocytic astrocytoma, n = 50; unbiopsied optic pathway glioma, n = 13; ganglioglioma, n = 3; and pleomorphic xanthroastrocytoma, n = 1) and WHO grade 2 (astrocytoma not otherwise specified, n = 4; pilomyxoid astrocytoma, n = 4; neurocytoma, n = 2; and oligogendroglioma, n = 1) tumors. Hydrocephalus was present at diagnosis in 31 patients, ventriculoperitoneal shunts were required for 29 patients, and NF-1 was documented for 13 patients.
CRT
CRT was indicated for the study patients on the basis of symptoms at the time of initial evaluation, neuroimaging evidence of tumor progression, or risk of residual tumor progression at a critical site after decompressive surgery. Among the patients treated primarily with CRT, 27 of 43 received treatment within 90 days of diagnosis, including four of seven patients who were diagnosed by magnetic resonance imaging (MRI), 22 of 33 who underwent one surgical procedure, and one of 13 who underwent more than one surgical procedure. None of the patients in this series were treated with CRT after progression that occurred after presumed prior gross tumor resection. All patients had imaging-measurable disease at the time of CRT. The protocol used the International Commission on Radiation Units and Measurements report-5030 definitions for gross tumor (ie, gross tumor volume [GTV]), CTV, and planning target volume (PTV) margins. The GTV was the cystic and solid tumor present on multisequence MRI before RT. To define the GTV, MRI studies from the time of diagnosis (before any therapy) and within 2 weeks of RT were registered to the treatment-planning CT. MRI registration was used to plan treatment in all but seven patients. The CTV margin was 10 mm and was modified at tissue interfaces where invasion was unlikely. The PTV margin was 3 to 5 mm, depending on the integrity of immobilization. The methods of CRT (n = 75) and intensity-modulated RT (n = 3) for these patients have been previously described.31 A dose of 54 Gy was prescribed as 1.8-Gy fractions during a period of 6 weeks. One patient with optic nerve glioma received 50.4 Gy. There were no dose constraints. MRI was performed during weeks 3 and 5 to monitor for tumor enlargement. Target adjustments were made when clinically significant. The supratentorial target volumes were statistically larger than infratentorial target volumes (P = .012, P = .003, and P = .004; Table 2).
Table 2.
Pediatric Low-Grade Glioma Chemotherapy and Radiotherapy Series
| Author by Type of Treatment | Year of Study | Treatment Regimen | No. of Patients | Event- or Progression-Free Survival (%) |
||||
|---|---|---|---|---|---|---|---|---|
| 2-Year | 3-Year | 5-Year | 8-Year | 10-Year | ||||
| Chemotherapy | ||||||||
| Ater21 | 2008 | CV | 137 | 35 | ||||
| TPCV | 137 | 48 | ||||||
| Gnekow20 | 2004 | CV | 198 | 61 | ||||
| Massimino19 | 2002 | CisVP | 31 | 78 | ||||
| Prados18 | 1997 | TPCV | 42 | 50 | ||||
| Packer17 | 1997 | CV | 78 | 68 | ||||
| Radiation therapy | ||||||||
| Marcus27 | 2005 | 52.2 Gy | 50 | 82 | 65 | |||
| Saran26 | 2002 | 50-55 Gy | 14 | 87 | ||||
| Grabenbauer29 | 2000 | 45-60 Gy | 25 | 69 | ||||
| Erkal28 | 1997 | 50 Gy | 30 | 82 | 77 | |||
| Merchant | 2008 | 54 Gy | 78 | 85 | 74 | |||
C, carboplatin; V, vincristine; T, thioguanine; P, procarbazine; Cis, cisplatin; VP, etoposide.
Pre- and Post-Treatment Evaluations
All patients were prospectively assessed for deficits in neurologic, endocrine, and cognitive function. The results from this testing will be reported separately in a companion manuscript.33 Clinical and imaging examinations were performed every 3 months for the first 2 years, every 6 months through 5 years, and then yearly through 10 years. Magnetic resonance (MR) angiography was performed yearly to monitor for the development or progression of vasculopathy.
Statistical Analysis
The Kaplan-Meier34 estimates of event-free and overall survival rates since CRT administration were reported. Event-free survival and overall survival were measured from the CRT start date to the date of any event, death, or follow-up. The log-rank test was used to compare the difference between the survival curves. The cumulative incidence of local failure was defined as the incidence of local failure in which any other event or distant failure were competing risks. The incidence of local failure was measured from the CRT start date to the date of local failure by using Gray's method.35 The same methods were used to determine the incidence of vasculopathy. The t test was used to compare the mean difference between two groups. The significance level for statistical tests was .05.
RESULTS
Disease Control
The mean and standard error (SE) 5- and 10-year event-free (87.4% ± 4.4% and 74.3% ± 15.4%, respectively) and overall (98.5% ± 1.6% and 95.9% ± 5.8%, respectively) survival estimates were determined for all 78 patients (Fig 1). Thirteen patients experienced disease progression within a median time of 83 months (range, 24 to 130 months) according to last MRI and within 89 months (range, 28 to 137 months) according to last contact. Four patients experienced treatment failure and developed metastatic disease at 4, 5, 12, and 85 months. One patient experienced marginal failure, which occurred at 7 months; of the remaining eight patients who experienced treatment failures, the failures were infield and local and occurred at 18, 35, 43, 45, 55, 61, 85, and 86 months. The marginal failure occurred near the optic chiasm in the lone patient with optic nerve glioma who was included in this series. The cumulative incidence of local failure was determined with distant failure as a competing risk. The 5-, 8-, and 10-year cumulative incidences (± SEs) of local failure were 8.7% ± 3.5%, 16.4% ± 5.4%, and 16.4% ± 5.4%, respectively.
Fig 1.
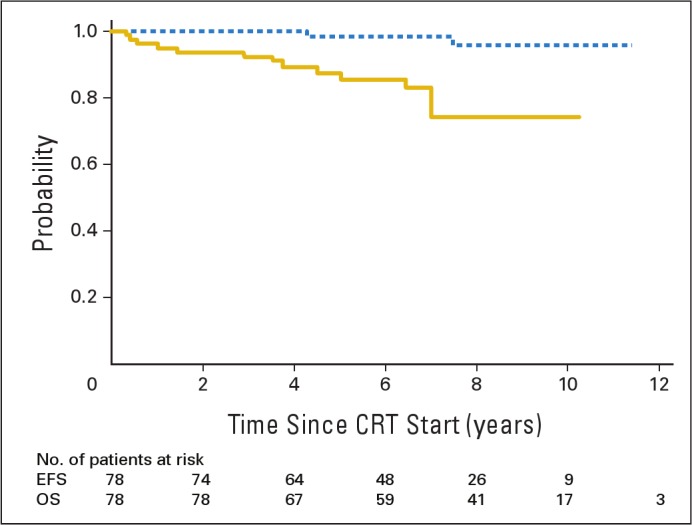
Event-free survival (EFS; gold line) and overall survival (OS; blue line) for pediatric patients with low-grade glioma. Numbers indicate patients at risk. CRT, conformal radiation therapy.
By tumor grade (WHO 1 v 2), the 5- and 10-year estimates for event-free survival were 87% ± 5% and 77% ± 17% for WHO grade 1 versus 91% ± 9% and 64% ± 27% for WHO grade 2 (P = .37). These differences were not significant. The cumulative incidence of local failure by tumor grade was not statistically significant (P = .43). The incidences (± SEs) at 5, 8, and 10 years were 8.7% ± 3.8%, 15.2% ± 5.7%, and 15.2% ± 5.7%, respectively, for patients with WHO grade 1 and were 9.1% ± 9.1%, 20.5% ± 13.9%, and 20.5% ± 13.9%, respectively, for patients with WHO grade 2 tumors (Fig 2). None of the patients with NF-1 experienced disease progression or secondary malignancy. There was one male patient who experienced secondary malignancy. He was 16 years old at the time of CRT, which was administered for a centrally located, WHO grade 2 glioma. He developed high-grade glioma within the high-dose volume 78 months after CRT.
Fig 2.
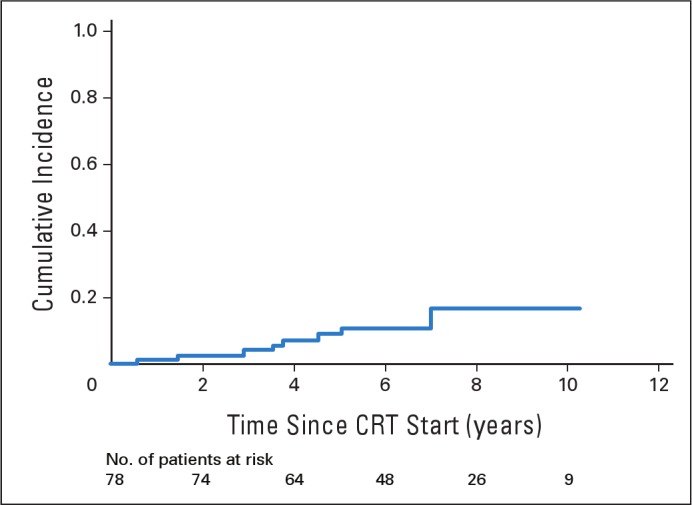
The cumulative incidence of local failure by tumor grade for pediatric patients with low-grade glioma. Numbers indicate patients at risk. CRT, conformal radiation therapy.
Symptoms During and 12 Months After CRT
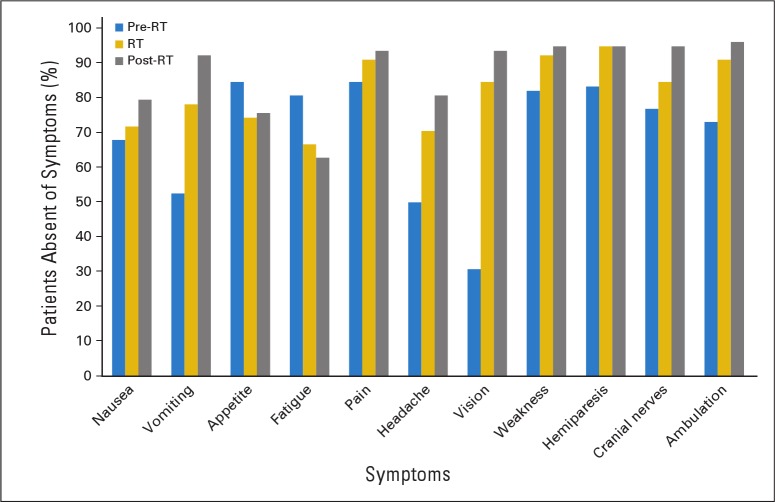
Patient symptoms were monitored and managed during and after CRT by the treating radiation oncologist. We considered three categories of symptoms. The first included nausea, vomiting, loss of appetite, and fatigue. The second included headache and pain other than headache. The third included visual impairment, cranial nerve deficits, motor weakness, and ataxic gait. Figure 3 shows the proportion of patients who were absent of symptoms before, during, and after CRT. There was improvement in symptoms in nearly every category, and the greatest improvement was noted for vomiting, headache, and visual complaints. Modest gains were noted for other categories, except for appetite and fatigue, for which the proportion of patients with symptoms increased with time. Appetite decrease during RT and did not improve or worsen during the ensuing 12 months, whereas fatigue continued to increase. Despite worsening fatigue, the proportion of patients with school-related difficulties reportedly increased from 10% at baseline to only 15% at 12 months. Antiemetic therapy was offered routinely to patients, and 69% (54 of 78) used a 5-hydroxytryptamine 3 receptor antagonist. Corticosteroids were not prescribed for asymptomatic patients but, when used, were carefully tapered at a rate to minimize exposure without compromising symptom control or neurologic function. Nevertheless, 50% (39 of 78) required corticosteroids at some point in time before, during, or after RT for ongoing symptoms related to tumor progression, pseudoprogression, and unexpected adverse events, including cyst expansion that required intervention. Expansion of a midbrain cyst in one patient required placement of a permanent ventriculoperitoneal shunt and intracystic catheter/reservoir that was used once for aspiration. This patient acquired a permanent unilateral cranial nerve III deficit from the event. On-treatment imaging at weeks 3 and 5 of CRT demonstrated active cyst expansion in 19 (24%) of 78 patients; two patients required modification of their treatment plans. At the completion of CRT and during the intervening 12 months, corticosteroids were required for 20 (26%) of 78 patients. Three patients had significant cyst expansion after CRT that required surgical intervention. Six patients required hospitalization, three during RT. One patient required a gastrostomy tube because of intractable nausea and anorexia after CRT. She recovered after 1 year. Figure 4 shows the proportion of patients who had increasing symptoms during and after CRT.
Fig 3.
Absent (pre-radiation therapy; RT) symptoms before, during, and after conformal RT for patients with pediatric low-grade glioma.
Fig 4.
Proportion of patients with increasing symptoms during and after conformal radiation therapy (RT) for pediatric patients with low-grade glioma.
Cerebral Vasculopathy
MR angiography revealed evidence of cerebral vasculopathy in four patients before CRT. Two of these patients, who both had NF-1, required revascularization surgery 9.5 and 68.5 months after CRT because of symptomatic ischemia and stroke. The other two, who were without NF-1, had no progression of vasculopathy 36 and 58 months after CRT.
When the patients were considered separately, five additional patients, including one with NF-1, developed MR angiographic evidence of vasculopathy 12, 26, 31, 85, and 99 months after CRT. Three required revascularization surgery at 21, 28, and 91 months. The shortest interval from CRT to revascularization surgery was noted for the lone patient with NF-1. CRT was indicated for this patient, who was 2.7 years old, because of her inability to tolerate chemotherapy. When treatment failure was a competing risk and when patients with pre-CRT vasculopathy were excluded, the cumulative incidence (± SE) of vasculopathy was 4.79% ± 2.73% at 7 years. We considered NF-1 status, sex, and age as covariates for prediction of the incidence. Only age proved clinically significant (P = .0105). The risk (± SE) at 6 years was 12.5% ± 12.6% for patients younger than 5 years of age (n = 8) compared with 3.8% ± 2.6% for those older than 5 years (n = 66) at the time of CRT.
DISCUSSION
Low-grade glioma (LGG) is the most common central nervous system neoplasm in children. It affects all ages and intracranial sites. Surgery alone is curative for focal, resectable tumors, which most often involve the cerebellum. Tumors that develop in central locations, including the diencephalon and optic pathways, often require a multimodality approach that uses surgery to decompress normal tissue structures and alleviate symptoms36 and adjuvant therapy in the form of chemotherapy or RT—depending on patient age, severity of symptoms, risks associated with additional progression, and other factors coincident with the overall treatment plan. The use of RT in children with LGG is controversial and is reserved for older children or for those who experience disease progression after combination chemotherapy. Fear of cognitive effects, endocrine deficiencies, hearing loss, secondary malignancies, neurovascular damage, and abnormalities in growth and development have led to the avoidance of RT and to a search for alternatives, especially for the youngest children who are most vulnerable. With the advent of three-dimensional treatment planning and delivery, there is an opportunity to reassess the role of RT and to address the controversy surrounding the age at which it might be considered safe or at which the benefits of treatment outweigh the potential risks.
Contrary to large, retrospective series that have reported 5-year PFS rates as low as 48%,37 recent institutional studies of focal irradiation for pediatric LGG have reported disease control rates that exceeded 80% when estimated at 5 years (Table 1); however, lacking is the evidence that function outcomes are preserved or improved. With the objective of reducing the adverse effects of irradiation in pediatric LGG, we designed a trial to test the hypothesis that irradiation with a 10-mm CTV margin would reduce adverse effects without affecting the rate of treatment failure in pediatric LGG.
Local control and PFS rates reported in series of pediatric LGG depend on the relative proportion of patients with WHO grade 1 and 2 tumors as well as with NF-1. The latter typically have better overall survival but a higher risk of complications. For series that include all types of patients, PFS is expected to be greater than 80% when measured at 5 years.26–29 Recent series that used highly focused conformal treatment methods have not reported their results separately on the basis of tumor grade. Those patients with WHO grade 2 tumors are expected to have lower PFS rates, as shown in a recent series of 52 patients with WHO grade 2 glioma who received irradiation as part of their initial management.16 The 5-year PFS rate was reported in a series of 52 patients who received irradiation by using conventional RT. The results (mean ± SE) at 5 and 10 years were 56% ± 5% and 42% ± 6%, respectively.
RT for optic pathway tumors in the setting of NF-1 was associated with vascular complications (ie, ischemic strokes) in 32% of patients in one series.38 Among those who had pre-CRT vasculopathy in the series in this study, only those with NF-1 worsened, which suggested that these patients had severe disease, whereas those without NF-1 did not; this difference implied that the anomalies may be attributed to tumor or surgery. The literature is lacking on the incidence of pre-irradiation vasculopathy in LGG.
The incidences of vasculopathy and revascularization procedures are of concern. Surveillance is important with consideration to early intervention. Late onset of vasculopathy indicates that, despite the median follow-up in this study of 89 months, additional events should be expected. Vasculopathy is a late complication39,40 and is more common in younger patients and in those with NF-1.6,41 Only one patient with NF-1 in this series was younger than 5 years of age; vasculopathy developed 1 year after CRT. There were seven patients with NF-1 who were older than 5 years who did not develop vasculopathy.
An array of techniques have been used to treat pediatric LGG, including CRT, intensity-modulated RT,31 stereotactic RT,27 and proton-beam RT.42 Hypofractionated irradiation43 also has been used along with stereotactic radiosurgery44 in selected instances. Although the technical requirements for treatment planning may be similar,45 the margins used to define the treatment volume vary widely. We now consider the CTV margin used for this study to be large and have reduced it to 5 mm.2 On the basis of our experience, in which changes in the PTV during treatment are common, vigilance is required.
This series confirms the rate of disease control expected from RT in the treatment of LGG. This series prospectively defines a targeting benchmark for disease control on the basis of a 10-mm CTV and associated treatment effects during the first year. We remain concerned about late treatment failures, vasculopathy and secondary malignancy. We recommend vigilance in baseline evaluation and follow-up. Patients with NF-1 appear to have a higher rate of baseline vasculopathy, and patients younger than 5 years of age are more susceptible.
Acknowledgment
This work was supported in part by the American Cancer Society and the American Lebanese Syrian Associated Charities.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas E. Merchant, Larry E. Kun, Shengjie Wu, Xiaoping Xiong
Administrative support: Thomas E. Merchant
Provision of study materials or patients: Thomas E. Merchant, Larry E. Kun, Robert A. Sanford, Frederick A. Boop
Collection and assembly of data: Thomas E. Merchant
Data analysis and interpretation: Thomas E. Merchant, Shengjie Wu, Xiaoping Xiong
Manuscript writing: Thomas E. Merchant, Larry E. Kun, Shengjie Wu
Final approval of manuscript: Thomas E. Merchant, Larry E. Kun, Shengjie Wu, Xiaoping Xiong, Robert A. Sanford, Frederick A. Boop
REFERENCES
- 1.Gnekow A, De Salvo GL, Thieme B, et al. SIOP-LGG 2004: Comprehensive treatment strategy for low-grade glioma in children and adolescents, including a randomized chemotherapy trial and a radiotherapy trial. Neuro Oncol. 2008;10:452–453. abstr LGG 21. [Google Scholar]
- 2.National Cancer Institute, National Institutes of Health. Phase II Study of Reduced-Field Conformal Radiotherapy in Young Patients With Low-Grade Gliomas. http://www.cancer.gov/clinicaltrials/COG-ACNS0221.
- 3.Brauner R, Malandry F, Rappaport R, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149:825–828. doi: 10.1007/BF02072067. [DOI] [PubMed] [Google Scholar]
- 4.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75:1051–1059. doi: 10.1002/1097-0142(19950215)75:4<1051::aid-cncr2820750423>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Chadderton RD, West CG, Schuller S, et al. Radiotherapy in the treatment of low-grade astrocytomas: II. The physical and cognitive sequelae. Childs Nerv Syst. 1995;11:443–448. doi: 10.1007/BF00334961. [DOI] [PubMed] [Google Scholar]
- 6.Kortmann RD, Timmermann B, Taylor RE, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part II: Treatment-related late toxicity. Strahlenther Onkol. 2003;179:585–597. doi: 10.1007/s00066-003-8104-0. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Savino PJ, Bilaniuk LT, et al. Chiasmatic gliomas of childhood. A reappraisal of natural history and effectiveness of cranial irradiation. Childs Brain. 1983;10:393–403. [PubMed] [Google Scholar]
- 8.Morris DE, Bourland JD, Rosenman JG, et al. Three-dimensional conformal radiation treatment planning and delivery for low- and intermediate-grade gliomas. Semin Radiat Oncol. 2001;11:124–137. doi: 10.1053/srao.2001.22060. [DOI] [PubMed] [Google Scholar]
- 9.Shaw EG, Wisoff JH. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol. 2003;5:153–160. doi: 10.1215/S1152-8517-02-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kortmann RD, Timmermann B, Taylor RE, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain: Part I. Treatment modalities of radiation therapy. Strahlenther Onkol. 2003;179:509–520. doi: 10.1007/s00066-003-9104-9. [DOI] [PubMed] [Google Scholar]
- 11.van der Wal EJ, Azzarelli B, Edwards-Brown M. Malignant transformation of a chiasmatic pilocytic astrocytoma in a patient with diencephalic syndrome. Pediatr Radiol. 2003;33:207–210. doi: 10.1007/s00247-002-0828-y. [DOI] [PubMed] [Google Scholar]
- 12.Ishii N, Tada M, Hamou MF, et al. Cells with TP53 mutations in low grade astrocytic tumors evolve clonally to malignancy and are an unfavorable prognostic factor. Oncogene. 1999;18:5870–5878. doi: 10.1038/sj.onc.1203241. [DOI] [PubMed] [Google Scholar]
- 13.Allen JC. Initial management of children with hypothalamic and thalamic tumors and the modifying role of neurofibromatosis-1. Pediatr Neurosurg. 2000;32:154–162. doi: 10.1159/000028922. [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Castroviejo I, Pascual-Pascual SI, Velázquez-Fragua R, et al. Neurofibromatosis type 1 and optic pathway gliomas: A series of 80 patients [in Spanish] Rev Neurol. 2008;46:530–536. [PubMed] [Google Scholar]
- 15.Guillamo JS, Créange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): A retrospective study of 104 patients. Brain. 2003;126:152–160. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- 16.Mishra KK, Puri DR, Missett BT, et al. The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol. 2006;8:166–174. doi: 10.1215/15228517-2005-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 18.Prados MD, Edwards MS, Rabbitt J, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 19.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 20.Gnekow AK, Kortmann RD, Pietsch T, et al. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy: Report from the multicenter treatment study for children and adolescents with a low grade glioma, HIT-LGG 1996, of the Society of Pediatric Oncology and Hematology (GPOH) Klin Padiatr. 2004;216:331–342. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 21.Ater J, Holmes E, Zhou T, et al. Results of COG protocol A9952: A randomized phase 3 study of two chemotherapy regimens for incompletely resected low-grade glioma in young children. Neuro Oncol. 2008;10:451. abstr LGG18. [Google Scholar]
- 22.Wallner KE, Gonzales MF, Edwards MS, et al. Treatment results of juvenile pilocytic astrocytoma. J Neurosurg. 1988;69:171–176. doi: 10.3171/jns.1988.69.2.0171. [DOI] [PubMed] [Google Scholar]
- 23.Wisoff JH, Sanford R, Holmes E, et al. Impact of surgical resection on low grade gliomas of childhood: A report from the CCG9891/POG 9130 low grade astrocytoma study. Presented at the 10th International Symposium on Paediatric Neuro-Oncology; June 9-12, 2002; London, England. [Google Scholar]
- 24.Beebe DW, Ris MD, Armstrong FD, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: Evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130) J Clin Oncol. 2005;23:5198–5204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 25.Rey-Casserly C, Chordas C, Liptak C, et al. IQ versus executive function ratings as predictors of adaptive skills in children treated for low-grade gliomas only with surgery. Neuro Oncol. 2008;10:450. abstr LE9. [Google Scholar]
- 26.Saran FH, Baumert BG, Khoo VS, et al. Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int J Radiat Oncol Biol Phys. 2002;53:43–51. doi: 10.1016/s0360-3016(02)02734-7. [DOI] [PubMed] [Google Scholar]
- 27.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: Final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61:374–379. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45:11–15. doi: 10.1016/s0167-8140(97)00102-3. [DOI] [PubMed] [Google Scholar]
- 29.Grabenbauer GG, Schuchardt U, Buchfelder M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG): Radiographic response, vision and late toxicity. Radiother Oncol. 2000;54:239–245. doi: 10.1016/s0167-8140(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 30.Washington, DC: International Commission on Radiation Units and Measurements; 1993. ICRU Report 50, September 1993: Dose Specification for Reporting External Beam Therapy With Photons and Electrons. [Google Scholar]
- 31.Merchant TE, Zhu Y, Thompson SJ, et al. Preliminary results from a Phase II trial of conformal radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52:325–332. doi: 10.1016/s0360-3016(01)01807-7. [DOI] [PubMed] [Google Scholar]
- 32.Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK, et al. Surgical neuropathology update: A review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008;132:993–1007. doi: 10.5858/2008-132-993-SNUARO. [DOI] [PubMed] [Google Scholar]
- 33.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 35.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- 36.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83:583–589. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 37.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 38.Guillamo JS, Créange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): A retrospective study of 104 patients. Brain. 2003;126:152–601. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- 39.Mori K, Takeuchi J, Ishikawa M, et al. Occlusive arteriopathy and brain tumor. J Neurosurg. 1978;49:22–35. doi: 10.3171/jns.1978.49.1.0022. [DOI] [PubMed] [Google Scholar]
- 40.Singh R, Trobe JD, Hayman JA, et al. Ophthalmic artery occlusion secondary to radiation-induced vasculopathy. J Neuroophthalmol. 2004;24:206–210. doi: 10.1097/00041327-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 42.Hug EB, Muenter MW, Archambeau JO, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol. 2002;178:10–17. doi: 10.1007/s00066-002-0874-2. [DOI] [PubMed] [Google Scholar]
- 43.Roberge D, Souhami L, Olivier A, et al. Hypofractionated stereotactic radiotherapy for low grade glioma at McGill University: Long-term follow-up. Technol Cancer Res Treat. 2006;5:1–8. doi: 10.1177/153303460600500101. [DOI] [PubMed] [Google Scholar]
- 44.Hadjipanayis CG, Kondziolka D, Flickinger JC, et al. The role of stereotactic radiosurgery for low-grade astrocytomas. Neurosurg Focus. 2003;14:e15. doi: 10.3171/foc.2003.14.5.16. [DOI] [PubMed] [Google Scholar]
- 45.Kooy HM, van Herk M, Barnes PD, et al. Image fusion for stereotactic radiotherapy and radiosurgery treatment planning. Int J Radiat Oncol Biol Phys. 1994;28:1229–1234. doi: 10.1016/0360-3016(94)90499-5. [DOI] [PubMed] [Google Scholar]