Abstract
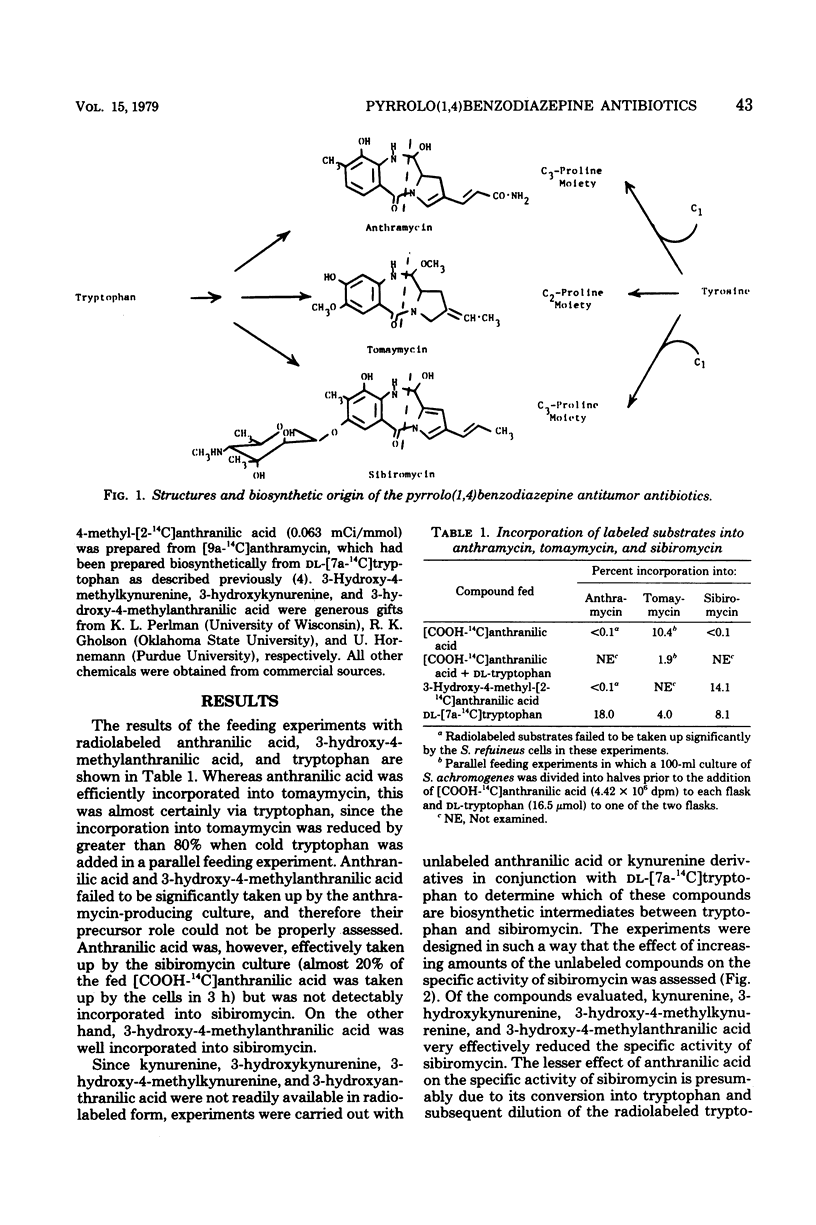
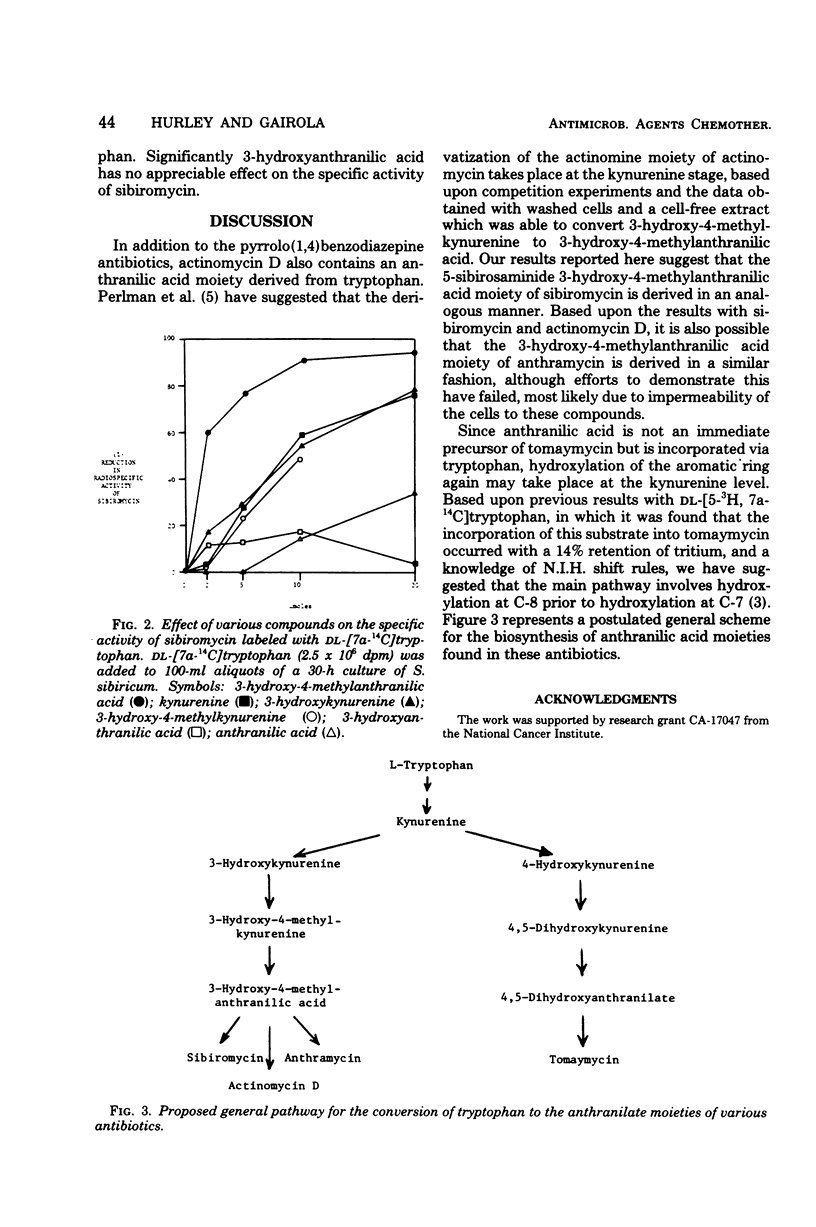
Biosynthetic intermediates between tryptophan and the anthranilate moieties of tomaymycin and sibiromycin have been suggested, based upon a combination of feeding experiments with either carbon-14-labeled substrates or competition experiments between radiolabeled tryptophan and unlabeled intermediates. In the case of sibiromycin and tomaymycin, substitution of the aromatic ring most likely takes place at the kynurenine stage. Feeding experiments with the anthramycin culture were inconclusive, most likely because of the cell impermeability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hurley L. H., Gairola C., Das N. V. Pyrrolo[1,4]benzodiazepine antibiotics. Biosynthesis of the antitumor antibiotic 11-demethyltomaymycin and its biologically inactive metabolite oxotomaymycin by Streptomyces achromogenes. Biochemistry. 1976 Aug 24;15(17):3760–3769. doi: 10.1021/bi00662a019. [DOI] [PubMed] [Google Scholar]
- Hurley L. H. Pyrrolo(1,4)benzodiazepine antitumor antibiotics. Comparative aspects of anthramycin, tomaymycin and sibiromycin. J Antibiot (Tokyo) 1977 May;30(5):349–370. doi: 10.7164/antibiotics.30.349. [DOI] [PubMed] [Google Scholar]
- Hurley L. H., Zmijewski M., Chang C. J. Biosynthesis of anthramycin. Determination of the labeling pattern by the use of radioactive and stable isotope techniques. J Am Chem Soc. 1975 Jul 23;97(15):4372–4378. doi: 10.1021/ja00848a040. [DOI] [PubMed] [Google Scholar]
- Perlman D., Otani S., Perlman K. L., Walker J. E. 3-Hydroxy-4-methylkynurenine as an intermediate in actinomycin biosynthesis. J Antibiot (Tokyo) 1973 May;26(5):289–296. doi: 10.7164/antibiotics.26.289. [DOI] [PubMed] [Google Scholar]



