Summary
Background
Artemisinin-resistant falciparum malaria has arisen in western Cambodia. A concerted international effort is underway to contain artemisinin-resistant Plasmodium falciparum, but containment strategies are dependent on whether resistance has emerged elsewhere. We aimed to establish whether artemisinin resistance has spread or emerged on the Thailand–Myanmar (Burma) border.
Methods
In malaria clinics located along the northwestern border of Thailand, we measured six hourly parasite counts in patients with uncomplicated hyperparasitaemic falciparum malaria (≥4% infected red blood cells) who had been given various oral artesunate-containing regimens since 2001. Parasite clearance half-lives were estimated and parasites were genotyped for 93 single nucleotide polymorphisms.
Findings
3202 patients were studied between 2001 and 2010. Parasite clearance half-lives lengthened from a geometric mean of 2·6 h (95% CI 2·5–2·7) in 2001, to 3·7 h (3·6–3·8) in 2010, compared with a mean of 5·5 h (5·2–5·9) in 119 patients in western Cambodia measured between 2007 and 2010. The proportion of slow-clearing infections (half-life ≥6·2 h) increased from 0·6% in 2001, to 20% in 2010, compared with 42% in western Cambodia between 2007 and 2010. Of 1583 infections genotyped, 148 multilocus parasite genotypes were identified, each of which infected between two and 13 patients. The proportion of variation in parasite clearance attributable to parasite genetics increased from 30% between 2001 and 2004, to 66% between 2007 and 2010.
Interpretation
Genetically determined artemisinin resistance in P falciparum emerged along the Thailand–Myanmar border at least 8 years ago and has since increased substantially. At this rate of increase, resistance will reach rates reported in western Cambodia in 2–6 years.
Funding
The Wellcome Trust and National Institutes of Health.
Introduction
Artemisinin combination treatments are the recommended first-line therapy for falciparum malaria. Plasmodium falciparum parasites with reduced in-vivo susceptibility to artemisinin derivatives (eg, artesunate) have emerged in western Cambodia.1–3 This finding threatens worldwide initiatives to control and eliminate malaria.4 Resistance to the previous mainstays of antimalarial treatment—namely, chloroquine and sulfadoxine–pyrimethamine—also arose in western Cambodia5–7 and spread across southeast Asia into Africa, resulting in the deaths of millions of children.8,9 If resistance to artemisinin is confined to the Cambodia–Thailand border, regional elimination of falciparum malaria will probably be necessary for containment.10,11 However, if resistant parasites have already spread or emerged elsewhere, then the containment zone will need to be extended and the strategy reconsidered.3,7
Artemisinin resistance is characterised by slow parasite clearance.1,2,12 Clearance (assessed by microscopy) of sensitive P falciparum is achieved within 2 days in 95% of patients,13 whereas artemisinin-resistant infections remain slide-positive for 3 or more days; treatment failure is more common in such infections after artemisinin combination treatment. High-grade artemisinin resistance has not been reported. Laboratory studies and mathematical modelling suggest that slow clearance of resistant parasites mainly results from decreased susceptibility of ring-stage parasites to artemisinin and its derivatives.14 No laboratory assays reliably identify artemisinin-resistant parasites.2 Patients from western Cambodia with similar parasite clearance rates are infected with genetically indistinguishable parasite clones, suggesting that parasite genetics have a central role in determination of this trait,15 although the genes responsible are unknown.
On the northwestern border of Thailand, 800 km from western Cambodia, treatment failure rates with artesunate and mefloquine combinations have increased.16 In this area, patients without signs of severity but with more than 4% parasitaemia (ie, parasites present in 4% or more of red blood cells) are given oral artesunate regimens, which have proven superior to intravenous quinine.17,18 High admission parasitaemias allow for accurate assessment of parasite clearance rates. To assess whether artemisinin resistance has emerged in this strategically important area, we analysed over 10 years the longitudinal changes in parasite clearance rates in a large prospective series of hyperparasitaemic patients given artesunate. Parasites were genotyped to establish the contribution of parasite genetics to parasite clearance rates.
Methods
Study site and patients
Data were obtained from patients with a high parasite count (>4% infected red blood cells) but no signs of severe malaria routinely admitted to malaria clinics run by the Shoklo Malaria Research Unit, which span a 150 km region of the northwestern border of Thailand. Maela (refugee camp) and Wang Pha village are north of Mae Sot town (where the research unit is based), whereas Mae Kon Khen and Mawker Thai villages are south (appendix). Most patients came from adjacent Myanmar (Burma). We compared the parasite clearance data with results from 119 patients studied in Pailin, western Cambodia (2007–10), where artemisinin resistance has been confirmed.2 A subset of 30 parasite genotypes from Pailin was part of the genetic analysis. These studies were approved by the ethics review boards of the Faculty of Tropical Medicine, Mahidol University.
Falciparum malaria was diagnosed by microscopy of thick and thin peripheral blood smears stained with Giemsa. Parasite counts were read per 1000 red cells (thin film) or 500 white cells (thick film). Uncomplicated hyperparasitaemia was defined as 4% or more of red cells infected with malaria parasites without clinical evidence of severe malaria,19 and was the only criterion for inclusion in the analysis. Routine care was hospitalisation with a six hourly blood smear to monitor parasite clearance until smears were slide-negative. Treatment was with a 7 day regimen of oral artesunate (4 mg/kg initially, then 2 mg/kg once daily for 7 days), usually combined with either mefloquine (25 mg/kg in two divided doses), or doxycycline (4 mg/kg per day for 7 days) or clindamycin (5 mg/kg three times daily for 7 days) if mefloquine was contraindicated. In cases of clinical deterioration parenteral treatment was substituted. We monitored treatment until smears became slide-negative.
Procedures
DNA was extracted from admission blood spots by use of a two-step protocol to maximise DNA yield. Blood was eluted from six 3 mm diameter punches with the GenSolve kit (GenVault Corporation, Pleasanton, CA, USA), and DNA was then extracted with QIAamp 96 DNA blood kits (Qiagen, Valencia, CA, USA). Genotyping of 96 single nucleotide polymorphisms distributed across the P falciparum genome was done with the Illumina Goldengate platform (Illumina Inc, San Diego, CA, USA) (appendix). We judged samples to be multiple-clone infections if more than five single nucleotide polymorphisms showed heterozygous base calls.
We measured the heritability of parasite clearance half-life to assess the part that parasite genetics plays in determination of this trait. Multilocus parasite genotypes infecting two or more patients were identified. Such parasite genotypes are equivalent to identical twins reared apart and can be used to measure heritability of parasite clearance (panel 1).
Panel 1. Heritability.
Measurement of heritability—ie, the proportion of variance in a trait that is explained by genetics—is widely used in human and agricultural genetics.20 This measure is especially useful when the loci underlying trait variation are unknown and when the genetic and environmental contributions to trait variation are uncertain, as in the case of parasite clearance rates. Heritability can be measured in Plasmodium falciparum by comparing trait variation in genetically indistinguishable parasites infecting different patients, just as heritability in people can be measured with identical twins. This approach uses the fact that such parasites are frequently recovered from several patients in southeast Asian countries because of low transmission and consequently high rates of inbreeding.15 When resistance genes spread within a parasite population, heritability increases because genetic determinants have a growing role in determination of trait variation.
The geometric mean parasite clearance half-life in patients with artemisinin-resistant falciparum malaria in western Cambodia was 6·2 h (n=36, 95% CI 5·7–6·6),2,15 corresponding to a loge normalised distribution with a mean of 1·8 (SD 0·22). Hyperparasitaemic patients on the northwestern border of Thailand in 2001 were used as a reference artemisinin-sensitive population, with a mean loge parasite clearance half-life of 0·95 (0·33). The hypothesis that the changes in parasite clearance half-life in western Thailand resulted from emergence versus importation of parasites with the same resistance phenotype prevalent in western Cambodia was tested.
Statistical analysis
We assessed parasite clearance using a standardised fitting method that separates the variable initial lag phase, during which parasite counts level off or rise, from the subsequent log-linear decline.12 The slope of this phase was calculated and expressed as the parasite clearance half-life, which is the time taken for parasitaemia to fall by half during log-linear decline.21 Patients with parasite clearance curves showing a poor fit (R2<0·8) to the log-linear model were excluded from the analysis.
We used Stata (version 11) to examine the association between parasite clearance half-life and the following covariates: age, sex, history of malaria, treatment regimen (artesunate with mefloquine vs artesunate with other partner drugs or artesunate alone), date of admission, duration of fever before presentation (<3 days vs ≥3 days), admission parasitaemia, haematocrit (<30% vs ≥30%), gametocytaemia, blood transfusion, and parenteral rescue therapy.
Univariate regression analysis of all listed covariates was done on log-transformed parasite clearance half-lives, followed by stepwise multivariate analysis (excluding parenteral rescue therapy). Variables that only significantly improved the final model (likelihood ratio test p<0·05) were included (tables 1, 2).
Table 1.
Effect of covariates on parasite clearance half-life of Plasmodium falciparum examined by univariate analysis
| N | Geometric mean parasite clearance half-life (95% CI) | Range | % change (95% CI)* | p value | ||
|---|---|---|---|---|---|---|
| Artesunate and mefloquine treatment† | −4·3 (−7·8 to −0·7) | 0·018 | ||||
| No | 640 | 3·267 (3·162–3·374) | 0·730–11·613 | |||
| Yes | 2266 | 3·125 (3·072–3·180) | 0·588–12·545 | |||
| Admission‡ | 3·4 (2·8 to 4·0)‡ | <0·0001 | ||||
| 2001 | 156 | 2·628 (2·535–2·725) | 1·079–7·484 | |||
| 2010 | 156 | 3·674 (3·561–3·791) | 1·454–9·111 | |||
| Days fever | 9·0 (5·7 to 12·4) | <0·0001 | ||||
| <3 days | 1467 | 3·030 (2·966–3·095) | 0·847–11·613 | |||
| ≥3 days | 1401 | 3·301 (3·230–3·374) | 0·588–12·545 | |||
| Admission gametocytaemia | 13·9 (8·7 to 19·4) | <0·0001 | ||||
| No | 2563 | 3·108 (3·058–3·158) | 0·588–12·545 | |||
| Yes | 343 | 3·540 (3·387–3·700) | 0·730–10·146 | |||
| Admission haematocrit | −9·2 (−12·8 to −5·5) | <0·0001 | ||||
| <30% | 511 | 3·417 (3·295–3·543) | 0·730–9·804 | |||
| ≥30% | 2395 | 3·103 (3·051–3·155) | 0·588–12·545 | |||
| Sex | 0·5 (−2·6 to 3·8) | 0·74 | ||||
| Female | 1083 | 3·145 (3·068–3·225) | 0·730–11·613 | |||
| Male | 1823 | 3·162 (3·102–3·224) | 0·588–12·545 | |||
| History of malaria | −3·8 (−6·8 to −0·8) | 0·014 | ||||
| No | 1301 | 3·235 (3·162–3·310) | 0·805–12·545 | |||
| Yes | 1511 | 3·111 (3·046–3·177) | 0·588–11·613 | |||
| Parenteral rescue treatment | 19·7 (13·8 to 25·9) | <0·0001 | ||||
| No | 2616 | 3·100 (3·051–3·149) | 0·588–11·613 | |||
| Yes | 290 | 3·710 (3·537–3·892) | 0·906–12·545 | |||
| Blood transfusion | 11·8 (3·0 to 21·4) | 0·008 | ||||
| No | 2738 | 3·150 (3·101–3·200) | 0·588–12·545 | |||
| Yes | 104 | 3·522 (3·248–3·820) | 1·260–9·804 | |||
| Study sites | ||||||
| Mawkertai§ | 1035 | 2·945 (2·872–3·020) | 0·805–12·545 | .. | .. | |
| Maela | 439 | 2·940 (2·829–3·056) | 1·028–9·385 | −0·2 (−4·6 to 4·6) | 0·95 | |
| Mae Khon Ken | 148 | 2·958 (2·768–3·162) | 1·205–8·636 | 0·5 (−6·4 to 7·9) | 0·9 | |
| Wang Pha | 1283 | 3·444 (3·367–3·523) | 0·588–11·613 | 16·9 (13·1 to 21·0) | <0·0001 | |
% change refers to the change in parasite clearance half-life estimated from the regression model.
Patients not given artesunate and mefloquine received either artesunate with doxycycline or clindamycin, or artesunate alone for 7 days.
Day of admission was modelled as a continuous covariate; geometric mean was estimated at the start of the study (2001) and 10 years later (2010). Percentage changes are give for one year.
Mawkertai was used as a reference location for comparison with other sites.
Table 2.
Admission variables associated with parasite clearance half-life of Plasmodium falciparum in multivariate regression analysis
| % change in geometric mean parasite clearance half-life | 95% CI | p value | ||
|---|---|---|---|---|
| Study centre* | ||||
| Maela | 5·64 | 4·34 to 6·95 | <0·0001 | |
| Wang Pha | 2·60 | 1·11 to 4·11 | 0·00059 | |
| Mae Khon Khen | 1·65 | −6·09 to 10·02 | 0·68 | |
| Mawker Thai | 1·68 | 0·72 to 2·65 | 0·00062 | |
| Presence of gametocytaemia on admission | 5·55 | 0·57 to 10·78 | 0·029 | |
| Haematocrit <30% on admission | 7·33 | 3·10 to 11·73 | 0·00056 | |
| Fever ≥3 days | 7·37 | 4·17 to 10·67 | <0·0001 | |
Change per year estimated within each study location.
To assess heritability, variance of parasite clearance was compared within and between clonally identical parasites recovered from more than two patients. Heritability (H2) was estimated from the mean squares terms in ANOVA.15,21 H2 was estimated for normalised parasite clearance and for residuals remaining after regression of clearance rates against patient and environmental factors.
We examined the hypothesis that changes in parasite clearance resulted from importation or emergence of parasites with the western Cambodian phenotype. The clearance rate distribution of a mixture of the western Cambodia and western Thailand populations was calculated as: π×normal([log{x}–1·8]/0·22)+(1–π)× normal([log{x}–0·95]/0·33), where π is the proportion of infections derived from the resistant population. For each year, we used the Kolmogorov–Smirnov test (50 tests for each year) to compare recorded distributions of loge transformed half-lives with these theoretical distributions (for π between 0·01 and 0·50).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
Between July, 2001, and December, 2010, 3202 hyperparasitaemic patients were treated. Most patients were younger than 15 years (appendix). Most patients (2403) were given oral artesunate and divided doses of mefloquine. The remaining patients were given artesunate monotherapy (321 patients), artesunate in combination with either doxycycline (382 patients) or clindamycin (71 patients), or other combinations (25 patients). Patients who got blood transfusions within 24 hours of admission, or an incomplete course of or more than one parenteral dose of artesunate were excluded from the analysis. 2855 patients (1268 in Wang Pha, 975 in Mawker Thai, 467 in Maela, and 145 in Mae Khon Ken) remained eligible, of whom 1778 (62%) were men or boys. In 1759 of 2855 patients, artesunate monotherapy was given, or else the partner drug (mefloquine, doxycycline, or clindamycin) was given more than 48 h from the start of artesunate treatment. All patients survived, confirming the effectiveness of oral artesunate in uncomplicated hyperparasitaemia.18
Between 2001 and 2010, the median age of hyperparasitaemic patients increased slightly but significantly from 11 years to 15 years (p<0·0001). The proportion of patients developing severe malaria did not increase over time, nor did the ratio of severe to uncomplicated falciparum malaria cases, malaria mortality, nor the proportion of patients developing anaemia. We noted a significant increase in the proportion of patients with gametocytaemia on admission (p<0·0001; 7·5×10−6 test for trend).
Parasite clearance half-life lengthened from a geometric mean of 2·6 h (95% CI 2·5–2·7) in 2001, to 3·7 h (3·6–3·8) in 2010 (p<0·0001), compared with 5·5 h (5·2–5·9) in the 116 patients from western Cambodia between 2007 and 2010 (clearance data from three patients fitted poorly to the linear model [R2<0·8] and were thus excluded) (figures 1, 2A, table 1). We used a stringent threshold of a parasite clearance half-life of more than 6·2 h to categorise infections with slow or fast parasite clearance; the proportion of patients at our study centres with slow clearance rose from 0·6% in 2001, to 20% in 2010, compared with 42% of patients in western Cambodia in 2010 (figure 2B). Parasite clearance rates in patients given artesunate monotherapy or given artesunate alone for more than 48 h (n=1759) before receiving partner drugs were similar to those in patients who were given combination treatments immediately, suggesting that increasing resistance to the partner drug cannot explain the temporal changes reported.
Figure 1.

Parasite clearance half-life of Plasmodium falciparum on the Thailand–Myanmar border over 10 years
We plotted untransformed parasite clearance half-lives over time for all malaria infections with a good fit to a linear model (R2>0.8). Data from western Cambodia gathered between June, 2007, and November, 2010, are shown for comparison, compressed as if they had been obtained in a single year. The dotted red line shows a threshold value of 6·2 h, used to differentiate between fast-clearing and slow-clearing infections. The dotted blue boxes show the periods for which bloodspots were available. These two time windows form the basis of the genetic analysis.
Figure 2.

Change in parasite clearance half-life of Plasmodium falciparum 2001–10
(A) Geometric means of parasite clearance half-life in patients with hyperparasitaemia on the Thailand–Myanmar border. *Subset of infections treated with artesunate monotherapy (ART) for more than 48 h, for which 48 h clearance rates and parasite clearance half-lives were calculated. Error bars are 95% CIs and above them is the number of patients. (B) Proportion of patients with very slow clearance (parasite clearance half-life≥6·2 h). Error bars are binomial 95% CIs. Colour scheme is as per A.
No relation existed between parasite clearance half-life and age (p=0·439) or starting parasitaemia (p=0·598) in patients given artesunate monotherapy or given artesunate alone for more than 48 h before receiving partner drugs, nor in all patients (p=0·124 and p=0·569, respectively). The variable most strongly associated with increases in parasite clearance half-life was the date of patient admission (appendix). This trend was strongly significant in all clinics sampled except Mae Khon Ken, Thailand (table 2; appendix). Only 7·4% of the variation in half-life could be explained by other admission variables. Location had a significant effect on parasite clearance; infections in northern locations (ie, Maela and Wang Pha) had slower clearance than those sampled in the south (ie, Mawker Thai and Mae Khon Ken) (appendix; table 2).
Of 96 single nucleotide polymorphisms assessed, 93 gave robust genotype data. Of the 1583 infections genotyped, 1029 were single-clone infections (appendix). From these, 148 unique 93-locus parasite genotypes were identified, each infecting between two and 13 patients (29 from 2001 to 2004, and 119 from 2007 to 2010) (appendix). Patients harbouring parasites with the same 93-locus genotype had similar parasite clearance half-lives (figure 3), showing the important part played by parasite genotype. Between 2001 and 2004, parasite genotype had a significant effect on parasite clearance half-life (p=0·0166), even after adjusting for significant covariates (p=0·01). Two of the three patients with the longest parasite clearance half-lives recorded before 2004 had the same 93-locus genotype (figure 3). From 2007 to 2010, parasite genotype had a much stronger effect on parasite clearance half-life (p<0·0001) than it had between 2001 and 2004, and remained highly significant after adjusting for other covariates (F=5·219, df=118, p<0·0001). Mean H2 increased from 0·30 (SD 0·14) in 2001–04, to 0·66 (0·04) in 2007–10. After adjustment for the effects of covariates, H2 rose from 0·32 (0·14) between 2001–04, to 0·58 (0·05) in 2007–10 (appendix). The increase in H2 was tested by permutation and was significant both before (p<0·01) and after (p=0·04) adjustment for covariates (appendix).
Figure 3.
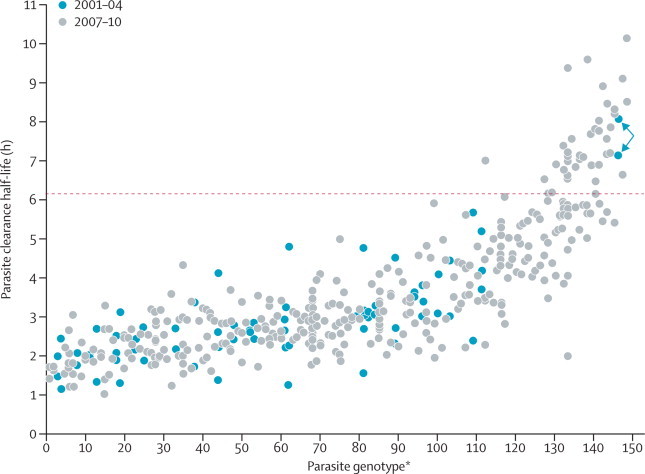
Effect of parasite genotype on parasite clearance rates
Clearance half-lives in patients infected with parasites bearing identical 93-locus genotypes. Red dotted line marks the 6·2 h threshold used to categorise slow-clearing parasites. The arrows show slow clearance parasite genotypes gathered in November, 2003. *Ranked by mean parasite clearance half-life.
To investigate whether the increase in H2 was driven by alleles conferring slow parasite clearance, we removed 30% of the slowest clearing infections from the 2007–10 dataset, which caused H2 to decrease from 0·66 (SD 0·04) to 0·17 (0·07). Removal of 30% of the fastest clearing infections increased H2 to 0·70 (0·05), whereas random removal of 30% of infections did not alter H2 (mean H2 from 100 randomisations 0·67 (0·03); range 0·56–0·74). These in-silico experiments show that high H2 is driven by parasite genes that determine slow parasite clearance.
Admixture of parasites with the Cambodian phenotype could explain the parasite clearance distributions in 2001–02, and 2009–10, but not those between 2003 and 2008 (p<0·01). This finding suggests that the gradual increases in parasite clearance half-life are unlikely to be explained by a single-step process of importation or de-novo selection of a parasite population with the same characteristics as the resistant parasites prevalent in western Cambodia.
None of the 93-locus genotypes was common to patients from the Thailand–Myanmar border and western Cambodia. Pairwise allele sharing (ps) between the 25 slowest and 25 fastest clearing parasites from the Thailand–Myanmar border was compared with 30 slow-clearing parasites from western Cambodia. Cambodian parasites were more closely related (ps=0·453) to slow-clearing parasites than to fast-clearing parasites (ps=0·450) from the Thailand–Myanmar border. However, permutation tests show that this difference is not significant (p=0·137, 10 000 permutations).
Discussion
This large prospective series of patients with hyperparasitaemia during 10 years provides clear evidence that parasite clearance responses after artesunate treatment are slowing on the northwestern border of Thailand (panel 2). The rate of decline is consistent with theoretical expectations and empirical data for the spread of resistance loci through a parasite population under drug selection.27–29 If the rate of decline continues then parasite clearance half-lives on the Thailand–Myanmar border will be similar to those in western Cambodia within 2–6 years.
Panel 2. Research in context.
Systematic review
We searched for trials on artemisinin resistance on PubMed using the term “resistance” in combination with “artemisinin”, “artesunate”, or “artemether”, and confined the search to clinical studies in which resistance had been suspected or confirmed on the basis of assessment of parasite clearance. Additionally, we searched for all clinical trials or assessments of artemisinin combination treatments. We identified 101 references. The only area where slow parasite clearance has been unambiguously shown previously is western Cambodia (four studies reporting parasite clearance).1,2,22–24 Artemisinin resistance has been suspected because of diminishing treatment responses to artemisinin combination therapies on the western border of Thailand16,25 and changes in parasite clearance rates on the Kenyan coast.26
Interpretation
Although artemisinin resistance has been suspected outside western Cambodia, this study provides the first unequivocal proof that resistance has emerged or spread westward. It confirms (with a large sample size) findings showing that this phenotype is explained mainly by a heritable genetic trait in the parasite population. The research suggests a potential transmission advantage associated with resistance to artesunate through increased gametocyte carriage on admission. These findings suggest that regional artemisinin resistance-containment strategies should be reviewed. Increasing the availability of artemisinin combination therapies in southeast Asia will reduce the incidence of malaria but provides a selection pressure driving artemisinin resistance. The development of strategies to reduce the selection and spread of artemisinin-resistant Plasmodium falciparum while continuing to drive down the incidence of malaria is of the highest priority.
Parasite clearance after treatment with artemisinin derivatives is determined mainly by drug effectiveness, although host immunity and the partner drug can also contribute.12 The frequency of malaria and the proportion of multiple-clone infections (appendix) in the study area in northwestern Thailand have fallen over the past 10 years; thus immunity to malaria has probably also declined. Because patients with hyperparasitaemia are unable to control their infection, changes in immunity are unlikely to have contributed substantially to the slowing of parasite clearance. The absence of any relation with age in this study provides strong support for this contention.
Similarly, although previous malaria exposure has a weak effect on parasite clearance half-life (table 1), this finding cannot explain the temporal trends noted (appendix). The artemisinin combination treatment partner drug mefloquine has been used in this region for more than 25 years, and resistance developed rapidly after initial deployment. The proportion of P falciparum isolates with increased pfmdr1 copy numbers (the main determinant of mefloquine resistance) has been increasing over the past decade.16 However, the temporal changes in parasite clearance half-lives for patients given artesunate monotherapy for more than 48 h before administration of partner drugs were closely related to those reported in the full dataset, showing that resistance to the partner drug does not contribute substantially to the changes recorded (figure 2).
The genotyping data provide compelling evidence that parasite genetic factors explain the fall in parasite clearance rates. In this low transmission setting, different patients are often infected with genetically very similar parasites, allowing assessment of the contribution of genetic factors to in-vivo phenotypes.15,30 After adjustment for covariates, the heritability of parasite clearance increased during the study period, from 32% between 2001 and 2004, to 58% between 2007 and 2010, which is consistent with an increasing prevalence of alleles determining slow parasite clearance.20 The increased contribution of genetics was confined largely to the parasites with the slowest clearance rates. The high heritability of slow parasite clearance suggests that this trait will spread rapidly under continued selection.
This study shows the importance of longitudinal detailed monitoring to detect the early emergence of antimalarial drug resistance. Hyperparasitaemic patients provide important information on temporal trends in artemisinin susceptibility because confounding by immunity and partner drug effects is minimised and clearance rates can be assessed accurately (>10 sequential parasite counts for individual patients in this analysis). The slope, and thus the derived parasite clearance half-life of the exponential decline (ignoring the variable lag phase) is judged the key pharmacodynamic determinant of the artemisinins.21 No evidence suggests saturation or density dependence in parasite clearance rates. Thus parasite clearances in patients with hyperparasitaemia can be compared with data from patients with lower parasitaemias, notably the artemisinin-resistant P falciparum infections in western Cambodia.
This study cannot establish whether resistance alleles present in western Cambodia and northwestern Thailand have a common origin. Parasites with identical 93-locus genotypes were not recorded at the two sites, nor were slow clearing parasites from northwestern Thailand more closely related to Cambodian parasites than were fast clearing parasites from western Thailand. The evolution of artemisinin resistance will possibly be understood when the genetic determinants of parasite clearance rates have been identified.7,31
Parasite clearance rates in northwestern Thailand are continuously distributed. Modelling of the changes in parasite clearance over time did not support selection of a single artemisinin-resistant subpopulation with the same clearance phenotype as in Cambodia—ie, a one-step process. Together with the finding that clonally identical parasites with slow parasite clearance were reported in different patients in northwestern Thailand 8 years ago, this result argues against recent importation of Cambodian parasites as the explanation for the reduction in parasite clearance.
The artesunate–mefloquine combination has been the first-line treatment for falciparum malaria on the western border of Thailand since 1994. Artesunate was added to a failing mefloquine regimen, and the high effectiveness of the combination (>90% day 42 cure rates) has been sustained since then, although evidence suggests that effectiveness is decreasing,16 with a day 42 cure rate in 86 patients in 2010 of 88·9% (95% CI 77·8–94·7). Declining artemisinin effectiveness will have an adverse effect on the treatment of uncomplicated falciparum malaria by slowing therapeutic responses and increasing treatment failure rates, and will reduce the remarkable life-saving effectiveness of artesunate in the treatment of severe malaria and hyperparasitaemic patients. The degree to which current rates of resistance compromise these benefits has not been established, although the proportion of patients developing severe malaria did not increase concomitantly with the decline in artesunate effectiveness in this location. No patient died in this series, whereas in the past the mortality rate in patients given quinine for uncomplicated hyperparasitaemia was 3%.
A potential radical approach to containment of artemisinin resistance is to try to eliminate P falciparum malaria from western Cambodia, but would this strategy be justified if resistance has already spread or emerged elsewhere?7 Genetically determined resistance to artemisinins is now prevalent on the Thailand–Myanmar border, contiguous with a malaria endemic area in which a large burden of uncontrolled disease exists, so containment efforts will need to be expanded and surveillance and control strategies re-examined. Whether the fitness and transmissibility of the resistant parasites in the two locations are similar cannot be established yet, and thus elimination of falciparum malaria in western Cambodia might still be beneficial through prevention of the spread of higher degrees of resistance.
Identification of a molecular marker will be crucial to monitor the distribution and spread of resistance and to understand the evolution of this trait and the mechanism of action of artemisinin. The large numbers of patients infected with malaria, high heritability, and the broad range of parasite clearance half-lives make the Thailand–Myanmar border region ideal for powerful association studies.32 Clinical studies are needed urgently to map further spread and to establish the effect of different degrees of artemisinin resistance on treatment effectiveness and transmissibility.
Acknowledgments
Acknowledgments
The clinical study was done as part of the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme supported by the Wellcome Trust. Molecular work was funded by National Institutes of Health grant R01 AI048071 awarded to TJCA and done in facilities constructed with support from Research Facilities Improvement Programme (grant number C06 RR013556) from the National Center for Research Resources, National Institutes of Health. We are grateful to all the patients and staff who contributed to data collection, to Verena Carrara for providing additional data and the maps, to Ian Cheeseman, to Christine Luxemburger, and to Ric Price. The genetic analysis was stimulated by discussions with Carol Sibley and Philippe Guerin (Worldwide Antimalarial Resistance Network).
Contributors
TJCA, StN, EAA, FN, and NJW contributed to study design. EAA, RM, AD, APP, CIM, KML, and FN collected clinical data. StN, ShN, and SA-S did molecular work. StN, KS, EAA, TJCA, and FN analysed the data. StN, TJCA, EAA, FN, PS, NPJD, and NJW wrote the manuscript. APP and SN contributed equally to this work. TJCA and FN also contributed equally.
Conflicts of interest
NJW is co-chairman of the WHO antimalarial treatment guidelines committee. All other authors declare that they have no conflicts of interest.
Supplementary Material
References
- 1.Noedl H, Se Y, Schaecher K. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Global malaria control and elimination: report of a meeting on containment of artemisinin tolerance. World Health Organization; Geneva: 2009. [Google Scholar]
- 4.Dondorp AM, Yeung S, White L. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 5.Verdrager J. Epidemiology of the emergence and spread of drug-resistant falciparum malaria in South-East Asia and Australasia. J Trop Med Hyg. 1986;89:277–289. [PubMed] [Google Scholar]
- 6.Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987;3:241–246. doi: 10.1016/0169-4758(87)90147-5. [DOI] [PubMed] [Google Scholar]
- 7.White NJ. Artemisinin resistance—the clock is ticking. Lancet. 2010;376:2051–2052. doi: 10.1016/S0140-6736(10)61963-0. [DOI] [PubMed] [Google Scholar]
- 8.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 9.Anderson TJ, Roper C. The origins and spread of antimalarial drug resistance: lessons for policy makers. Acta Trop. 2005;94:269–280. doi: 10.1016/j.actatropica.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Maude RJ, Pontavornpinyo W, Saralamba S. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malar J. 2009;8:31. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . Global plan for artemisinin resistance containment (GPARC) World Health Organization; Geneva: 2011. [Google Scholar]
- 12.Stepniewska K, Ashley E, Lee SJ. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2011;201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 14.Saralamba S, Pan-Ngum W, Maude RJ. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 2011;108:397–402. doi: 10.1073/pnas.1006113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson TJ, Nair S, Nkhoma S. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J Infect Dis. 2010;201:1326–1330. doi: 10.1086/651562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrara VI, Zwang J, Ashley EA. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–262. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 18.Luxemburger C, Nosten F, Raimond SD, Chongsuphajaisiddhi T, White NJ. Oral artesunate in the treatment of uncomplicated hyperparasitemic falciparum malaria. Am J Trop Med Hyg. 1995;53:522–525. doi: 10.4269/ajtmh.1995.53.522. [DOI] [PubMed] [Google Scholar]
- 19.WHO Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(suppl 1):1–90. [PubMed] [Google Scholar]
- 20.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era—concepts and misconceptions. Nav Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 21.Flegg JA, Guerin P, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bethell D, Se Y, Lon C. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One. 2011;6:e19283. doi: 10.1371/journal.pone.0019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J, Socheat D, Tan B. Randomized trials of artemisinin-piperaquine, dihydroartemisinin–piperaquine phosphate and artemether-lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia–Thailand border area. Malar J. 2011;10:231. doi: 10.1186/1475-2875-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noedl H, Se Y, Sriwichai S. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in Southeast Asia. Clin Infect Dis. 2010;51:e82–e89. doi: 10.1086/657120. [DOI] [PubMed] [Google Scholar]
- 25.Na-Bangchang K, Ruengweerayut R, Mahamad P. Declining in efficacy of a three-day combination regimen of mefloquine–artesunate in a multi-drug resistance area along the Thai–Myanmar border. Malar J. 2010;9:273. doi: 10.1186/1475-2875-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrmann S, Sasi P, Mwai L. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS One. 2011;6:e26005. doi: 10.1371/journal.pone.0026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antao T, Hastings IM. Environmental, pharmacological and genetic influences on the spread of drug-resistant malaria. Proc Biol Sci. 2011;278:1705–1712. doi: 10.1098/rspb.2010.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roper C, Pearce R, Bredenkamp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 29.Alifrangis M, Lusingu JP, Mmbando B. Five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe District, Tanzania: accumulation of the 581G mutation in the P falciparum dihydropteroate synthase gene. Am J Trop Med Hyg. 2009;80:523–527. [PubMed] [Google Scholar]
- 30.Anderson TJ, Williams JT, Nair S. Inferred relatedness and heritability in malaria parasites. Proc Bio Sci. 2010;277:2531–2540. doi: 10.1098/rspb.2010.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair S, Nash D, Sudimack D. Recurrent gene amplification and soft selective sweeps during evolution of multidrug resistance in malaria parasites. Mol Biol Evol. 2007;24:562–573. doi: 10.1093/molbev/msl185. [DOI] [PubMed] [Google Scholar]
- 32.Cheeseman I, Miller BA, Nair S. A major genome region underlying artemisinin resistance in malaria. Science. 2012 doi: 10.1126/science.1215966. published online April 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


