Abstract
Extracellular single unit activity was recorded from medial prefrontal cortex (mPFC) of postpartum dams over the course of 3 days while they engaged in spontaneous pup-directed behaviors and non-specific exploratory behavior. Out of 109 units identified over the course of the experiment, 15 units were observed to be pup-responsive and 15 increased their discharge rates non-specifically while not attending to pups. An association between neuronal activity and typical maternal behaviors (e.g., retrieval, pup-grooming, nursing) was not observed. Instead, brief bouts of snout contact with pups were accompanied by phasic increases and decreases in spike rates. The observed pup contact responsive cells might play a role in processing of sensory feedback from pups or the transmission of modulatory output to other subcortical maternal brain areas.
Keywords: prelimbic area, medial prefrontal cortex, neuronal firing rate, neuronal activity, maternal behavior, rat, motivation, goal-directed behavior, pups, open field behavior, excitatory neurons, glutamatergic neurons, principal cells, retrieval, rearing
1. INTRODUCTION
There is evidence that the cortex plays a role in specific aspects of maternal care, although some studies have emphasized the role of somatosensory and olfactory cortical networks in maternal care, lactation and maternal aggression instead of other neocortical sites (Afonso et al., 2007; Lonstein and Stern, 1997; Stern and Kolunie, 1993; Xerri et al., 1994). Several studies have reported that midline cortical areas (Slotnick, 1967; Slotnick and Nigrosh, 1975; Stamm, 1955), particularly the medial prefrontal region (mPFC)(Afonso et al., 2007), are important for the expression of maternal behaviors. Gathering pups within a nest requires intact midline cortical areas, whereas lesions in lateral cortical regions had no effect (Stamm, 1955). Retrieval behavior is especially sensitive to severing of the anterior cingulate and mPFC (Slotnick and Nigrosh, 1975). Maternal rats showing place preference for pups express increased levels of c-fos in infralimbic and anterior cingulate areas (Mattson and Morrell, 2005). However, mothers preferring cocaine-associated chambers show greater overall prefrontal cortical c-fos immunoreactivity, thus providing a neural substrate where hedonic stimuli compete for the mothers’ attention and motivation (Mattson and Morrell, 2005). Afonso et al. (2007) have shown that selective lesioning of the mPFC prior to pregnancy leads to reductions in sexual motivation and impairment in postpartum retrieval behavior. This has been further supported by recent work from our laboratory showing that tetrodotoxin inactivation or GABA-mediated inhibition of the mPFC results in a dramatic reduction maternal retrieval behavior (Febo et al., 2010). Rodent neuroimaging studies have reported increases in blood oxygen level dependent (BOLD) signal in the mPFC and orbital PFC of lactating rats responding to suckling stimulation from pups (Febo et al., 2005; Febo and Ferris, 2007; Ferris et al., 2005). Human functional MRI studies also have implicated prefrontal regions with maternal care (Bartels and Zeki, 2004; Lorberbaum et al., 2002; Nitschke et al., 2004; Ranote et al., 2004; Strathearn et al., 2008), therefore substantiating a role of this cortical region in primates as well.
The mPFC and other associated prefrontal areas are involved in a range of cognitive functions, and also share connectivity with subcortical areas controlling motivated behavior (Berendse et al., 1992; Floyd et al., 2001; Gabbott et al., 2005; Hoover and Vertes, 2007; Kita and Kitai, 1990; Vertes, 2002; Vertes, 2004). Synaptic targets for the various prefrontal areas and mPFC include subregions of the hypothalamus, midbrain, amygdala and the mesolimbic system (Gabbott et al., 2005; Vertes, 2004). The ventral and dorsal striatum, VTA, basolateral amygdala, septum and PAG, for example, receive mPFC input (Gabbott et al., 2005). In addition, the mPFC receives prominent inputs from mesencephalic dopamine neurons (Sesack et al., 1998); therefore, it might be part of a set of distributed neuronal populations that exert some form of modulatory control over the expression of maternal care towards pups. The present study tested whether there are, in fact, pup responsive neurons in the mPFC of maternal rats. Single unit activity was measured in mPFC of dams while they carried out typical patterns of postpartum maternal behavior. The present work was a first attempt to directly detect neurons within the mPFC of maternal rats and describe their relation to maternal responding.
2. MATERIALS AND METHODS
2.1 Subjects
Adult Long-Evans female rats (225–275 g; 70–102 days old; Charles River Laboratories, Wilmington, MA) were housed in pairs in a temperature-humidity and light:dark cycle controlled room (lights on 0700 hr–1900 hr). Water and Purina rat chow were provided ad libitum. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (7th Edition, 1996) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study.
2.2 Electrode implantation
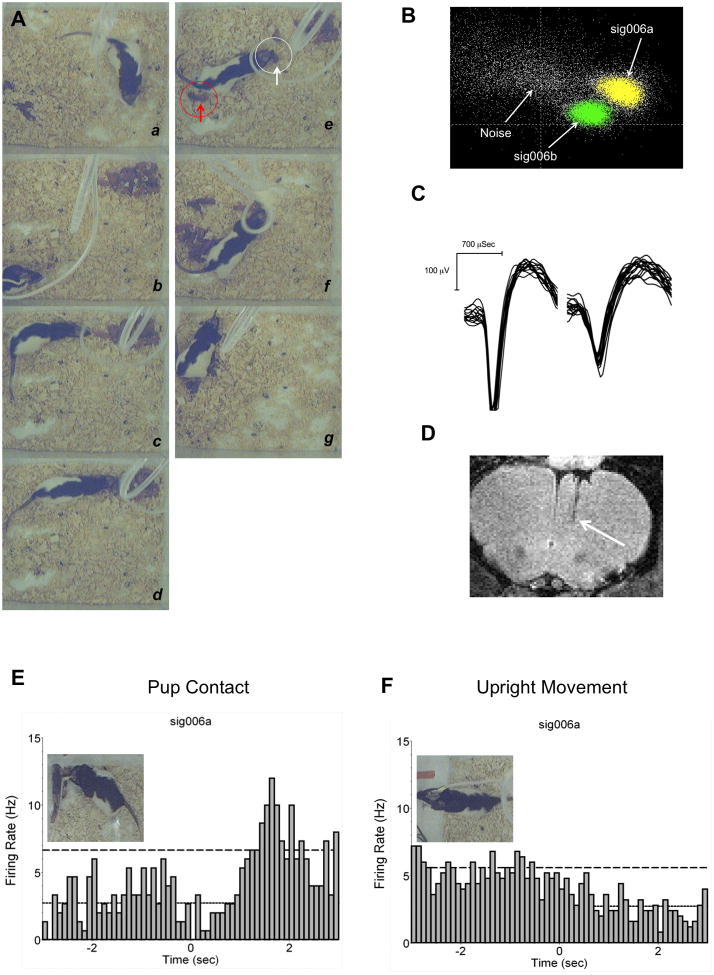
Neuronal recordings were carried out in 9 freely moving female rats. Females were housed with sexually experienced male rats for 5 days and then housed individually during pregnancy. Surgical procedures were carried out on postpartum days 2 and behavioral testing began 3–4 days later. The shorter recovery time allowed the early detection of units. For surgeries, rats were anesthetized with 2–4% isoflurane gas/air mixture and aligned on the stereotaxic apparatus (Kopf instruments, Tujunga, CA). Anesthesia and body temperature were maintained throughout the surgery. Microwire array electrodes were purchased from two companies, 2 from Neurolinc Corp. (New York, NY) and 7 from Plexon Inc. (Dallas, TX), but were constructed with exactly the same specifications (see (Nicolelis et al., 1997) for details of design). These consisted of eight Teflon-coated stainless steel wires with a 50 μm outer diameter arranged in a 2 × 4 array, which served to as recording channels. Overall impedance of the wires ranged from 0.3–1.2 MΩ at 1kHz (impedance check unit, FHC, Bowdoin, ME). Wires were arranged with 50 μm spacing between 4 rows and 250 μm between 2 columns. Electrodes ends were blunt and cut to insertion length and the tips extended 1–2 mm beyond a coating of polyethylene glycol, which stiffened the wires and aided their penetration through the cortical surface. The other end of the 8 electrode wires were soldered to the pins of a 10-pin Omnetics connector and coated with a protective layer of Epoxy. The remaining 2 pins were used as ground wires (1 inactive channel per array was chosen as a reference to subtract chewing artifacts using Plexon ref2 software). The skin overlying the incision site was cleansed using iodine solution, isopropyl alcohol and the skull area dried free of blood using sterile bone wax. Electrodes were slowly advanced through a 2 × 2 mm skull opening overlying the right mPFC. The dura was resected using a sterile 27 gauge sterile need bent at its tip. Warm sterile saline and sterile Gelfoam was used to bath the exposed cortical surface and maintain its humidity, while also helping dissolve the polyethylene glycol. Electrodes were secured on a stereotaxic holder (David Kopf Instruments) and slowly lowered manually into the mPFC (Bregma coordinates AP: +3.2 mm to +3.3, ML: −0.5 to −0.75 mm and DV: −4 mm for upper to −5 mm for deeper mPFC; intraraural line was set at −3.4 mm and flat vertical positioning was verified by sliding the anterior posterior micromanipulator along the surface of the skull) using a Kopf Model 1760 micromanipulator at slow 100–400 micron steps per 1–2 minutes. Once in place, Gelfoam was used to cover the skull opening surrounding the implanted wires. Electrodes were then anchored to the skull with dental cement and 4 miniature stainless steel self-tapping screws (Thread 0.06 in. 1/8 in. length; J.I. Morris Precision Screws, Southbridge, MA). A ground wire was secured around 2 screws that made contact internally with the cortical surface. Rats were given 3–4 days of recovery before recordings. Electrode placement was verified by ex vivo T2 weighted anatomical MRI scans on a 7T Bruker USR system (Fig. 1D).
Figure 1.
In vivo electrophysiology in awake behaving postpartum rats. A) Digital still frames collected during an actual experiment. Sequence of behaviors observed as follows: a – baseline exploration and activities before pups, b– pups placed in corner of cage opposite to area where dam is huddled, c – approach towards pups that can result in simple mouthing, sniffing or, d – picking up and retrieving a pup. e - shows an actual pup being retrieved (white circle and arrow) and one already in the nest (red circle and arrow). In f all pups are in the nest and the last pup is being retrieved. g, lactation. B) Clustering in PCA feature space was used to detect units on single wires. First and second principal components were used. Shown are 2 units and noise cluster. C) waveform amplitudes exceeded the cut-off threshold of 60 μV. D) Anatomical MRI scans were used to locate microwire placement sites. Arrow indicates the site where electrode tips were observed. E–F) Single unit firing of a representative neuron in the medial prefrontal cortex during pup contact. Images show examples of upright movement and brief pup contact. Perievent time histogram are for a neuron during pup contact and upright movement. Spike counts binned at 100 msec.
2.3 Single unit recordings
In vivo recordings of awake behaving maternal rats was carried out in a clear Plexiglas arena (42 cm L × 42 cm W × 30 cm H) containing the rats’ home cage bedding. Rats were tested across three consecutive daily sessions, once clearly defined units were detected on at least one of the recording channels. The chamber was located inside a custom designed sound attenuation box (30′ H × 27′ L × 23′ W, Med-Associates, St. Albans, VT) that had independently controllable lighting and circulating air. The sound attenuation box ceiling accommodated an electrical commutator device near a digital camera centralized over the test arena. The entire test environment was placed inside a large Benchtop Faraday cage (TMC, Peabody, MA), made with a stainless steel base plate and surrounded by copper wire mesh to minimize electrical interference (60-Hz noise) during recordings. On each recording session, rats were given a 20–30 min baseline acclimatization period followed by 20 minutes of baseline recording and 40–80 minutes of recordings after the pups were placed in to the cage. Neural signals from individual wires were amplified by an 8-channel operational-amplifier headstage mounted on to the electrode connector pins on the animals head before reaching a 16-channel preamplifier box (Plexon Inc., Dallas, TX). Here, the neural signals were further amplified (100x gain) and filtered (150 Hz–9 kHz). Additional filtering, amplification and analog-to-digital conversion was carried out using a multichannel acquisition processor (MAP, Plexon, Dallas, TX) controlled from the MAP server installed externally on a Pentium Core 2 Duo CPU. The MAP box generated an internal 40kHz clock signal that synchronized neural and behavior signals acquired from a high-speed digital camera (acquisition speed of 30 frames per second; Model DFK 21F04, The Imaging Source, Charlotte, NC) and behavioral software suite run independently on another CPU (CinePlex, Plexon, Dallas, TX).
2.4 Single unit detection and sorting
Online waveform detection was carried out using Sort Client (Plexon), with the assistance of 2 oscilloscopes (Model HM507, Hameg, Germany) that allowed an independent visualization of broadband signal, waveform amplitude and background noise and an audio monitor to help identify spikes on each channel individually (Model 3300 A-M Systems, Carlsborg, WA). A 50–60 μV waveform amplitude cutoff was applied, along with a 1.2ms refractory interspike interval threshold, and the waveform window view was set at 1.2–1.4ms. On each individual channel, a sample of 500 waveforms was collected and online principal components analysis (PCA) algorithm based on waveform features was performed (Wheeler, 1999). Spikes were sorted online using voltage-time windows (Nicolelis et al., 1997) and classification of individual units per channel was confirmed online from their clustering in 2D PCA space (Fig. 1B). The waveform detection parameters were stored on the first day of recordings, then updated and used for each subsequent session. Further offline classification of spikes was carried out by importing digitally recorded neural data to Offline Sorter and WaveTracker (Plexon Inc.). A template matching routine was used to drop waveforms that did not fall under the user-specified fit tolerance for waveform shape and amplitude [(Nicolelis et al., 1997); Fig. 1C]. The fit tolerance adjustment was guided by visualizing the first 2 principal components in PC space, interspike interval (ISI) histograms and autocorrelograms. The latter two statistical methods allowed us to discriminate units based on their absolute refractory periods and the peaks of interspike delays in firing, which generally occurred at 10–100 ms. Post hoc inspection of sorted spikes to confirm consistency across session was done using WaveTracker software (Plexon Inc., Dallas, TX).
2.5 Time-stamp generation
Digital video streams were acquired at 30 Hz and synchronized with neural signals. Several maternal behavior events were identified by a trained observer and time-stamped using Cineplex markup software. During the 40–80 min pup period, mothers mostly spent the first half showing frequent bouts of retrieving, mouthing, sniffing pups, pushing nesting material and the second half hovering, crouching over pups, or sleeping. Time-stamped behaviors included, initial pup exposure, initial contact with pups, pup mouthing, picking up pups during retrieval, grooming, nesting and non-specific upright movement (rearing activity) as a control behavior. Baseline, pup and nursing intervals were also identified. Care was taken to add a timestamp at the starting video frame during the initiation of each of the maternal behavior events (Fig. 1A). The number of behavioral markers for any given behavior was not fixed and varied across sessions. Timestamps for the classified spikes and behavioral event data were then exported to Neuroexplorer for single unit analysis.
2.6 Statistical analysis
Neural datasets were imported into Neuroexplorer to generate perievent time histograms (PETH) and raster plots that aligned spike timestamps around specified maternal behavioral events. This allowed inspection of unit firings around the time of behavior expression across all sessions independently. Spikes were binned at 100 msec intervals (unless otherwise specified in graphs) and analyzed around a 6 second window flanking the behavioral event of interest. Significant changes in firing rates were considered above or below 99% confidence intervals (2 or more spikes surpassing 99% C.I.). Unit firing rates were converted to Z-scores as an additional method of detecting significant changes in unit firing (spikes were considered significant if above or below 2.58 standard deviations). Significant changes in firing rates of single units for the different behavioral events were confirmed by a Wilcoxon matched pairs test to compare baseline vs. specific epochs of maternal behavior. Two-way repeated measures analysis of variance (ANOVA, α < 0.05) with a Bonferroni posthoc test was used to compare pup responsive (pup contact) and rearing responsive (upright movement) units. Data for the 3 recording days were analyzed separately. Firing characteristics of individual units were estimated separately across test days for a 300 second baseline, pup, retrieval and nursing intervals. All behavior, single unit and population statistical analysis was done using Statistical Package for the Social Sciences (SPSS, Chicago, IL) and GraphPad Prism (La Jolla, CA).
3. RESULTS
3.1 Electrode implantation, single unit features and variations in maternal behavior across subjects
Fig. 1D shows a representative MRI scans indicating placement of electrodes into mPFC. Electrodes were mostly observed to be within deeper layers (IV–V) of the prelimbic region. Peak interspike delays around 10–20 ms were observed from autocorrelograms and ISI histograms, with mean firing rates of 1.4 ± 0.8S.D. spikes per second (range: 0.2Hz–5.3Hz). No high frequency units with firing rates above 10Hz were observed. The examined population of units is thus assumed to be mostly regular spiking cells (Jung et al., 1998). Waveform amplitudes with a signal-to-noise ratio above 2 to 1 were around 60 μV–270 μV (Fig. 1C). Statistically significant differences in mean firing rates were not observed between baseline, pup and lactation epochs (300 second sample each; Table 1). A total of 109 units were detected over the course of 3 days. The range of units (active wires) per animal was 4–27, thus yields were uneven. Units that were detected more than once over the course of the 3-day tests were used only once, during the first trial. Identification of these units was aided by their firing characteristics and detection on the same wires over several experiment days. Six out of the nine rats yielded units responsive to pup contact (showed significant increases or decreases in firing rates) and upright movements (rearing activity). Therefore, the data are presented for 6 out of 9 animals although the total unit count takes into consideration those detected across all subjects.
Table 1.
Firing characteristics of single units recorded from mPFC of lactating rats. Data are binned for 300 seconds per rat for each behavioral period. Number of units varies according to whether or not rats expressed retrieval or lactation during testing. All data presented as mean ± standard error.
| ISI Peak Spikes (ms) | Mean Frequency (Sp/s) | Mean ISI (ms) | # of Bursts | % Spikes in Bursts | Bursts per Minute | # of Units | |
|---|---|---|---|---|---|---|---|
| Baseline | 17.1 ± 2.3 | 1.3 ± 0.1 | 2.2 ± 0.5 | 17.7 ± 3.6 | 8.7 ± 1.0 | 3.5 ± 0.7 | 66 |
| Pups | 14.5 ± 2.3 | 1.5 ± 0.2 | 1.9 ± 0.4 | 21.4 ± 4.8 | 8.1 ± 1.0 | 4.3 ± 1.0 | 66 |
| Retrieval | 20.0 ± 4.4 | 1.2 ± 0.3 | 1.8 ± 0.5 | 14.0 ± 6.5 | 7.9 ± 1.5 | 3.2 ± 1.3 | 28 |
| Lactation | 17.1 ± 2.7 | 1.4 ± 0.2 | 1.4 ± 0.3 | 22.7 ± 5.0 | 9.8 ± 1.3 | 4.5 ± 1.0 | 38 |
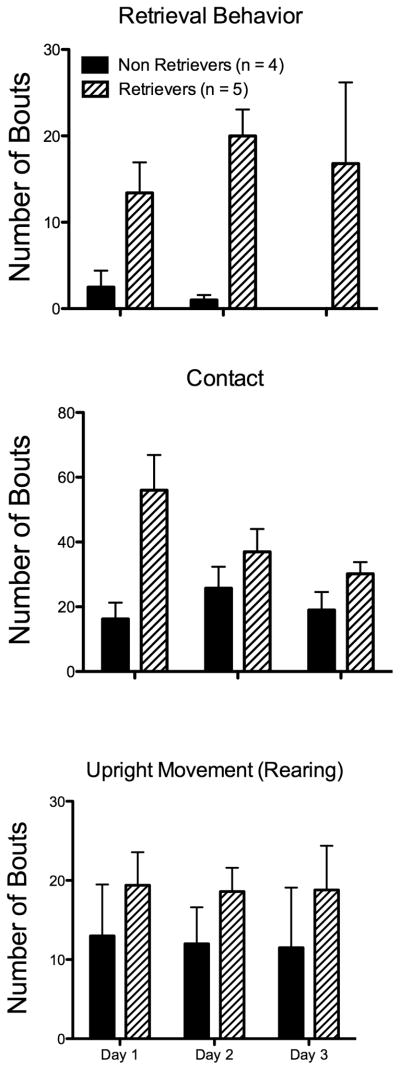
There was significant variation in maternal behaviors over the 3 trials. It was observed that a group of rats showed consistent levels of retrieval behavior over sessions (n = 5), while another subgroup consistently did not show retrieval (n = 4)(Fig. 2). However, the establishment of brief snout contact with pups (similar to that reported previously by (Lonstein and Stern, 1997) and upright rearing were observed to be consistent across animals (Fig. 2). For pup contact behavior, mothers would clearly establish some form of snout contact on a specific pup. It was easily distinguishable from prolonged licking behavior, but given the overlying angle of the videos brief sniffing and mouth contact were not distinguishable and were thus grouped.
Figure 2.
Maternal behavior in the test cage environment. Top, animals were grouped according to the expression of retrieval behavior. Four out of 5 rats did not retrieve pups and therefore this behavior could not be used to generate timestamps for the neuronal analysis. Middle, rats showed significant amounts of spontaneous pup contact regardless of whether or not they expressed retrieval behavior or other pup-directed behaviors. This pup directed exploratory behavior was used for the neuronal analysis. Bottom, upright movement (upward ‘rearing’ activity) was consistently expressed by animals and was used as a spontaneously expressed control behavior.
3.2 Unit firing during maternal behavior
Contrary to previous work (Febo et al., 2010), no relationship between maternal retrieval behavior and mPFC unit firing rates was observed. Furthermore, no interactions between neuronal activity and bouts of grouping, grooming, or burrowing under bedding were noted. In other words, PETH did not show any changes in firing rates occurring at times when these behaviors were expressed by dams.
3.3 Medial PFC neuronal activity during contact with pups
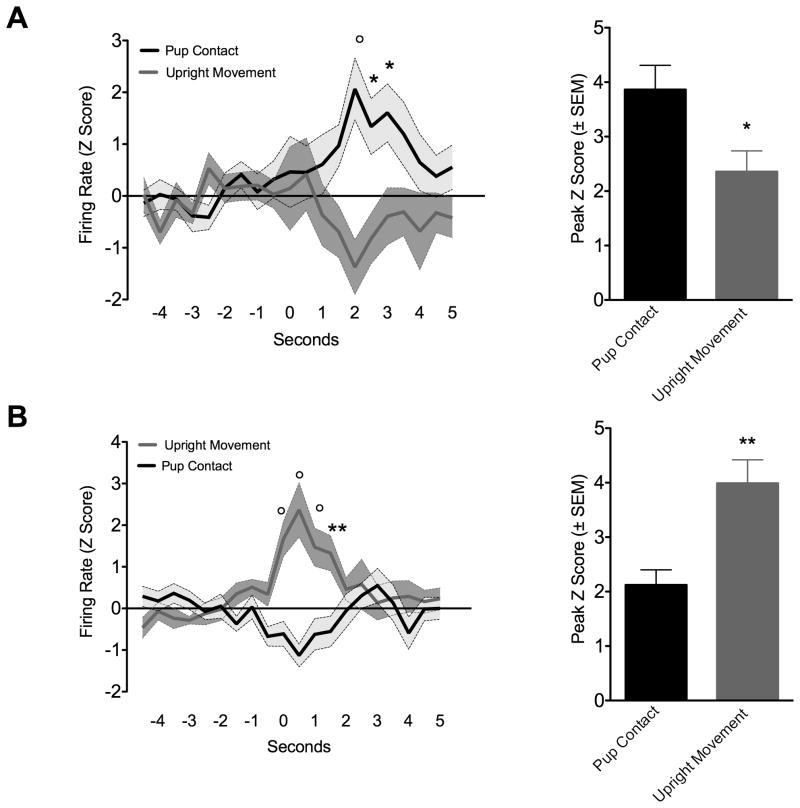
Despite the variations in typical maternal behavior patterns, rats showed consistency in pup directed exploratory behavior (pup contact) and rearing (upright movement). Rats frequently explored the test arena with a high number of bouts of upright standing and upward sniffing. Between these motor events, rats would frequently direct their attention to pups, navigate towards them, establish brief contact and then turn away again. The brief nosing-sniff contact was used to generate timestamps. To compare to a non-specific exploratory/motor behavior, rearing activity was used to generate timestamps (Fig. 1E and 1F). A representative unit is shown in Fig. 1E and 1F. This neuron was analyzed around the maternal pup-contact and also around the upright movement event. The behavior marker event occurrence is at 0 seconds. Following pup-contact there is an increase in mean firing rate. The peak response occurred at around 2 seconds after contact with pups and not at the exact moment of contact. The same neuron did not show any change in firing when analyzed around the time of rearing (Fig. 1F). Eleven units showed a similar relation between pup-contact and increased firing (increased firing responses) were observed out of the total 109 presumed regular spiking cells (Fig. 3A). Fifteen cells were observed to show the opposite relation with pup-contact vs upright movements. These latter cells showed significant increases in firing during upright movement behavior, but no change or even a slight non-significant decrease during pup-contact (Fig. 3B). Fig. 3A (top right) shows mean firing rates (Z-scores) during these peak periods in neurons that are pup responsive (n= 11) and rearing responsive (n= 15). Again, pup responsive neurons did not show increased spike frequency when analyzed around rearing behavior and vice versa (Fig. 3B, bottom right). An additional subset of neurons showed significant decreases in firing activity at the time of pup-contact (n = 4) or upright movement (n = 4) (data not shown).
Figure 3.
Mean single unit firing (mean Z scores ± standard error) in medial prefrontal cortex of maternal rats during up contact and upright movement. Perievent time histograms for each unit was analyzed around spontaneous pup contact and the non-specific exploratory behavior. Spike counts are binned at 500ms. A) Units that were pup responsive, as indicated by increased discharges during pup contact but not rearing. Bar graphs on right summarize peak firing averaged over 2 seconds (in Z scores ± standard error). B) Units that were not pup responsive, as indicated by increased discharges during upright movement and not pup contact. Bar graphs on right summarize peak firing averaged over 2 seconds (in Z scores ± standard error). Symbols at each time point of line graphs denote significantly different from control behavior (two way analysis of variance with repeated measures; circle p<0.001; asterisk p < 0.05). Asterisk over bars denotes significant difference (Wilcoxon’s matched paried t-test *p = 0.02, **p = 0.001).
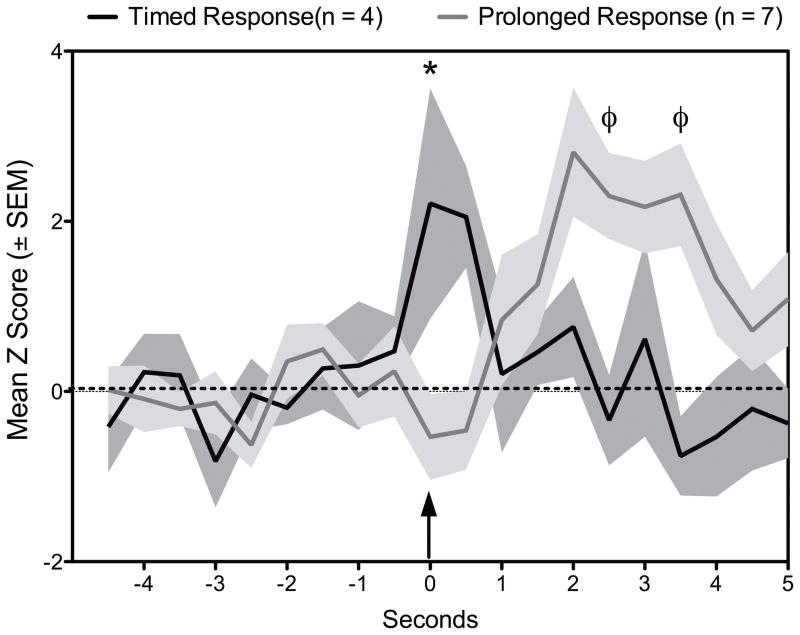
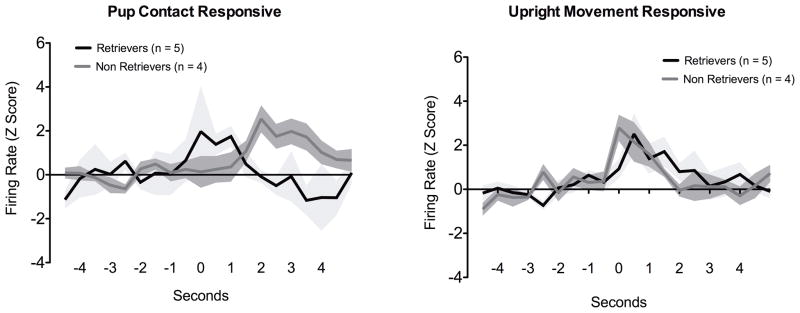
A closer look at the units showed increases in firing during pup contact suggest that there are two types of responses, an immediate or timed response (n = 4) and a slightly lagging or prolonged firing response (n = 7) (Fig. 4 and Fig. 6). The timed responses showed increased firing rates at the time of the behavioral event (pup contact), whereas the prolonged responses occurred with a slight delay after the behavioral response. In contrast to pup contact, rearing responsive units mostly fired during the execution of the behavior (Fig. 4 and Fig. 6). Finally, we analyzed the pup contact and upright movement neurons according to whether they belonged to retrievers or non-retrievers shown in Fig. 2. Results from this analysis is shown in Fig. 5. Increased firing for upright movement were similar between these two groups. However, the firing profile for pup contact differed between retrievers and non-retrievers (Fig. 5). Retrievers showed a brief increase in activity while non-retrievers showed a prolonged response similar to that observed in Fig. 4. Proportions of units showing differential firing patterns between retrievers and non-retrievers is shown in Fig. 6. While units responsive during upright movement were roughly similar between retrievers and non-retrievers, only 2 neurons of retrievers showed changes in firing during pup contact.
Figure 4.
Average changes in firing rate (mean z score ± standard error) during pup contact. Pup responsive units are classified as occuring during pup contact and following contact. Arrow indicates time of observation of the behavior event (pup contact). N’s indicate number of units and not number of animals. Symbols denote significant differences from * post-contact responsive and φ contact responsive (Wilcoxon’s matched paried t-test p < 0.05).
Figure 6.
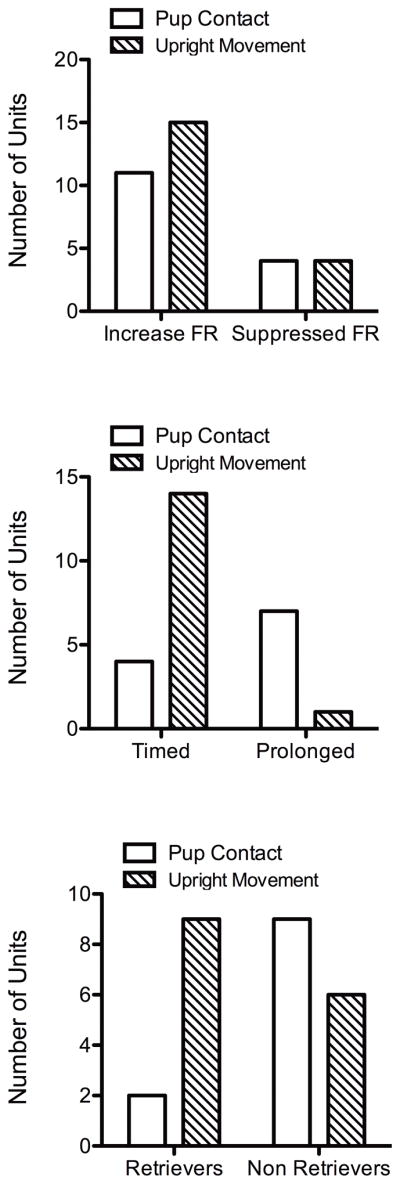
Changes in single unit firing during and following pup contact. Top) Proportion of units showing increased and suppressed firing responses (FR) during pup contact and rearing activity. Middle) Proportion of units showing increased firing during the expression of either pup contact or rearing activity (timed) and units showing changes following the expression of these behaviors (prolonged). A contingency test showed significant differences between pup contact and control units (χ2 = 9.7, p = 0.002). Bottom) Proportion of single units that are responsive during pup contact and upright movement, subdivided into animals showing retrieval or that failed to do so. A significantly greater proportion of units responsive during pup contact belonged to the non retrieving maternal animals (χ2 = 4.5, p = 0.03).
Figure 5.
Mean single unit firing (mean Z scores ± standard error) in medial prefrontal cortex of maternal rats during up contact and upright movement. Datasets were subdivided according to whether or not animals displayed retrieval behavior. Perievent time histograms for each unit was analyzed around spontaneous pup contact and the non-specific exploratory behavior. Spike counts are binned at 500ms. Data presented as normalized Z scores ± standard error.
4. DISCUSSION
The present study provides evidence of pup-responsive neurons in mPFC of maternal rats. Activity of the identified subset of neurons appears to be associated with brief contact with pups and not with the expression of other typical patterns of maternal behavior that are observable in the rat. Lonstein and Stern (1997) (Lonstein and Stern, 1997) reported that brief ‘sniffing-nosing’ occurred even in rats that did not show other typical maternal behaviors because of perioral anesthetization. Here, this behavior occurred regardless of whether or not retrieval behavior was carried out during the test days. Different firing patterns were identified between pup responsive and non-specific units. Changes in extracellular firing rates (increases or decreases) occurred during onset and several seconds after pup contact. Upright movement (rearing), which was used as a control motor behavior, showed a correlation with another subset of units. Activity for this subset of single units occurred during almost exactly at the moment of the behavioral event. It is hypothesized that the observed firing patterns of pup responsive cells are perhaps related to the processing of sensorimotor feedback from pups at the moment of contact and is not associated with anticipatory or motor activity related to the expression of maternal behaviors. This hypothesis would still need to be corroborated in future experiments, since the present results fell short of proving that feedback from pups is indeed involved. One interesting finding was that most ‘pup-contact’ responsive neurons occurred in the absence of retrieval behavior (Fig. 5), while this was not the case with the non-specific exploratory behavior. Although highly speculative at this time, this additional finding could lend support to the notion that sensorimotor processing is occurring in mPFC and, perhaps, a decision of whether or not to attend pups occurs as a result of this activity.
There is evidence that the mPFC, as part of the mesocortical dopamine system, might play a role in maternal responsiveness. Using functional MRI, Febo and Ferris (2007), Febo et al. (2005) and Ferris et al. (2005) previously showed increases in BOLD signal intensity in mPFC of rat dams receiving suckling stimulation from pups. BOLD activation in mPFC using this imaging paradigm is significantly reduced by pre-gestational cocaine exposure, which may implicate this cortical region in deficits in maternal responding following cocaine exposure and withdrawal. Changes in extracellular dopamine levels have also been reported within mPFC of maternal rats in response to pups, thus providing a possible substrate mediating or modulating the increased BOLD signal intensity with suckling stimulation (Febo and Ferris, 2007). Using a different neuroimaging paradigm where dams’ are presented with a male nest intruder in the presence of pups, Nephew et al (2009) show that there is significantly increased BOLD signal in the mPFC (Nephew et al., 2009). This paradigm simulates an often-used behavioral test for maternal aggression. Although within the context of the magnet environment it could not be ascertained whether the mothers were aggressive, it is very likely that the paradigm involves a heightened emotional state. Greater BOLD activation with intruder presentation vs pups alone was also observed in other prefrontal areas such as the anterior cingulate, orbital and insular regions (Nephew et al., 2009). Interestingly, the greater BOLD activation in these prefrontal areas was no longer significant when the intruder was presented in the absence of pups (Nephew et al., 2009). This could signify that the presence of the pups themselves and not the intruder might drive activity in these prefrontal areas in the mother. Using the same neuroimaging paradigm, we failed to observe any modulatory actions of the vasopressin V1a receptors on mPFC BOLD activation (Caffrey et al., 2010). Oxytocin receptor blockade did not significantly reduce mPFC BOLD response to suckling (Febo et al., 2005). Therefore, neuropeptides do not appear to have a direct impact on neural activity within this prefrontal region of maternal rats. It might be true, on the other hand, that ascending midbrain monoamine neurotransmitters are more important in the modulation of mPFC BOLD response to suckling pups or to a nest intruder in maternal rodents.
Recent studies have provided additional support for a role of the prefrontal cortex in maternal behavior in the rat. Tetrodotoxin infusion into the mPFC was observed to reduce the latency to retrieve pups (Febo et al., 2010). Similarly, GABA agonist infusion into the same area of mPFC was observed to result in reduced retrieval and grouping of pups within a nest area in the rat’s home cage (Febo et al., 2010). This was not accompanied by equivalent reductions in motor performance or attention to pups within the test cage (Febo et al., 2010). N-Methyl-D-Aspartate mediated neurotoxic lesions of mPFC in virgin rats impaired their expression of proceptive sexual behaviors without affecting their sexual performance (Afonso et al., 2007). This is consistent with past research in the field arguing that separate brain circuits control motivation and performance during sex in rats (Everitt, 1990). These same female rats showed significant changes in postpartum maternal behaviors, including licking, nest building and retrieval (Afonso et al., 2007). These cited results, along with our imaging findings cited above point to an interesting relation between prefrontal cortical neurotransmission and maternal behavior. Rats showing high rates of retrieval show low BOLD signal and low basal dopamine in the medial PFC (Febo and Ferris, 2007). Neuronal activity in mPFC is important for maternal retrieval and high GABA receptor stimulation in the mPFC reduces maternal behavior (Febo et al., 2010). Therefore, dopamine may play a key role in maternal motivation that is counteracted by increased GABA neurotransmission. We have identified a subset of excitable neurons in this region that may need further scrutiny for their mediation of some aspect of maternal behavior.
Prefrontal cortical cell firing is modulated during distinct phases of goal directed behavior. Neuronal firing rates were increased or decreased during maintenance, extinction and reinstatement phases in operant tasks to obtain water reinforcement (Peters et al., 2005). The peak responses in this latter study occurred at the moment that the behavioral response was recorded (lever press in an operant chamber) and might be involved in the execution of the goal-directed response. This varies from the present study in which increased firing rates were mostly observed following pup contact. Prelimbic neurons exhibit spatially dependent firing rates during the active search for a reward (Hok et al., 2005) and this may also be important during distinct phases of goal directed behavior. Similar findings have been reported using an 8-arm radial maze task (Jung et al., 1998). It was not possible in the present study to determine whether or not the firing rates of maternal rats were dependent on the location of pups versus the pups themselves. However, it is noteworthy that anticipatory responses that precede pup contact were not clearly identified in the present study. Future experimental paradigms should take the dynamics of approach and acquisition of pups into account. Ablation of cells within the mPFC can also result in changes in effort dependent motivated behavior. Rats sustaining lesions of the medial PFC will choose more easily obtainable food pellet rewards over a higher number of pellets that are harder to reach (Walton et al., 2002). The same rats showed pre-lesion preferences for the greater magnitude-harder to obtain rewards (Walton et al., 2002). Discharge patterns of the mPFC have been shown to be dependent on behavior execution during spatial tasks to obtain a reward of greater magnitude (Kargo et al., 2007). It will be interesting in future studies to compare the value of pups, which are a highly valued reward for the mother, versus other competing rewards at the time of lactation. It may be possible that the pattern of neuronal activity observed in the present study is due to the processing of incoming sensory information that was preprocessed at other secondary association cortices. The neuronal processing in the mPFC may act within the known functions of mPFC, particularly decision making or the execution of a behavior related to the goal at hand (pup care).
Single units classified as being ‘pup-responsive’ showed distinct firing patterns. A subset of cells showed increased firing rates during the execution of the pup-contact event; whereas other cells showed increased activity after the event had occurred. These responses are reminiscent of cells that are involved in task execution and cells that have some form of delayed onset with respect to the behavioral event or stimulus presentation. The former type of responses have been described for other goal directed behaviors in which an animal is trained in a task and neurons are observed to fire during task execution or during conditioned responding (Peters et al., 2005). It is unclear what the delayed type of response might be with regards to the pup contact behavior described here. Delayed response cells have been described in working memory tasks in the rat. Information processed during such a firing pattern is perhaps incorporated into working memory (Jung et al., 1998) or conveyed to other subcortical areas such as the dorsal striatum (Febo, 2011). Other work has shown that delayed response neurons fire when a sequence of stimuli predictive of a reward are presented (Cowen and McNaughton, 2007). Neurons in mPFC under these circumstances are sensitive to task sequence and the development of a behavioral strategy in order to achieve a goal. Despite the fact that these neuronal firing patterns are registered in the mPFC, it is unclear whether this is associated with the responses observed during pup contact in the present work.
A word of caution needs to be noted with regards to the present methods that were employed to record in vivo single unit firing. Microwire arrays that were used here are similar to those previously reported and that are commercially available (Nicolelis et al., 1997). These are non-drivable (fixed) electrode arrays provide stability over long periods of time for chronic recordings, but restrict the amount of neurons that can be sampled per animal. The uneven yield due to the electrode recording method, along with the variability of dams’ behavior over the study sample, reduced the power of the present analysis and restricted the assumptions that could be declared about the entire sample of rats used in the study. Future studies will be designed to address these concerns by the construction of standard drivable electrode bundles and the optimization of the maternal behavior paradigm used here. Moreover, in order to take into account the selectivity of the neuronal responses to pups, other alternate control (salient) stimuli could be employed. The present study limited the comparison to a non-specific but highly reproducible spontaneous behavior in the rat, upright movements. Finally, behavior-neuronal response timing was not controlled because of what was just mentioned, the animal generated its own responses and this was not controlled. The present study used digital video analysis to mark the behavioral events of interest, however, there may be variability in the exact timing of neuronal responses with regards to the behavioral events.
The findings that the mPFC has a role in the expression of maternal behaviors and that neurons in these region respond to pups may have importance for psychiatric conditions involving impairments in prefrontal cortical function (Febo and Ferris, 2007). The mPFC has been highly studied for its role in cognition, mood and motivation in rodents and primates and has been implicated in major depression, bipolar disorder and schizophrenia. Estrogen receptors α (Montague et al., 2008) and β (Shughrue et al., 2002) have been detected in the PFC of female rats and primates by immunohistochemistry and mRNA hybridization methods and are believed to be involved in major depression and other psychiatric illnesses (Perlman et al., 2005). Furthermore, drugs of abuse can have a strong impact on the physiology of neurons within these regions or dopamine neurons that provide modulatory input (Beyer and Steketee, 1999; Castner et al., 2005; Goto and Grace, 2005; Goto and Grace, 2006; Williams and Steketee, 2005a; Williams and Steketee, 2005b).
Highlights.
First study to register from medial prefrontal neurons of behaving maternal rats.
Shows relation between direct interactions with pups and increased neuronal firing.
Pup responsive neurons were distinguished from those that were not pup responsive.
Implicates prefrontal neurons in maternal goal-directed behavior.
Acknowledgments
Support was provided by NIH grant DA019946. Its contents are solely of the responsibility of the author and do not represent the official views of the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–26. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–47. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Dopamine depletion in the medial prefrontal cortex induces sensitized-like behavioral and neurochemical responses to cocaine. Brain Res. 1999;833:133–41. doi: 10.1016/s0006-8993(99)01485-7. [DOI] [PubMed] [Google Scholar]
- Caffrey MK, Nephew BC, Febo M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology. 2010;58:107–16. doi: 10.1016/j.neuropharm.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57:743–51. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98:303–16. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Febo M. A bold view of the lactating brain: functional magnetic resonance imaging studies of suckling in awake dams. J Neuroendocrinol. 2011;23:1009–19. doi: 10.1111/j.1365-2826.2011.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Felix-Ortiz AC, Johnson TR. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Res. 2010;1325:77–88. doi: 10.1016/j.brainres.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–44. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Ferris CF. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience. 2007;148:400–12. doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–28. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–66. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry. 2006;60:1259–67. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A. 2005;102:4602–7. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8:437–50. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Szatmary B, Nitz DA. Adaptation of prefrontal cortical firing patterns and their fidelity to changes in action-reward contingencies. J Neurosci. 2007;27:3548–59. doi: 10.1523/JNEUROSCI.3604-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298:40–9. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Somatosensory contributions to c-fos activation within the caudal periaqueductal gray of lactating rats: effects of perioral, rooting, and suckling stimuli from pups. Horm Behav. 1997;32:155–66. doi: 10.1006/hbeh.1997.1416. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–45. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135:315–28. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20:893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. Eur J Neurosci. 2009;30:934–45. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LM. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron. 1997;18:529–37. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 2005;58:812–24. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–9. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Prog Brain Res. 2002;139:15–29. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- Slotnick BM. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29:204–36. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975;88:118–27. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. J Comp Physiol Psychol. 1955;48:347–56. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol Behav. 1993;54:861–8. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–87. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–1003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler BC. Automatic discrimination of single units. In: Nicolelis MAL, editor. Methods for neural ensemble recordings. Methods and New Frontiers in Neuroscience. CRC Press; 1999. pp. 61–78. [Google Scholar]
- Williams JM, Steketee JD. Time-dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology. 2005a;48:51–61. doi: 10.1016/j.neuropharm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Effects of repeated cocaine on the release and clearance of dopamine within the rat medial prefrontal cortex. Synapse. 2005b;55:98–109. doi: 10.1002/syn.20093. [DOI] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]