Abstract
There is a need for understanding and treating post-traumatic stress disorder (PTSD), in soldiers returning to the United States of America after combat. Likewise, it would be beneficial to finding a way to reduce violence committed by soldiers, here and abroad, who are suspected of having post-traumatic stress disorder (PTSD). We hypothesize that even before combat, soldiers with a childhood background of violence (or with a familial susceptibility risk) would benefit from being genotyped for high-risk alleles. Such a process could help to identify candidates who would be less suited for combat than those without high-risk alleles. Of secondary importance is finding safe methods to treat individuals already exposed to combat and known to have PTSD. Since hypodopaminergic function in the brain’s reward circuitry due to gene polymorphisms is known to increase substance use disorder in individuals with PTSD, it might be parsimonious to administer dopaminergic agonists to affect gene expression (mRNA) to overcome this deficiency.
Keywords: Post-Traumatic Stress Disorder (PTSD), Reward Deficiency Syndrome (RDS), Gene testing, Dopamine, KB220
Possible Genotyping for Risk of PTSD
Post-traumatic stress disorder (PTSD) is a well known prevalent anxiety disorder marked by behavioral, physiologic, and hormonal alterations. PTSD is disabling and commonly follows a chronic course. The etiology of PTSD is unknown, although exposure to a traumatic event constitutes a necessary, but not sufficient, factor. Although the biological underpinnings of immediate and protracted trauma-related responses are extremely complex, 40 years of research on humans and other mammals has demonstrated that trauma (particularly trauma early in the life cycle) has long-term effects on neurochemical responses to stressful events. These effects include the magnitude of the catecholamine response and the duration and extent of the cortisol response. In addition, a number of other biological systems are involved, including mesolimbic brain structures and various neurotransmitters. In contrast to other major psychiatric disorders, large studies have not been executed in which underlying genes for PTSD have been identified, but clues are emerging. Complementary approaches for locating involved genes include association-based studies employing case-control or parental genotypes for transmission disequilibrium analysis, and quantitative trait loci studies in nonhuman animal models. Our laboratory and many others are in the process of identifying susceptibility genes that will increase our understanding of traumatic stress disorders and help to elucidate their molecular basis. However, there are serious ethical and political concerns as to whether or not governments should genotype soldiers for specific genetic polymorphisms of any trait, including those linked to a high risk of PTSD exposed to traumatic events like combat.
PTSD Prevalence
Military conflicts inevitably produced war-related illness among our troops and veterans. Although the biological underpinnings of immediate and protracted trauma-related responses are extremely complex, 40 years of research on humans and other mammals have demonstrated that trauma has long-term effects on neurochemical responses to stressful events [1].
These effects include the magnitude of the catecholamine response and the duration and extent of the cortisol response. In addition, a number of other biological systems are involved, including mesolimbic brain structures and various other neurotransmitters. Understanding many of the genetic and environmental interactions contributing to stress-related responses has provided a map for the diagnosis and treatment of individuals vulnerable to PTSD (Table 1).
Table 1.
Genes and PTSD.
|
|
High rates of PTSD in veterans have been found throughout history. People have recognized that exposure to combat situations can negatively impact the mental health of those involved. Further it is well understood that there are high rates of Substance Use Disorder in veterans. The diagnosis historically referred to as “combat fatigue,” “shell shock,” or “war neurosis” originates from observations of the effect of combat on soldiers and the grouping of symptoms that are now used to characterize PTSD. Interestingly, with regard to Vietnam veterans, approximately 15% of men and 9% of women were found to have PTSD prior to combat with approximately 30% of men and 27% of women having PTSD at some point in their life following Vietnam. In the Persian Gulf war, PTSD prevalence was 9% to approximately 24% [2]. According to the Department of Veterans Affairs (VA) in June 2010, there were 171,423 deployed Iraq and Afghanistan war veterans diagnosed with PTSD, out of a total of 593,634 patients treated in VA facilities (www.va.gov).
Neurogenetic Abnormalities in PTSD
One study of Army soldiers screened up to four months after returning from deployment to Iraq showed that 27% met criteria for alcohol abuse and were at increased risk for related harmful behaviors (e.g., drinking and driving, using illicit drugs) [3]. In another study based on DRD2 allelic association, 35 PTSD patients with the A1(+) (A1A1, A1A2) allele consumed more than twice the daily amount of alcohol than the 56 patients with the A1(−) (A2A2) allele, and this difference was highly significant [4]. When the hourly rate of alcohol consumed was compared, A1(+) allelic patients consumed twice the rate of the A1(−) allelic patients [4].
Serotonin is an important neurotransmitter involved in brain reward circuitry, and serotonergic system dysfunction has been implicated in PTSD. Genetic polymorphisms associated with serotonin signaling may predict differences in brain circuitry involved in emotion processing and deficits associated with PTSD. In healthy individuals, common functional polymorphisms in the serotonin transporter gene (SLC6A4) have been shown to modulate amygdala and prefrontal cortex activity in response to salient emotional stimuli. Similar patterns of differential neural responses to emotional stimuli have been demonstrated in PTSD, but genetic factors influencing these activations have yet to be examined. Morey et al. [5] found that the SLC6A4 SNPs rs16965628 and 5-HTTLPR are associated with a bias in neural responses to traumatic reminders and cognitive control of emotions in patients with PTSD.
The opioid receptor mu-1 (OPRM1) gene may play a role in both PTSD and alcohol use. Nugent et al. [6] examined the association between PTSD and drinking motives as well as variation in the OPRM1 as a predictor of both PTSD and drinking motives in a sample of 201 persons living with HIV (PLH) reporting recent binge drinking. Self-reported PTSD symptom severity was significantly associated with drinking motives for coping, enhancement, and socialization. OPRM1 variation was associated with decreased PTSD symptom severity as well as enhancement motives for drinking.
GABAergic systems have been implicated in the pathogenesis of anxiety, depression, and insomnia. These symptoms are part of the core and comorbid psychiatric disturbances in PTSD. Feusner et al. [7] concluded in a population of PTSD patients, heterozygosity of the GABRB3 major (G1) allele confers higher levels of somatic symptoms, anxiety/insomnia, social dysfunction and depression than found in homozygosity.
Variations in genes related to the dopaminergic pathway have been implicated in neuropsychiatric disorders such as schizophrenia, substance misuse, Alzheimer’s disease and PTSD. In one particular study, Voisey et al. [8] reported that the 957C>T polymorphism in the D2 dopamine receptor (DRD2) gene is one of the genetic factors for susceptibility to PTSD. Lawford et al. [9] identified clusters of patients with PTSD according to symptom profile and examined the association of the A1 allele of the DRD2 gene with these clusters. They found that veterans with the DRD2 A1 allele — compared to those without this allele — were significantly more likely to be found in the high than the low psychopathology cluster group. In agreement with those findings, Comings et al. [10] suggested that a DRD2 variant in linkage disequilibrium with the D2A1 allele confers an increased risk to PTSD, and the absence of the variant confers a relative resistance to PTSD. Moreover, Valente et al. [11] found a statistical association between DAT1 3’UTR VNTR nine repeats and PTSD (OR = 1.82; 95% CI, 1.20–2.76). This preliminary result confirmed previous reports supporting a susceptibility role for allele 9 and PTSD.
van Zuiden et al. [12] investigated whether glucocorticoid receptor (GR) pathway components assessed in leukocytes before military deployment represent preexisting vulnerability factors for development of PTSD symptoms. In predeployment high GR number, low FKBP5 mRNA expression, and high GILZ mRNA expression were independently associated with increased risk for a high level of PTSD symptoms. Childhood trauma also independently predicted development of a high level of PTSD symptoms. Additionally, the investigators observed a significant interaction effect of GR haplotype BclI and childhood trauma on GR number.
Nuclear factor-κB (NF -κB) is a ubiquitously expressed transcription factor for genes involved in cell survival, differentiation, inflammation, and growth. Cohen et al. examined the role of NF-κB pathway in stress-induced PTSD-like behavioral response patterns in rats. Extreme behavioral responder animals displayed significant upregulation of p50 and p65 with concomitant downregulation of I-κBα, p38, and phospho -p38 levels in hippocampal structures, compared with minimal behavioral responders and controls. Immediate post-exposure treatment with high-dose corticosterone and a selective NF-κB inhibitor (pyrrolidine dithiocarbamate) significantly reduced prevalence rates of extreme responders and normalized the expression of those genes. The authors suggested that stress-induced upregulation of NF-κB complex in the hippocampus may contribute to the imbalance between what are normally precisely orchestrated and highly coordinated physiological and behavioral processes, thus associating it with stress-related disorders.
Testing Genetic Antecedents
Our laboratory, proposed in a recent article [13] that successful treatment of PTSD will involve preliminary genetic testing for specific polymorphisms. Due to the complex etiology of PTSD, for which experiencing a traumatic event results in a specific phenotype, it may be difficult to identify even via genetic analysis. The consensus of the literature suggests that interactions between different genes (e.g. DRD2 and DAT1 genes) and between them and the environment make certain people vulnerable to developing PTSD [14]. Certainly gene-environmental studies are needed that focus more narrowly on specific, distinct endophenotypes and on influences from environmental factors. However testing for polymorphisms of genes that have already been found to associate with PTSD will provide a powerful tool to diagnose suspected veterans after deployment. Results of our research suggest the following genes should be tested: serotoninergic, dopaminergic (DRD2, DAT, DBH), glucocorticoid, GABAergic (GABRB), apolipoprotein systems (APOE2), brain-derived neurotrophic factor, Monamine B, CNR1, Myo6, CRF-1 and CRF-2 receptors, and neuropeptide Y (NPY) [15].
Natural Dopaminergic Activation
Early detection is especially important, because early treatment can improve outcome and potentially reduce vulnerability to PTSD prior to exposure to trauma, as well as help to heal those who have developed PTSD symptomatology. When genetic testing reveals deficiencies, vulnerable individuals can be recommended for treatment with “body friendly” [16] pharmacologic substances and/or nutrients. One goal of our research has been to develop a treatment that would up-regulate the expression of reward genes in order to bring about a feeling of well being (e.g., by enhancing dopaminergic function) as well as a reduction in the frequency and intensity of the symptoms of PTSD. Specifically, we developed KB220Z a natural dopaminergic activator having not only important anti-stress properties but anti-craving actions as well. Our published research [17] has consistently shown anti-anxiety effects of KB220 variant in double-blinded placebo controlled studies in highly addicted subjects (Figure 1).
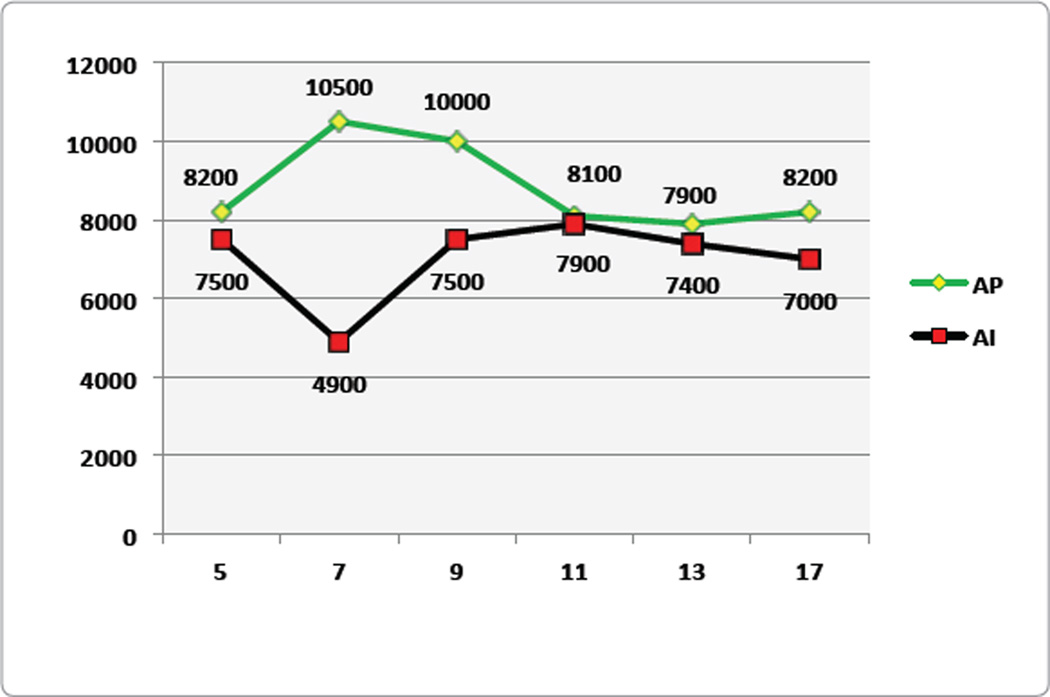
Figure 1.
Skin conductance levels for Placebo (AP, n = 12) and Experimental (AI, n=15) groups. (Modified from Blum et al., 2009, with permission.)
Figure 1 shows the skin conductance values for the KB220 polydrug (AI) and placebo (AP) groups. On Day 7, the difference between the groups was significant (p < 0.025; within groups, p < 0.025). After the effect of the first seven days, the striking finding was that the curves for the two groups mirrored one another, although the KB220 groups are lower. This may indicate a commonality in response to the dynamics of the treatment program, or it may indicate characteristic changes in the population with recovery groups. In contrast, distinctive changes occurred within groups. By Day 9, the polydrug-KB220 group showed a marked time-dependent significant improvement. At Day 13, a second significant change occurred with respect to days 11, 17, and 21. In contrast to the AP group, the AI group showed a significant change, which occurred later at Day 13, and continued through Day 21.
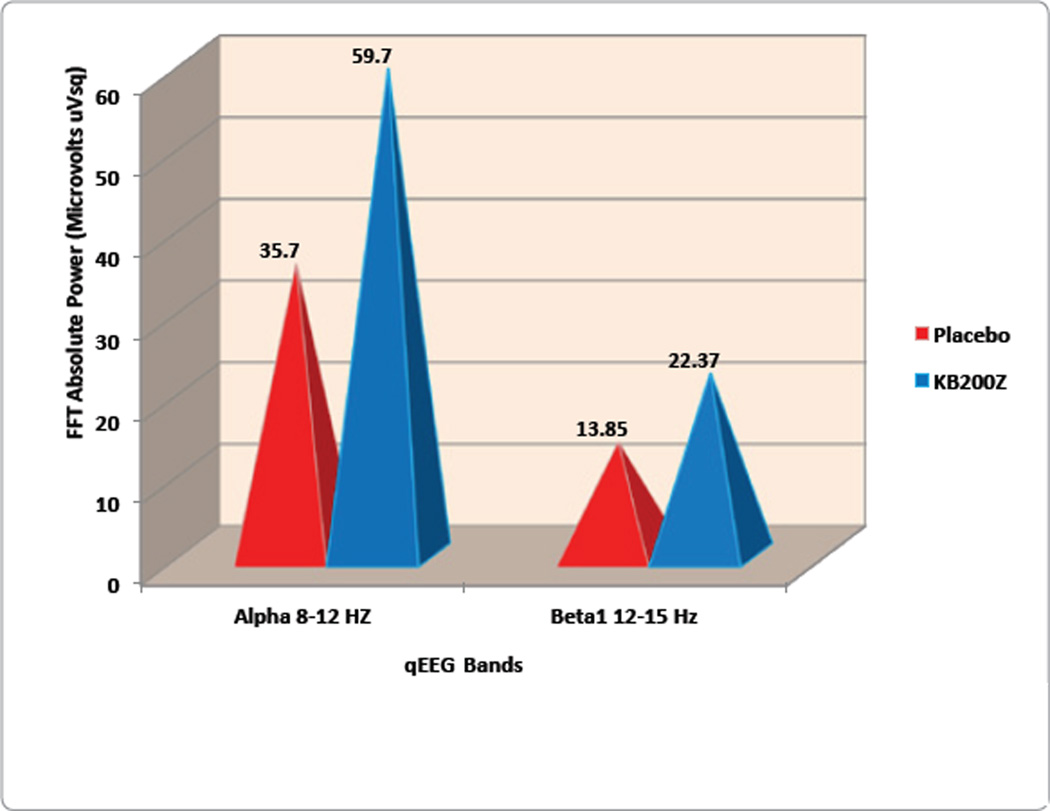
In an attempt to overcome qEEG abnormalities in cohort of abstinent psycho stimulant abusers, our laboratory studied the effects of KB220Z [18]. Positive outcomes were demonstrated by qEEG imaging in a randomized, triple-blind, placebo-controlled, crossover study involving oral KB220Z. Results showed an increase of alpha waves and low beta wave activity in the parietal brain region (Figure 2). Significant differences observed between placebo and KB220Z™ consistently occurred in the frontal regions after Week 1, and then again after Week 2 of analyses. This is the first report to demonstrate involvement of the prefrontal cortex in the qEEG response to a natural putative D2 agonist (KB220Z™), especially evident in individuals with the dopamine D2 A1 allele.
Figure 2.
qEEG Study: KB220Z compared to placebo in psychostimulant addicts. (Blum et al. IIOAB Letters 1(1) 2011; derived from Blum et al., Postgraduate Medicine, 2010.).
Our Proposal
An understanding of the many genetic and environmental interactions contributing to stress-related responses will provide a diagnostic and treatment map, which will illuminate the vulnerability and resilience of individuals to PTSD. We propose that successful treatment of PTSD will involve preliminary genetic testing for specific polymorphisms. Early detection is especially important, because early treatment can improve outcome. When genetic testing reveals deficiencies, vulnerable individuals can be recommended for treatment with “body friendly” pharmacologic substances and/or nutrients. Consensus suggest the following genes should be tested: serotoninergic, dopaminergic (DRD2, DAT, DBH), glucocorticoid, GABAergic (GABRB), apolipoprotein systems (APOE2), brain-derived neurotrophic factor, Monamine B, CNR1, Myo6, CRF-1 and CRF-2 receptors, and neuropeptide Y (NPY). Treatment in part should be developed that would up-regulate the expression of these genes to bring about a feeling of well being, as well as a reduction in the frequency and intensity of the symptoms of PTSD.
Conclusions
Soldiers involved in combat consistently show high rates of PTSD. There has been some difficulty in accurately diagnosing the true endophenotype of PTSD in returning veterans [19]. We propose that by incorporating genetic testing of a number of reward gene polymorphisms already known to associate with PTSD [13], diagnosis will become more evident and accurate. Additionally, we propose that the existing qEEG abnormalities observed in PTSD, which are further enhanced with increasing substance use disorders in individuals with PTSD, can be attenuated by the administration of the natural dopaminergic activator KB220Z, a promising genetically designed therapeutic safe substance. Our proposal is based on over 27 clinical trials with KB220 variants (a key ingredient in SynaptaGenX ™ distributed by Nupatways, Indianapolis, Indiana). KB220 variants have documented effects in overcoming qEEG abnormalities in protracted abstinent psychostimulant abusers, and documented anti anxiety effects as well. Thus, after confirmatory large clinical trials including neuroimaging, KB220Z may be confirmed as a helpful adjunctive intervention for PTSD, because it not only activates dopaminergic pathways, but it also potentially could enhance dopamine D2 receptor density, thereby attenuating the negative behavioral effects of subjects diagnosed with PTSD.
Acknowledgements
Support for the writing of this paper came from the US Department of Veterans Affairs Medical Research Service and NIAAA grants R01-AA07112 and K05-AA00219 to MO-B. Additional funding came from grants awarded to PATH Research Foundation from LifeExtension Foundation, LifeGen Inc., Lederach, PA, USA. and Dominion Diagnostics, Inc. Thomas Simpatico is the recipient of SAMHSA Award #SM058809: Veteran’s Jail Diversion & Trauma Recovery Grant.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of Interest
Kenneth Blum is an officer and stock holder of LifeGen, Inc., Lederach, PA, USA. LifeGen Inc. is the worldwide exclusive distributor of products related to patents concerning Reward Deficiency Syndrome. John Giordano is a LifeGen partners.
References
- 1.Miller G. The invisible wounds of war. Healing the brain, healing the mind. Science. 2011;333:514–517. doi: 10.1126/science.333.6042.514. [DOI] [PubMed] [Google Scholar]
- 2.Tull M. About.com Guide. 2009 Jul 22; 2009. [Google Scholar]
- 3.Beevers CG, Lee HJ, Wells TT, Ellis AJ, Telch MJ. Association of predeployment gaze bias for emotion stimuli with later symptoms of PTSD and depression in soldiers deployed in Iraq. Am J Psychiatry. 2011;168:735–741. doi: 10.1176/appi.ajp.2011.10091309. [DOI] [PubMed] [Google Scholar]
- 4.Young RM, Lawford BR, Noble EP, Kann B, Wilkie A, et al. Harmful drinking in military veterans with post-traumatic stress disorder: association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002;37:451–456. doi: 10.1093/alcalc/37.5.451. [DOI] [PubMed] [Google Scholar]
- 5.Morey RA, Hariri AR, Gold AL, Hauser MA, Munger HJ, et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in post-traumatic stress disorder. BMC Psychiatry. 2011;11:76. doi: 10.1186/1471-244X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugent NR, Lally MA, Brown L, Knopik VS, McGeary JE. OPRM1 and Diagnosis-Related Post-traumatic Stress Disorder in Binge-Drinking Patients Living with HIV. AIDS Behav. 2011 doi: 10.1007/s10461-011-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feusner J, Ritchie T, Lawford B, Young RM, Kann B, et al. GABA(A) receptor beta 3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry Res. 2001;104:109–117. doi: 10.1016/s0165-1781(01)00296-7. [DOI] [PubMed] [Google Scholar]
- 8.Voisey J, Swagell CD, Hughes IP, Morris CP, van Daal A. The DRD2 gene 957C>T polymorphism is associated with post-traumatic stress disorder in war veterans. Depress Anxiety. 2009;26:28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- 9.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to post-traumatic stress disorder: a study and replication. Biol Psychiatry. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 11.Valente NL, Vallada H, Cordeiro Q, Miguita K, Bressan RA, et al. Candidate-gene approach in post-traumatic stress disorder after urban violence:association analysis of the genes encoding serotonin transporter, dopamine transporter, and BDNF. J Mol Neurosci. 2011;44:59–67. doi: 10.1007/s12031-011-9513-7. [DOI] [PubMed] [Google Scholar]
- 12.van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, et al. Glucocorticoid receptor pathway components predict post-traumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2011;71:309–316. doi: 10.1016/j.biopsych.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Bowirrat A, Chen TJ, Blum K, Madigan M, Bailey JA, et al. Neuropsychopharmacogenetics and Neurological Antecedents of Post-traumatic Stress Disorder: Unlocking the Mysteries of Resilience and Vulnerability. Curr Neuropharmacol. 2010;8:335–358. doi: 10.2174/157015910793358123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey JN, Goenjian AK, Noble EP, Walling DP, Ritchie T, et al. PTSD and dopaminergic genes, DRD2 and DAT, in multigenerational families exposed to the Spitak earthquake. Psychiatry Res. 2010;178:507–510. doi: 10.1016/j.psychres.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Blum K, Fornari F, Downs BW, Wait RL, Giordano J, et al. Genetic Addiction Risk Score (GARS): Testing for polymorphic predisposition and risk to Reward Deficiency Syndrome(RDS), Chapter 19. In: King C, editor. Gene Therapy Applications. 2011. InTech, Rijeka, Croatia. [Google Scholar]
- 16.Downs BW, Chen AL, Chen TJ, Waite RL, Braverman ER, et al. Nutrigenomic targeting of carbohydrate craving behavior: can we manage obesity and aberrant craving behaviors with neurochemical pathway manipulation by Immunological Compatible Substances (nutrients) using a Genetic Positioning System (GPS) Map? Med Hypotheses. 2009;73:427–434. doi: 10.1016/j.mehy.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum K, Chen A, Chen TJH, Bowirrat A, Waite RL. Putative Targeting of dopamine D2 receptor function in reward deficiency syndrome (RDS) by Synapatmine Complex™ Variant (KB220): Clinical trial showing anti-anxiety effects. Gene Ther Mol Biol. 2009;13:214–230. [Google Scholar]
- 18.Chen TJ, Blum K, Chen AL, Bowirrat A, Downs WB, et al. Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptagen: proposing an addiction candidate gene panel map. J Psychoactive Drugs. 2011;43:108–127. doi: 10.1080/02791072.2011.587393. [DOI] [PubMed] [Google Scholar]
- 19.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin MM, Parisi JA, Gold MS, Gold AR. War-related illness symptoms among Operation Iraqi Freedom/Operation Enduring Freedom returnees. Mil Med. 2010;175:155–157. doi: 10.7205/milmed-d-90-00153. [DOI] [PubMed] [Google Scholar]
- 21.Braverman ER, Blum K. Substance use disorder exacerbates brain electrophysiological abnormalities in a psychiatrically-ill population. Clin Electroencephalogr. 1996;27:5–27. doi: 10.1177/1550059496027s0402. [DOI] [PubMed] [Google Scholar]
- 22.Jokić -Begić N, Begić D. Quantitative electroencephalogram (qEEG) in combat veterans with post-traumatic stress disorder (PTSD) Nord J Psychiatry. 2003;57:351–355. doi: 10.1080/08039480310002688. [DOI] [PubMed] [Google Scholar]