Abstract
Work from our laboratory in both in-patient and outpatient facilities utilizing the Comprehensive Analysis of Reported Drugs (CARD)™ found a significant lack of compliance to prescribed treatment medications and a lack of abstinence from drugs of abuse during active recovery. This unpublished, ongoing research provides an impetus to develop accurate genetic diagnosis and holistic approaches that will safely activate brain reward circuitry in the mesolimbic dopamine system. This editorial focuses on the neurogenetics of brain reward systems with particular reference to genes related to dopaminergic function. The terminology “Reward Deficiency Syndrome” (RDS), used to describe behaviors found to have an association with gene-based hypodopaminergic function, is a useful concept to help expand our understanding of Substance Use Disorder (SUD), process addictions, and other obsessive, compulsive and impulsive behaviors. This editorial covers the neurological basis of pleasure and the role of natural and unnatural reward in motivating and reinforcing behaviors. Additionally, it briefly describes the concept of natural dopamine D2 receptor agonist therapy coupled with genetic testing of a panel of reward genes, the Genetic Addiction Risk Score (GARS). It serves as a spring-board for this combination of novel approaches to the prevention and treatment of RDS that was developed from fundamental genomic research. We encourage further required studies.
Introduction
There has been over half a century of dedicated and rigorous scientific research on the brain’s mesolimbic system, a critical site for experiences of well-being. These investigations have provided insight into the addictive brain and the neurogenetic mechanisms involved in the quest for happiness. This part of the brain is a reward center where chemical messengers including serotonin, enkephalin, γ-aminobutyric acid (GABA), dopamine (DA), acetylcholine (ACH) and many second messenger proteins work in concert to provide a net release of DA at the nucleus accumbens (NAc). The idea that the synthesis, vesicular storage, metabolism, receptor formation, and catabolism of neurotransmitters are controlled by genes is well understood [1–3]. Polymorphic versions of these genes have certain variations that can disrupt the neurochemical events that culminate in neuronal release of DA. A breakdown in the cascade “The Brain Reward Cascade” [4] of these neuronal events will eventually lead to DA dysfunction. Two prominent functions of the DA molecule are the experience of pleasure (reward) and the reduction of stress. DA dysfunction then can result in a deficiency in reward and a predisposition to substance-seeking in an attempt to ameliorate hypodopaminergic function [5].
Neurogenetic considerations
Certainly, Homo sapiens have a biological predisposition to drink, eat, reproduce, and desire pleasurable experiences. The mechanisms involved in reward from these natural processes may be impaired due to polymorphic genetic antecedents provoked by epigenetic, environmental factors that can result in multiple impulsive, compulsive, and addictive behaviors. From the many genes known to predispose individuals to excessive cravings and result in SUD, some of the most prominent are the following polymorphisms: the serotonergic 2A receptor (5-HTT2a); serotonergic transporter (5HTTLPR.); DA D1 receptor(DRD1); DA D2 receptor (DRD2); DA D3 receptor (DRD3); DA D4 receptor (DRD4); DA transporter (DAT1); and the catechol-O-methyltransferase (COMT), monoamine-oxidase (MOA); Mu-opiate receptor (MOR); GABA–B3 genes [6–8] (Table 1 GARS). Individuals are predisposed to self-medicate with any substance or behavior that will activate DA release. This can occur if they possess, for example, an increased rate of mitochondrial DA breakdown, due to having high MOA activity or an increased rate of synaptic DA breakdown due to having high catabolic genotype of the COMT gene. However, slower breakdown of DA due to polymorphisms in both the MOA and or COMT may lead to hyperactivity as seen in Attention Deficit Hyperactivity Disorder (ADHD).
Table 1.
Proposed Genetic Addiction Risk Score (GARS).
| Dopamine D1 Receptor Gene |
| Dopamine D2 Receptor Gene Dopamine D3 Receptor Gene |
| Dopamine D4 Receptor Gene Dopamine Transporter Gene |
| Serotonin 2a Receptor Gene |
| Serotonin Transporter Gene Mu-opiate Receptor Gene |
| GABA –B3 Receptor Gene PENK Gene |
| Mono-Amine –Oxidase A Gene Catecholamine –Methyl-Transferase Gene |
| Cytochrome P450 Gene |
An association, between common genetic variants of the DAD2 receptor gene (DRD2) polymorphisms [9,10] and other reward genes [6–8] (hypodopaminergic function) and impulsive, compulsive, and addictive behaviors has been identified [6,7,11]. Thus the term Reward Deficiency Syndrome (RDS), first coined in our laboratory, in 1995, was designated to cover all conditions genetically associated with hypodopaminergic function [8].
Most addictions, including alcohol, opiates, psychostimulants (cocaine, methamphetamine), nicotine, glucose, gambling, sex addiction, excessive spending, and even uncontrolled internet gaming are associated with the release of DA in the mesocorticolimbic system or reward pathway of the brain [4,5,12–14] figure 1. While activation of this dopaminergic system results in feelings of reward and pleasure [15–17] reduced activity (hypodopaminergic functioning) can trigger drug-seeking behavior [18–22]. Mechanisms of hypodopaminergic functioning including reduced DA receptor density, blunted response to DA, or enhanced DA catabolism in the reward pathway, can be induced by variant alleles or defined polymorphisms [23]. Cessations of chronic drug use also can generate a hypodopaminergic state that prompts drug-seeking behaviors in an attempt to address the unwanted withdrawal-induced state [24].
Figure 1.
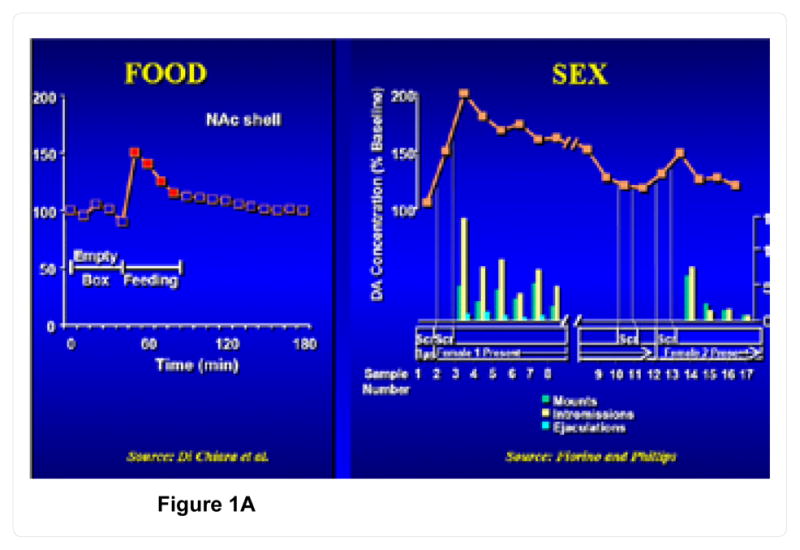
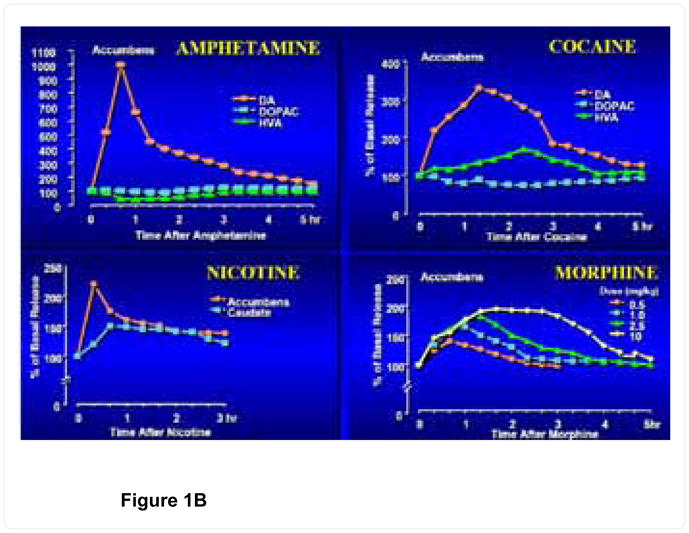
Figure 1A. Natural rewards and dopamine release.
Figure 1B. Unnatural rewards and dopamine release.
[Modified from Di Chiara G and Imperato, 1988 and Fiorino & Phillips, 1999]
Dopaminergic mechanisms
While a feeling of wellbeing can be produced by acute use of psychoactive substances, sustained and prolonged abuse results in tolerance and discomfort [25]. For example, opioid desensitization/tolerance mechanisms have focused on adaptations that occur on the level of the mu-opioid receptor (MOR) itself. These include opioid receptor phosphorylation [26]. Recent research has revealed augmented isoform-specific synthesis of adenylyl cyclase and their phosphorylation as well as augmented phosphorylation of the G(beta) subunit of G(beta gamma). The effect of these changes is to shift mu-opioid receptor-coupled signaling from predominantly G(i alpha) inhibitory to (G(i)-derived) G(beta gamma) stimulatory adenylyl cyclase signaling [26]. Polymorphisms related to MOR have been associated with excessive drug (ethanol) seeking behavior that interacts with dopaminergic pathways in the NAc [27].
Moreover, excessive cravings caused by carrying the DRD2 A1 allelic genotype, a deficit in DA receptors, are compounded by consequential drug seeking behavior. Conversely, normal densities of DA receptors result in low craving behaviors [19]. Reduction of craving to prevent or treat SUD could result from proliferation of DAD2 receptors in genetically predisposed individuals [28,29] and those with hypodopaminergic function secondary to stress or the toxic effects of the abused substances [30]. Boundy et al. [31,32] have shown, in vitro, that constant stimulation of the DA receptor system with low doses of a D2 agonist results in significant proliferation of D2 receptors, in spite of genetic antecedents [33]. Messenger RNA expression causes proliferation of D2 receptors induced by negative feedback mechanisms, in the mesolimbic system signaled by gentle chronic D2 receptor stimulation [31,32]. This neuro-molecular finding serves as the basis for naturally inducing DA release, to produce the same induction of D2-directed mRNA and thus proliferation of D2 receptors in humans and a resultant attenuation of craving behavior [34,35]. This has been proven with work showing a form of gene therapy [36]. In nonhuman animals DNA-directed overexpression of the DRD2 receptors induces a significant reduction in both alcohol and cocaine craving induced behavior [37–39].
Our most recent findings, derived from a small unpublished pilot study showing a clear difference between placebo and KB220Z™ in terms of BOLD activation of the dopaminergic pathways of the caudate-accumbens area are encouraging. Moreover, we also observed, an attenuation of the hyperactivity in the putamen of abstinent heroin-dependent subjects. The experiment will continue, by adding additional heroin–dependent subjects, until statistical power is sufficient for demonstrating significant results. We did, however, observe statistically significant results (P <.05) in three important brain regions of interest (ROI) when we evaluated placebo compared to the KB220Z™ treatment group in 10 subjects at rest. Currently, albeit knowing that there is a lower D2R availability in the putamen of abstinent heroin dependent subjects, we do not understand the mechanism by which KB220Z™ administration (post one–hour) induced an attenuation of this hypo state. This will be the subject of further investigation and it may involve abnormal white matter synapses.
In ongoing research, we will explore the role of KB220Z compared to placebo, both its impact on white matter and on cue-induced craving behavior. This additional experiment is crucial since the structure and function of white matter synapses has become increasingly important in disease. While vesicular neurotransmitter release is the province of gray matter, synaptic style release of glutamate occurs deep in white matter. As white matter becomes increasingly well-recognized as a substrate for disease, dysregulation of white matter synaptic transmission will play a role a number of impulsive/compulsive/addictive RDS behaviors [34,35].
Interestingly, current cocaine-dependent users show reductions in white matter integrity, especially in connections to cortical regions associated with cognitive control that have been associated with inhibitory dysfunction [40]. In a diffusion tensor imaging study, by Bell, et al. [40] former cocaine dependant groups with different durations of abstinence were observed to show white matter fractional anisotropy differences bilaterally in the inferior longitudinal fasciculus, right anterior thalamic radiation, right ventral posterolateral nucleus of the thalamus, left superior corona radiata, superior longitudinal fasciculus bilaterally, right cingulum and the white matter of the right precentral gyrus [40]. The findings suggested that specific white matter abnormalities discriminate as a function of abstinence duration and therefore, might represent brain changes that mark recovery from addiction. Similar findings have been found in heroin –dependent subjects from research in Liu’s group [41,42]. They found that fractional anisotropy was significantly decreased in specific brain regions of heroin-dependent patients (P<0.001 uncorrected) including the frontal gyrus, the parietal lobule, the insula, and the corpus callosum. Thus, micro structural abnormality is present in the white matter of several specific brain regions of heroin-dependent patients.
Based on the current literature and our pilot findings discussed herein, we are poised to further evaluate the effectiveness of KB220Z on micro structural disruption of white matter in heroin addicts revealed by diffusion tensor imaging. Certainly, a combination of findings that includes BOLD activation of dopaminergic pathways in the caudate-accumbens; attenuation of abnormal hyperactivity of the putamen in heroin-dependent subjects and a potential reduction of micro structural white matter abnormalities by KB220Z should ultimately support its utilization as a novel safe DA agonist for prevention, tertiary treatment and relapse attenuation in RDS victims, especially carriers of reward gene polymorphisms. Currently there are many clinical trials showing significant benefits of KB220 and variants over four decades of research (Table 2).
Table 2.
Phase 1 and Phase 2 Clinical Trials of Neuro-nutrient Amino Acid Therapy (NAAT).
| PUBLISHED REFERENCE |
|---|
| L-DOPA: effect on ethanol narcosis and brain biogenic amines in mice. Blum K, Calhoun W, Merritt J, Wallace JE. Nature. 242: 407–409, 1973. |
|
Ethanol narcosis in mice: serotonergic involvement. Blum, K.; Wallace, J.E.; Calhoun, W.; Tabor, R.G. & Eubanks, J.D. Experientia 30:1053–1054, 1974. Enkephalinase inhibition: Regulation of ethanol intake in mice. Blum K, Wallace JE, Trachtenberg MC. Briggs AH, Dellallo L. Alcohol: 4; 449–456, 1987. |
| Improvement of inpatient treatment of the alcoholic as a function of neurotransmitter restoration: a pilot study. Blum, K.; Trachtenberg, MC.; Ramsay JC. The International journal of the addictions. 23: 991–998, 1988. |
|
Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: double-blind placebo-controlled study of the nutritional adjunct SAAVE. Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, Samuels AI, Cataldie L. Alcohol. 5(6): 481–93. 1988. Reduction of both drug hunger and withdrawal against advice rate of cocaine abusers in a 30 day inpatient treatment program by the neuronutrient Tropamine. Blum, K.; Allison, D.; Trachtenberg, M.C.; Williams, R.W. & Loeblich, L.A. Current Therapeutic Research 43: 1204–1214. 1988. Neurodynamics of relapse prevention: a neuronutrient approach to outpatient DUI offenders. Brown, R.J.; Blum, K. & Trachtenberg, M.C. Psychoactive Drugs 22: 173–187, 1990. Neuronutrient effects on weight loss in carbohydrate bingers; an open clinical trial. Blum K, Trachtenberg MC, Cook DW. Curr Ther Res.48: 217–233, 1990. |
| NeuRecover-SATM in the Treatment of Cocaine Withdrawal and Craving: A Pilot Study. Cold, Julie A. Clinical Drug Investigation. 12 (1):1–7, 1996. |
| Enhancement of attention processing by Kantroll in healthy humans: a pilot study. DeFrance, J.F.; Hymel, C.; Trachtenberg, M.C.; Ginsberg, L.D.; Schweitzer, F.C.; Estes, S.; Chen, T.J.; Braverman, E.R.; Cull, J.G. & Blum, K. Clinical Electroencephalography 28: 68–75, 1997. |
| Clinical evidence for effectiveness of Phencal™ in maintaining weight loss in an open-label, controlled, 2-year study. Blum K, Cull JG, Chen TJH, Swan SG, Holder JM, Wood R, Braverman ER, Bucci LR, Trachenberg MG. Current Therapeutic Research 55(10) 745–763, 1997. |
|
1st Conference on Reward Deficiency Syndrome: Genetic Antecedents and Clinical Pathways. San Francisco, California, USA. November 12–13, 2000. Abstracts. Amino-acid precursor and enkephalinase inhibition therapy: evidence for effectiveness in treatment of “Reward Deficiency Syndrome (RDS) with particular emphasis on eating disorders. Julia Ross. Mol Psychiatry. Feb; 6(1 Suppl 1):S1–8, 2001. Narcotic antagonists in drug dependence: pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Chen, T.J.; Blum, K.; Payte, J.T.; Schoolfield, J.; Hopper, D.; Stanford, M. & Braverman, E.R. Medical Hypotheses 63 (3): 538–48, 2004. |
| Reward deficiency syndrome in obesity: a preliminary cross-sectional trial with a Genotrim variant. Blum K, Chen TJ, Meshkin B, Downs BW, Gordon CA, Blum S, Mengucci JF, Braverman ER, Arcuri V, Varshavskiy M, Deutsch R, Martinez-Pons M. Adv Ther. 23(6):1040–51, 2006. |
|
Gene \Narcotic Attenuation Program attenuates substance use disorder, a clinical subtype of reward deficiency syndrome. Chen, T.J.; Blum, K.; Waite, R.L.; Meshkin, B.; Schoolfield, J.; Downs, B.W.; Braverman, E.E.; Arcuri, V.; Varshavskiy, M,; Blum, S.H.; Mengucci, J.; Reuben, C. & Palomo, T. Advances in Therapy 24: 402–414, 2007. Synaptamine (SG8839), TM An Amino-Acid Enkephalinase Inhibition Nutraceutical Improves Recovery of Alcoholics, A Subtype of Reward Deficiency Syndrome (RDS). Blum, K.; Chen, T.J.H.; Downs, B.W.; Meshkin, B.; Blum, S.H.; Martinez Pons, M.; Mengucci, J.F.; Waite, R.L.; Arcuri, V.; Varshofsiky, M. & Braverman, E.R. Trends in Applied Sciences Research 2 (3): 132–138, 2007. |
| Chromium Picolinate (Crp) A putative Anti-Obesity Nutrient Induces Changes In Body Composition As Function Of The Taq1 Dopamine D2 Receptor Gene. |
| Chen TJH, Blum K, Kaats G, Braverman ER, Eisenberg A, Arcuri V, Varshavsky M, Mengucci JF, Blum AH, Downs BW, Meshkin B, Williams L, Schoolfield J, Whitel L. Gene Therapy and Molecular Biology 11; 161–170, 2007. A short term pilot open label study to evaluate efficacy and safety of LG839, a customized DNA directed nutraceutical in obesity: Exploring Nutrigenomics. Blum K, Chen TJH, Williams L, Chen ALC, Downs WB, Waite RL, Huntington T, Sim S, Prihoda T, Rhoads P, Reinking J, Braverman D, Kerner M, Blum SH, Quirk B, Eric R Braverman ER. Gene Therapy and Molecular Biology 12, page 371–382, 2010. |
|
LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Blum, K.; Chen, A.L.; Chen, T.J.; Rhoades, P.; Prihoda, T.J.; Downs, B.W.; Waite, R.L.; Williams, L.; Braverman, E.R.; Braverman, D.; Arcuri, V.; Kerner, M.; Blum, S.H. & Palomo, T. Advances in Therapy 25 (9): 894–913, 2008. Dopamine D2 Receptor Taq A1 allele predicts treatment compliance of LG839 in a subset analysis of pilot study in the Netherlands. Blum K, Chen, TJH, Chen ALC, Rhodes P, Prihoda TJ, Downs BW, Bachi D, Bachi M, Blum SH, Williams L, Braverman ER, Kerner M, Waite RL, Quirk B, White L & Reinking J. Gene Therapy Molecular Biology 12, 129–140, 2008. |
|
Putative targeting of Dopamine D2 receptor function in Reward Deficiency Syndrome (RDS) by Synaptamine Complex™ Variant (KB220): Clinical trial showing anti-anxiety effects. Blum K, Chen ALC, Chen TJH, Bowirrat A, Waite RL, Kerner M, Blum SH, Downs BW, Savarimuthu S, Rhoades P, Reinking J, Braverman ER, DiNubile N, Braverman D, Oscar-Berman M. Gene Therapy Molecular Biology 13, 214–230, 2009. Sustainable Weight Loss and Muscle Gain Utilizing the Rainbow Diet™: Targeting Noradrenergic and dopaminergic Mechanistic Sites, Hormonal Deficiency Repletion Therapy and Exercise: A case report. Braverman ER, Braverman D, Acrui V, Kerner M, Downs B.W., Blum K. The American Journal of Bariatric Medicine. 25 (2)18–28, 2010. |
| Acute intravenous synaptamine complex variant KB220™ “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: part 1, pilot study with 2 case reports. Miller DK, Bowirrat A, Manka M, Miller M, Stokes S, Manka D, Allen C, Gant C, Downs BW, Smolen A, Stevens E, Yeldandi S, Blum K. Postgrad Med. Nov; 122(6):188–213, 2010. |
| Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D2 agonist therapy: part 2. Blum K, Chen TJ, Morse S, Giordano J, Chen AL, Thompson J, Allen C, Smolen A, Lubar J, Stice E, Downs BW, Waite RL, Madigan MA, Kerner M, Fornari F, Braverman ER. Postgrad. Med. Nov; 122(6):214–26, 2010. |
| “Dopamine Resistance” in brain reward circuitry as a function of DRD2 gene receptor polymorphisms in RDS: Synaptamine complex variant (KB220) induced “Dopamine Sensitivity” and enhancement of happiness. Blum, K.; Stice, E.; Liu, Y.; Giordano, J.; Morse, S.; Downs, B.W.; Waite, R.L.; Madigan, M.; Braverman, E.R.; Kerner, M.; Oscar-Berman. M.; Miller, D.; Stokes, S.; Gant, C.; Thompson, T.; Allen, C.; Smolen, A., Bowirrat, A. & Gold, M. XIX World Congress of Psychiatric Genetics, September 10–14th. Washington DC, 2011. |
| Neurotransmitter-precursor-supplement Intervention for Detoxified Heroin Addicts. Chen D, Liu Y, He W, Wang H, Wang Z. Huazhong University of Science and Technology and Springer-Verlag Berlin Heidelberg Med Sci 32(3):422–427, 2012. |
|
Early Intervention of Intravenous KB220IV- Neuroadaptagen Amino-Acid Therapy (NAAT)™ Improves Behavioral Outcomes in a Residential Addiction Treatment Program: A Pilot Study Miller M, Chen ALC, Stokes SD, Silverman S, Bowirrat A, Manka M, Manka D Miller DK, Perrine K, Chen TJH, Bailey JA, Downs BW, Waite RL, Madigan MA, Braverman ER, Damle U, Kerner M, Giordano J, Morse S, Oscar-Berman M, Barh D, Blum K. Journal of Psychoactive Drugs (in press December issue 2012). |
Source: Modified from Blum, et al. Omics-Addiction Research & Therapy (2012). Proceedings of Conference, August 2012 with permission
Conclusions
While it is true that Homo sapiens in evolutionary terms are changing very slowly, it is also true that certain genetic traits such as genes that regulate pleasure-seeking may be the exception [34,35]. At this juncture, we do not know whether the DRD2 A1 allele is an older gene allele or if it is newer than the DRD2 A2 allele. Understanding this will help clarify the nature of the relationship humans have with pleasure-seeking and perhaps how it benefits our survival. Certainly carriers of the DRD2 A1 allele are more aggressive than carriers of the DRD2 A2 allele [43].
The initial work of Blum, et al. [4] and others including brain imaging studies [44] that have helped clarify addiction mechanisms also have helped to amend the public’s view of drug addiction. Public opinion has moved from the idea that addiction is a moral problem, to an understanding that genetic predisposition and pathological physical changes that occur during active addiction make it extremely difficult for addicts to give up their substance abuse. We must reflect on the question of how we address the legality, of the natural pursuit of pleasure.
Hypodopaminergic function stimulates cravings, which in turn affects attention to goals and maintenance of cognitive control needed for overriding compulsions to use drugs and the ability to make action plans and then monitor action [45]. With drug use there is a steady influx of DA, but it becomes the sole focus of the addict’s attention. The central goal, is obtaining more drugs. They are motivated by their craving for drugs, even though the drugs have long stopped providing pleasure. Victims of SUD are caught in a spiral of physical brain changes and the psychological consequences of those changes that lead to further physical and psychological changes and consequences.
For approximately one-third of Americans, DA is a key genetically induced deficient neurotransmitter resulting in aberrant craving behavior and excessive pleasure seeking. Finding ways to increase DA D2 density, instead of blocking dopaminergic function, may be the best strategy to unlock the elusive addiction riddle and attenuate abuse [34,46].
Certainly, new treatment and diagnostic (genetic) approaches are required in view of our most recent unpublished work derived from studies with CARD.™ We evaluated both compliance and abstinence during treatment using 5,838 specimens from 2,919 patients located in various treatment settings across six eastern states in years 2010 and 2011. Preliminary, we found compliance to prescribed medications in our sample during treatment to be 67.2% while 60.8% of these patients were found to be still abusing drugs. In Opiate Treatment Programs whereas 87.4% of 1298 patients were compliant to Buprenorphine only 53.1% were abstinent, as measured by the first and last urine samples. For Methadone, 91.6% of 693 patients were compliant but only 50.9% were abstinent, again as measured by the first and last urine samples [47].
Finally, for the first time, we are proposing a new paradigm shift called “Reward Deficiency Solutions System”™ that includes the coupling of:
Genotyping of individuals for candidate reward genes to determine stratification of genetic risk for all RDS behaviors (GARS)™ [48,49]
The use of natural D2 agonist therapy (e.g. KB220Z™) to activate dopaminergic pathways in the NAc (affecting abnormal craving) and other brain regions (affecting decision –making)
And the use of CARD™ during active recovery to assess compliance to prescribed treatment medications and abstinence from drugs of abuse.
These tools provide the clinician the means to engender better diagnosis and recovery rates. Further research in terms of reinforcement experiments in nonhuman animal models [50] and human trials will assist in promotion of these novel strategies for the early diagnosis, prevention, treatment and attenuation of relapse in RDS [51,52] including process addictions [53].
Acknowledgments
The authors appreciate the expert editorial input from Margaret A. Madigan. Marlene Oscar-Berman is the recipient of grants from the National Institutes of Health, NIAAA RO1-AA07112 and K05-AA00219 and the Medical Research Service of the US Department of Veterans Affairs.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of Interest
Kenneth Blum, PhD., holds a number of US and Foreign patents related to diagnosis and treatment of RDS, which has been exclusively licensed to LifeGen, Inc. Lederach, PA. Mary Houser is Vice President of Dominion Diagnostics Inc., and along with Lifegen, Inc., they are actively involved in the commercial development of GARS. Kenneth Blum, Thomas Simpatico, John Femino, are paid consultants of Dominion Diagnostics, Inc. John Giordano is also a partner in LifeGen, Inc. There are no other conflicts of interest and all authors read & approved the manuscript.
References
- 1.Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 2.Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- 3.Archer T, Oscar-Berman M, Blum K. Epigenetics in Developmental Disorder: ADHD and Endophenotypes. J Genet Syndr Gene Ther. 2011;2:1000104. doi: 10.4172/2157-7412.1000104. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–2060. [PubMed] [Google Scholar]
- 5.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 6.Blum K, Chen TJ, Morse S, Giordano J, Chen AL, et al. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D agonist therapy: part 2. Postgrad Med. 2010;122:214–226. doi: 10.3810/pgm.2010.11.2237. [DOI] [PubMed] [Google Scholar]
- 7.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum K, Wood RC, Braverman ER, Chen TJ, Sheridan PJ. The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes’ theorem. Funct Neurol. 1995;10:37–44. [PubMed] [Google Scholar]
- 9.Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, et al. The human dopamine D2 receptor gene is located on chromosome 11 at q22–q23 and identifies a TaqI RFLP. Am J Hum Genet. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- 10.Hauge XY, Grandy DK, Eubanks JH, Evans GA, Civelli O, et al. Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics. 1991;10:527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- 11.Blum K, Braverman ER, Wood RC, Gill J, Li C, et al. Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report. Pharmacogenetics. 1996;6:297–305. doi: 10.1097/00008571-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Blum K, Noble EP, Sheridan PJ, Finley O, Montgomery A, et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- 13.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 14.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg DT, Campbell B, Mackillop J, Lum JK, Wilson DS. Season of birth and dopamine receptor gene associations with impulsivity, sensation seeking and reproductive behaviors. PLoS One. 2007;2:e1216. doi: 10.1371/journal.pone.0001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Baler RD. Neuroscience. To stop or not to stop? Science. 2012;335:546–548. doi: 10.1126/science.1218170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective p factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND. Drug abuse and mental illness: progress in understanding comorbidity. Am J Psychiatry. 2001;158:1181–1183. doi: 10.1176/appi.ajp.158.8.1181. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Fowler JS, Wang GL. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dackis C, Gold MS. Neurotransmitter and neuroendocrine abnormalities associated with cocaine use. Psychiatr Med. 1985;3(4):461–483. [PubMed] [Google Scholar]
- 23.Hietala J, West C, Syvälahti E, Någren K, Lehikoinen P, et al. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- 24.Hietala J, Syvälahti E, Vuorio K, Någren K, Lehikoinen P, et al. Striatal D2 dopamine receptor characteristics in neuroleptic-naive schizophrenic patients studied with positron emission tomography. Arch Gen Psychiatry. 1994;51:116–123. doi: 10.1001/archpsyc.1994.03950020040004. [DOI] [PubMed] [Google Scholar]
- 25.Braverman ER, Blum K. Substance use disorder exacerbates brain electrophysiological abnormalities in a psychiatrically-ill population. Clin Electroencephalogr. 1996;27:5–27. doi: 10.1177/1550059496027s0402. [DOI] [PubMed] [Google Scholar]
- 26.Gintzler AR, Chakrabarti S. Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci. 2006;79:717–722. doi: 10.1016/j.lfs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 27.McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, et al. Genetic moderators of naltrexone’s effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006;30:1288–1296. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 28.Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007;9:E1–10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bromwell K, Gold MS. Food and Addiction: A comprehensive handbook. Oxford University Press; Oxford, England & New York, USA: 2012. [Google Scholar]
- 30.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 31.Boundy VA, Lu L, Molinoff PB. Differential coupling of rat D2 dopamine receptor isoforms were expressed in Spodoptera frugiperda moth caterpillar cells. J Pharmacol Exp Ther. 1996;276:784–794. [PubMed] [Google Scholar]
- 32.Boundy VA, Pacheco MA, Guan W, Molinoff PB. Agonists and antagonists differentially regulate the high affinity state of the D2L receptor in human embryonic kidney 293 cells. Mol Pharmacol. 1995;48:956–964. [PubMed] [Google Scholar]
- 33.Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blum K, Downs WB, Waite RL, Heaney WJ. Genetic Risk Analysis In Reward Deficiency Syndrome. 2012. [Google Scholar]
- 35.Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des. 2012;18:113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szybalska EH, Szybalski W. Genetics of human cell line. IV. DNA-mediated heritable transformation of a biochemical trait. Proc Natl Acad Sci U S A. 1962;48:2026–2034. doi: 10.1073/pnas.48.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–486. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, et al. Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. 2005;77:130–319. doi: 10.1016/j.lfs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 39.Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 40.Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114:159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71:223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Tian J, Yuan K, Liu P, Zhuo L, et al. Distinct resting–state brain activities in heroin-dependent individuals. Brain Res. 2011;1402:46–53. doi: 10.1016/j.brainres.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 43.Chen TJ, Blum K, Mathews D, Fisher L, Schnautz N, et al. Are dopaminergic genes involved in a predisposition to pathological aggression? Hypothesizing the importance of “super normal controls” in psychiatric genetic research of complex behavioral disorders. Med Hypotheses. 2005;65:703–707. doi: 10.1016/j.mehy.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 44.Oberlin BG, Dzemidzic M, Bragulat V, Lehigh CA, Talavage T, et al. Limbic responses to reward cues correlate with antisocial trait density in heavy drinkers. Neuroimage. 2012;60:644–652. doi: 10.1016/j.neuroimage.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 46.Blum K, Chen AL, Giordano J, Borsten J, Chen TJ, et al. The addictive brain: all roads lead to dopamine. J Psychoactive Drugs. 2012;44:134–143. doi: 10.1080/02791072.2012.685407. [DOI] [PubMed] [Google Scholar]
- 47.Blum K, Giordano J, Han D. Coupling the Genetic Addiction Risk Score (GARS), Comprehensive Analysis of Reported Drugs (CARD) and KB220Z showing reward circuitry activation of Dopaminergic pathways with KB220Z for in treatment of Reward Deficiency Syndrome (RDS): A Paradigm Shift. Keynote Presented at International Conference on Genetic Syndromes & Gene Therapy; November 19th; San Antonio, Texas. 2012. [Google Scholar]
- 48.Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, et al. Sex, drugs, and rock ‘n’ roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms. J Psychoactive Drugs. 2012;44:38–55. doi: 10.1080/02791072.2012.662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchis-Segura C, Grisel JE, Olive MF, Ghozland S, Koob GF, et al. Role of the endogenous opioid system on the neuropsychopharmacological effects of ethanol: new insights about an old question. Alcohol Clin Exp Res. 2005;29:1522–1527. doi: 10.1097/01.alc.0000174913.60384.e8. [DOI] [PubMed] [Google Scholar]
- 51.Blum K, Chen AL, Oscar-Berman M, Chen TJ, Lubar J, et al. Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: selecting appropriate phenotypes for reward dependence behaviors. Int J Environ Res Public Health. 2011;8:4425–459. doi: 10.3390/ijerph8124425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blum K, Payne JE. Alcohol & the Addictive Brain: New Hope for Alcoholics from Biogenetic Research. The Free Press Simon & Schuster, Inc; New York: 1991. [Google Scholar]
- 53.Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


