Abstract
Study Objectives:
Evaluate frequency of diagnostic testing for obstructive sleep apnea (OSA), prevalence of OSA, and factors independently associated with OSA status in adults undergoing bariatric surgery.
Design, Settings and Interventions:
Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) is an observational cohort of 2,458 adults undergoing bariatric surgery at 10 U.S. hospitals. Within 30 days prior to surgery, researchers determined if participants had a diagnostic polysomnography (PSG) in the previous 12 months. When available, apnea-hypopnea index (AHI) was recorded. Based on medical records and participant report, research clinicians recorded OSA status and positive airway pressure (PAP) use. Participants completed the Berlin Questionnaire (BQ). Multivariable logistic regression was used to determine factors independently associated with AHI-confirmed OSA status.
Results:
28.7% (n = 693) of participants had a PSG within 12 months before surgery. Of subjects with AHI available (n = 509), 80.7% (n = 411) had OSA (AHI ≥ 5); 83.0% (n = 341) reported PAP use. In participants without a known AHI (n = 1,949), 45.4% (n = 884) had self-reported OSA; 81.2% (n = 718) reported PAP use. Self-reported history of snoring and pauses in breathing (odds ratio [OR] = 10.0; 95%, confidence interval [CI] = 4.8–20.6), male sex (OR = 5.1; 95% CI = 1.7–15.3), older age (OR = 1.4; 95% CI = 1.2–1.6 per 5 years), and larger sagittal abdominal diameter (OR = 1.8; 95% CI = 1.2–2.5 per 5 cm) were independently associated with a greater odds of confirmed OSA.
Conclusions:
Preoperative diagnostic testing for OSA was infrequent. Prevalence estimates of OSA differed greatly between those with and without a past-year AHI (81% vs. 46%, respectively). Most BQ responses did not differentiate OSA status, but endorsement of snoring and pauses in breathing was independently associated with presence of OSA.
Citation:
Khan A; King WC; Patterson EJ; Laut J; Raum W; Courcoulas AP; Atwood C; Wolfe BM. Assessment of obstructive sleep apnea in adults undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. J Clin Sleep Med 2013;9(1):21–29.
Keywords: Obesity, sleep apnea, polysomnography, bariatric surgery, Berlin Questionnaire
Approximately 113,000 adults undergo bariatric surgery annually in the United States.1 Although the reported prevalence of obstructive sleep apnea (OSA) in bariatric surgery patients has been highly variable (ranging from 41% to 98%),2–9 it is clearly substantially higher than the 2% to 4% prevalence in the general adult population.10,11 There is evidence to suggest that OSA is independently associated with increased risk of perioperative and postoperative complications,12,13 including following bariatric surgery.2 In addition, among patients with OSA undergoing general surgery, treatment of OSA has been associated with a decreased risk of perioperative complications.14,15 Thus, it has been argued that preoperative assessment and treatment of OSA is indicated in this population.4,8 However, to date, neither the American Academy of Sleep Medicine or the American Society for Metabolic and Bariatric Surgery has published guidelines regarding screening, testing, or treatment for OSA prior to bariatric surgery.
It is very difficult to make a diagnosis of OSA based on history and physical examination.16,17 The standard method for diagnosing OSA is a polysomnogram (PSG), which requires an overnight stay in a sleep laboratory. OSA is primarily treated with the use of positive airway pressure (PAP), either continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP), during sleep. Most bariatric centers refer patients with clinical symptoms of OSA for PSG but do not routinely refer all patients for preoperative testing, given the inconclusiveness of evidence supporting the benefits of preoperative treatment, and the knowledge that OSA often improves or resolves postoperatively.18–22 As an example, in a study of 1,095 patients who underwent laparoscopic Roux-en-Y gastric bypass at an academic medical center in the US, screening for OSA was not useful in predicting postoperative pulmonary complications, provided all patients were observed in a monitored setting and their pulmonary status optimized by aggressive incentive spirometry and early ambulation.22 In addition, many patients may be unable or unwilling to undergo PSG or use PAP due to the cost, time, and discomfort associated with testing and treatment.23 For example, in a study by Chung and colleagues, of 2,467 general surgery patients invited to undergo PSG as part of preoperative work up, only 211 underwent PSG (8.6%).
BRIEF SUMMARY
Current Knowledge/Study Rationale: The prevalence of obstructive sleep apnea and the role of screening questionnaires in patients presenting for bariatric surgery is not clear.
Study Impact: This study shows that 80% of subjects who had polysomnography within 12 months from bariatric surgery had sleep apnea. 60% of those subjects had moderate or severe sleep apnea. Endorsement of snoring and pauses in breathing in history was independently associated with presence of sleep apnea.
The cost and inconvenience of PSG has led to the development of several screening tools designed to determine patient risk of OSA. The Berlin Questionnaire (BQ), one of the most widely used screening tools, has been validated as a predictor of OSA in the primary care setting24 and is now commonly used to assess need for PSG.24–28 It has, however, not performed as well in the general surgery population,23,27 and its utility in the bariatric surgery population is unproven. Given that obesity is an indicator of increased OSA risk per the BQ scoring scheme, the discriminatory ability of the BQ may be worse among bariatric surgery patients, all of whom are obese. However, perhaps the utility of the BQ could be improved in this population with an alternative scoring scheme, or if it was used in conjunction with participant characteristics, such as age, sex, BMI, and neck circumference, which are associated with OSA status and severity in bariatric surgery patients.5,8,29,30
To address short comings in the literature, this study aimed to (1) determine the frequency of diagnostic testing for OSA in preparation for bariatric surgery in a large cohort of adults (n = 2,458) undergoing bariatric surgery at ten hospitals throughout the United States, (2) report the prevalence of OSA and PAP use among patients who did and did not undergo PSG in preparation for surgery, (3) determine whether BQ responses and scores (using traditional and alternative scoring algorithms) differ by OSA status and PAP use, and (4) determine factors independently related to confirmed OSA status.
METHODS
Participants
The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) is a prospective, multicenter observational cohort study designed to assess the risks and benefits of bariatric surgery.31 Between February 1, 2006, and February 17, 2009, patients ≥ 18 years old, seeking bariatric surgery by participating surgeons at 10 hospitals throughout the United States, were invited to participate if they had not had previous weight loss surgery. Of those consenting, 2,458 participants proceeded to surgery before the end of recruitment. All centers had institutional review board approval, and all participants provided informed consent. The study is registered at www.clinicaltrials.gov (NCT00465829).
Measures
Assessment of Obstructive Sleep Apnea
Within 30 days prior to scheduled surgery, participants attended a research visit. Researchers determined whether participants had a diagnostic PSG in preparation for bariatric surgery as part of clinical care within the previous 12 months. When PSG test results could be obtained from the medical record, the apnea-hypopnea index (AHI) of the diagnostic study was recorded. OSA severity was defined by AHI as mild (AHI 5 to < 15), moderate (AHI 15 to < 30), or severe (AHI ≥ 30); an AHI < 5 was considered indication of no OSA. In addition, based on medical records and participant-report, research-certified clinicians recorded whether participants ever had a diagnosis of OSA, and if so, whether they currently used CPAP or BPAP therapy or had ever had surgery (i.e., uvulopalatopharyngoplasty) for OSA.
Berlin Questionnaire
The BQ is a validated screening tool designed to assess the risk for sleep disordered breathing. It consists of 5 questions concerning snoring (category 1), 4 questions addressing daytime sleepiness (category 2); one question assessing history of high blood pressure, and 2 questions assessing height and weight, which are used to calculate body mass index (BMI) as a measure of obesity (category 3).24 In LABS-2, participants completed the 9 snoring and sleepiness BQ items based on their current symptoms, even if they were treated with PAP. Hypertension, height, and weight were assessed by research-certified clinicians.32 Per the traditional BQ scoring algorithm, patients were classified as high risk of sleep disordered breathing if ≥ 2 categories were positive. In addition, 3 alternative scoring schemes that are less influenced by diagnosis of obesity were applied (Table 1).
Table 1.
Berlin Questionnaire scoring schemes to define high risk for obstructive sleep apnea

Additional Measures
Given the difficulty of identifying the narrowest point of the torso on adults with severe obesity, waist circumference was measured at the midpoint between the highest point of the iliac crest and lowest part of the costal margin in the mid-axillary line with a fiberglass retractable tape measure to the nearest tenth of a centimeter. Neck circumference was measured in the standing position at the end of exhalation at the midpoint between the participant's chin and clavicle. In addition, given the difficulty of measuring sagittal abdominal diameter ([SAD] a measure of visceral obesity) in adults with severe obesity, SAD was measured with the participant lying flat, as opposed to standing, as the distance from the table to the highest point of the abdomen, to the nearest quarter inch with a SAD ruler. This value was multiplied by 2.54 to convert inches to centimeters.
Age, sex, race, ethnicity, marital status, education, employment status, household income, and smoking status were assessed by questionnaire. Chronic medical conditions and medication use were determined with a combination of self-report, clinical assessment, and medical chart review. An index of comorbidities was created as the number of the following 10 comorbidities (range 0–10): diabetes, hypertension, ischemic heart disease, congestive heart failure, history of stroke, pulmonary hypertension, asthma, history of deep vein thrombosis or pulmonary embolism, venous edema with ulcerations, and inability to walk 200 feet (61 meters).
Depressive symptoms were assessed with the Beck Depression Inventory (BDI), version 1.0.33 BDI scores were used to categorize participants as not depressed (0–9), or having mild to moderate (10–18), moderate to severe (19–29), or severe (30–63) depressive symptoms.
Statistical Analysis
Descriptive statistics were used to report frequency of preoperative diagnostic testing for OSA, and prevalence of OSA and PAP use by testing status. The relationship between OSA severity (i.e., mild, moderate, and severe) and PAP use was determined with the Jonckheere-Terpstra Trend test among subjects with confirmed OSA (i.e., past-year AHI ≥ 5). Other analyses were limited to subjects with confirmed OSA status (i.e., known past-year AHI) and a non-missing BQ score (n = 427). Potential selection bias was examined by comparing select characteristics of LABS-2 participants with this data (n = 427) to those without (n = 2,031) using Pearson χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Pearson χ2 test was used to compare BQ responses and scores between those without OSA, with untreated OSA, and with treated (by PAP) OSA. Multivariable logistic regression was used to identify participant characteristics (i.e., sex, age, race, ethnicity, marital status, education, smoking status, BMI, waist circumference, neck circumference, resting heart rate, comorbidities [considered individually and as an index], and depressive symptoms) and BQ variables (from the traditional and 3 alternative scoring schemes) that were independently associated with confirmed OSA (AHI ≥ 5). Nonsignificant factors (p < 0.05) were dropped from the model. The C-static was computed from the model of predicted relative likelihoods as a measure of the model's discriminator capacity. Predicted probabilities from the logistic model were used to classify participants into 2 groups (high risk and low risk of OSA) for different cut points. The predictive ability of the multivariable score with each cut point was determined. Data analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary NC, 2000).
RESULTS
LABS-2 participants (n = 2,458) were 78.6% female, with a median age of 45.5 years (range: 18–78 years) and BMI of 45.9 kg/m2 (range: 33.0–94.3 kg/m2). One-eighth (11.9%) of participants were ≥ 60 years. Less than one-third (28.7%; n = 693, 46 missing) of LABS-2 participants had a diagnostic PSG within the 12 months prior to bariatric surgery. However, there was significant site variation (range: 5% to 71%, median: 17.5%). Of those who underwent a diagnostic PSG, an AHI was available for 509 participants, of whom 80.7% (n = 411) had OSA (AHI ≥ 5) (Figure 1). PAP use was reported by 83.0% (n = 341) of participants with confirmed OSA; there was no statistically significant trend between OSA severity and PAP use (p = 0.15). There was significant site variation with respect to OSA prevalence and PAP use (i.e., 60% to 100% of those with a past-year diagnostic test had OSA (AHI ≥ 5), of whom 50% to 93% reported PAP use). Among the 1,949 participants without an available AHI from the previous 12 months, 45.4% (n = 884) self-reported OSA, 81.2% (n = 718) of whom reported PAP use (Figure 1). Overall, 1.5% (n = 37) of participants ever had surgery for OSA.
Figure 1. Confirmed and self-reported obstructive sleep apnea and self-reported PAP use in LABS-2 participants.
AHI, apnea hypopnea index; OSA, obstructive sleep apnea; PAP, positive airway pressure. *Includes participants who underwent diagnostic polysomnography in the past 12 months in preparation for bariatric surgery, but whose results could not be obtained (n = 184), and participants who did not undergo polysomnography in the past 12 months (n = 1,765).
Of the 509 participants with an available past-year AHI, 473 (92.9%) completed at least part of the BQ. However, due to missing items, risk for sleep disordered breathing could only be calculated for 427 participants using the traditional scoring scheme, who were thus the focus of BQ analysis. Characteristics of those who were included (n = 427) versus those excluded (n = 2,031) from BQ analysis are shown in Table 2. Participants included in analyses had significantly smaller sagittal abdominal diameter (females only), significantly fewer had hypertension (55.0% vs. 61.1%; p = 0.02) or venous edema (4.4% vs. 7.5%; p = 0.02), and significantly more reported OSA (81.0% vs. 46.7%; p < 0.0001) and CPAP or BPAP use (67.9% vs. 38.1%; p < 0.0001). There were no statistical differences between groups in the percentage of participants with other individual comorbidities (data not shown).
Table 2.
Characteristics of LABS-2 participants included and not included in Berlin Questionnaire analysis

Table 3 shows BQ items and scores by confirmed OSA status and self-report of PAP use. Four of 427 participants with an AHI < 5 but self-reported PAP use were excluded. For most BQ items and scores, there was no significant difference between those without OSA (n = 77; 18.2%), those with treated OSA (n = 286; 67.6%), and those with untreated OSA (n = 60; 14.2%; Table 3). However, there was a significant difference in the percentage of participants who reported pauses in breathing. Only 4.6% of participants without OSA reported pauses in breathing at least daily, compared to 21.6% among those untreated for OSA and 43.9% treated for OSA (p < 0.01). The same trend held for the alternative score snoring and pauses in breathing, which utilized the following 2 BQ items: Do you snore? and Has anyone noticed that you quit breathing during your sleep?; only 9.3% of participants without OSA were high risk, compared to 40.0% untreated for OSA and 64.0% treated for OSA (Table 3).
Table 3.
Berlin Questionnaire by confirmed* obstructive sleep apnea status (N = 427)
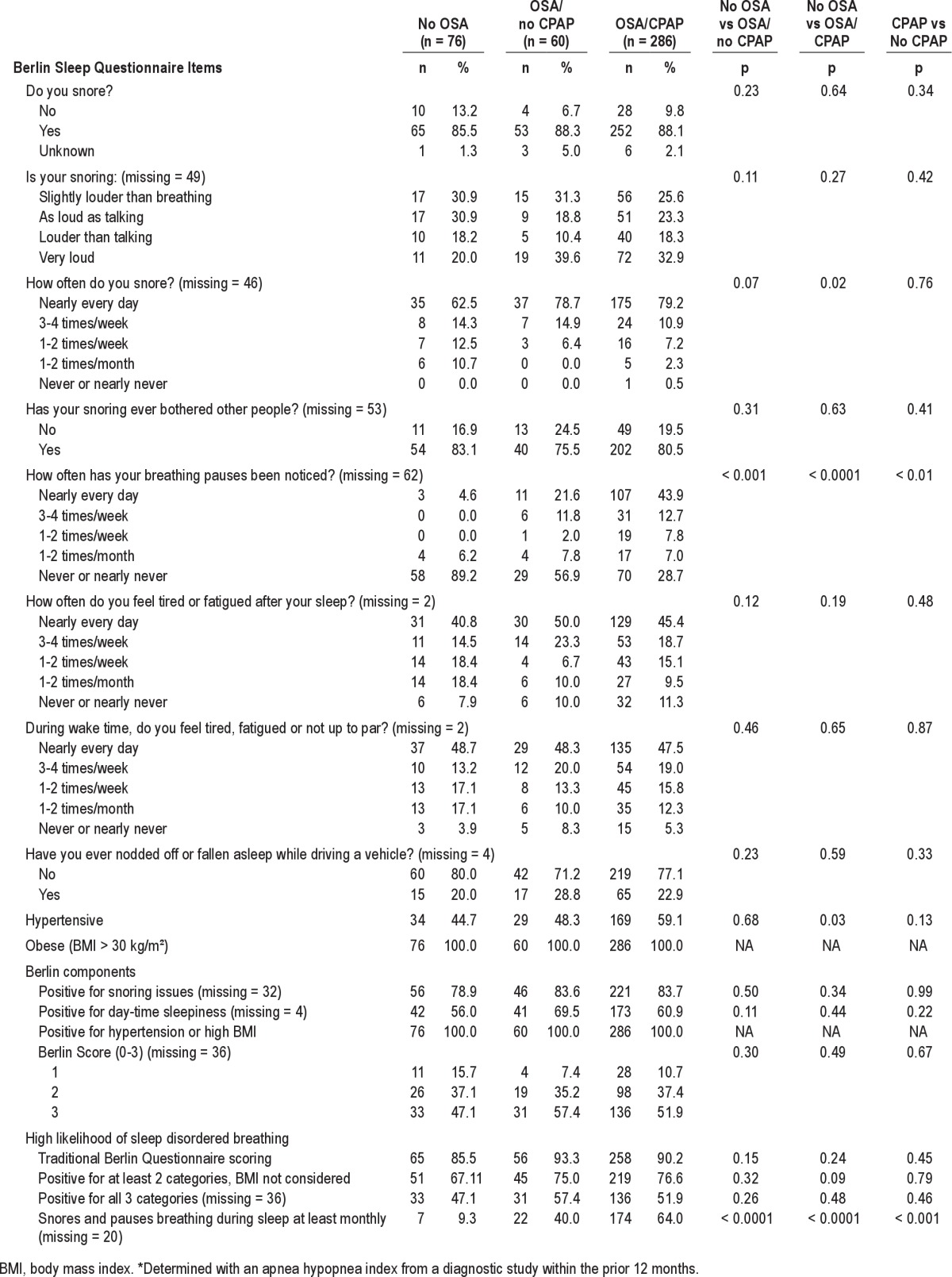
Multivariable logistic regression revealed that snoring and pauses in breathing (OR = 10.0; 95% CI = 4.8–20.6), male sex (OR = 5.1; 95% CI = 1.7–15.3), older age (OR = 1.4; 95% CI = 1.2–1.6 per 5 years), and larger SAD (OR = 1.8; 95% CI = 1.2–2.5 per 5 cm) were independently associated with a greater odds of confirmed OSA, whereas race, ethnicity, marital status, education, smoking status, resting heart rate, comorbidities, and depressive symptoms were not. Predictive accuracy of models in which SAD was replaced as an independent variable by either BMI or waist circumference were very similar (C = 0.85 and C = 0.84, respectively vs. C = 0.87 with SAD), whereas neck circumference was not independently related to OSA status (data not shown). When analysis was restricted to those not using PAP, results were similar (data not shown).
Table 4 provides the predictive ability of the multivariable score with various cut points. A cut point of 0.5 provided the highest NPV, i.e., proportion of low risk participants who did not have OSA. However, a NPV of 60.4% means that 39.6% of participants who were classified as low risk in fact had OSA. The corresponding PPV of 86.5% was better; 13.5% of participants classified as high risk did not have OSA.
Table 4.
Predictive ability of a multivariable score based on the Berlin Questionnaire and participant characteristics* to identify obstructive sleep apnea (AHI ≥ 5) in adults undergoing bariatric surgery**

DISCUSSION
Our study found that diagnostic testing for OSA in the year prior to surgery was performed in less than one-third (29%) of adults undergoing bariatric surgery at ten hospitals in the United States, indicating that, in general, PSG is not part of standard preoperative work-up. However, there was significant site variation, with frequency ranging from 5% to 71%. Prevalence of OSA was 81% among LABS-2 participants with a known past-year AHI (40.6% mild, 23.8% moderate, and 35.5% severe), compared to only 45% among those without a known past-year AHI based on participant interview and medical records review by research-clinicians. A majority of participants determined to have OSA without a past-year AHI reported PAP use, suggesting that they likely underwent PSG testing in the past. It is unknown whether participants without a known past-year AHI who were determined not to have OSA were ever evaluated by PSG making it possible that many participants were in fact undiagnosed. Thus, it is difficult to conclude whether actual prevalence of OSA in the LABS-2 cohort is closer to 53%, as determined in the entire cohort from a combination of assessment methods, or 81%, as determined in the subsample who had past-year AHI available. It is interesting to note that this prevalence of OSA in the subsample with past-year AHI is consistent with several small single-center studies that determined OSA status via PSG (prevalence range: 72–98%),3–8 while the prevalence in the group without a known past-year AHI is in line with a large cohort study in which OSA prevalence (41%) was determined with a set of screening questions.9
Self-reported PAP compliance (i.e., use among those with OSA), which was similar between participants with and without past-year AHI (83.0% and 81.2%, respectively), was higher than objectively determined compliance reported in the general population, which is between 40% and 60% one year after starting PAP.34,35 Differences in assessment method may explain this discrepancy since patient self-report of PAP compliance is generally higher than compliance based on electronic data downloaded from PAP machines.36 However, it is also possible that this population is more compliant in general, or was more compliant than usual in the immediate period leading up to surgery. While it might be expected that those with more severe symptoms are more compliant, there was not a significant relationship between OSA severity and reported PAP use in this sample.
The BQ was a product of a 1996 conference on sleep disorders in primary care, which involved 120 U.S. and German pulmonary and primary care physicians.24 Questions were selected to elicit factors or behaviors that predicted the presence of sleep-disordered breathing based on literature. In a validation study of the BQ in a primary care setting, 744 patients returned the questionnaire of whom 279 (37.5%) reported, high risk of sleep disordered breathing. In a subset of 100 patients who underwent limited channel PSG, BQ predicted a respiratory disturbance index ≥ 5 with a sensitivity of 86%, a specificity of 77%, a PPV of 89%, a NPV of 71% and a likelihood ratio (LR) of 3.79,24 suggesting that it does a fair job of detecting important symptom distributions and permits risk grouping in primary care. However, in a study of 177 patients presenting for general surgery, 61% of whom were high risk according to the BQ, the BQ's predictive ability declined. Sensitivity was 68.9%, specificity was 56.4%, PPV was 77.9% and NPV was 44.9% in predicting AHI ≥ 5.27 Thus, 55.1% of low risk patients in fact had OSA.
Given the varied utility of the BQ in various populations, and the high prevalence of OSA in bariatric surgery patients, our study aimed to examine whether BQ responses or risk assignment differed between patients with no OSA, untreated OSA and treated OSA. Using the traditional BQ scoring scheme, there was not a significant difference in the percentage of high-risk participants between groups. However, with the traditional BQ scoring scheme, all bariatric surgery patients are positive for the obesity/hypertension category regardless of hypertension status. Thus, they are categorized as high risk if they are positive for snoring issues or daytime sleepiness, which may be problematic as excessive daytime sleepiness is more common in obese compared to non-obese adults in the absence of OSA.37 In an attempt to increase the BQ's ability to differentiate participants based on their OSA status, three alternative scoring schemes were examined (Table 1). Only 1 alternative scheme produced a risk assignment that differed significantly between groups. When participants were categorized based on just two items from the BQ (Do you snore? and Has anyone noticed that you quit breathing during your sleep?), 12.5% without OSA were high risk vs. 40.0% with untreated OSA and 64.0% treated OSA. Further analysis was conducted to determine whether the BQ could be used in conjunction with patient characteristics to create a risk score with adequate predictive ability, and this did not result in reportable findings. Of the many variables considered, sex, age, SAD, and the BQ risk assignment based on the snoring and pauses in breathing score had an independent relationship with OSA. However, although the C-statistic of the multivariable model was representative of excellent discriminative ability (C = 0.87), applying the optimal cut point to the score resulted in a NPV of 60% (i.e., 40% of participants (in this case 19/48) who were classified as low risk in fact had OSA). The corresponding PPV of 86.5% was better (i.e., only 13.5% of participants classified as high risk did not have OSA). However, considering that 19.8% of participants in the sample did not have OSA, a scheme that considered everyone high risk would result in a PPV of 80.2%. Thus, it appears that a score based on the BQ and patient characteristics is inadequate for determining whether bariatric surgery candidates, with an OSA prevalence of > 80%, can forgo PSG testing.
In a comparable analysis, Sareli and colleagues explored whether they could predict which preoperative patients did not have OSA by using the Epworth Sleepiness Scale (ESS) and the multivariable apnea prediction (MAP) score in combination with select patient characteristics.8 Results were very similar; male sex, age, and BMI (which was an optional replacement for SAD in our best multivariable model) were independently related to OSA status when considered in conjunction with the MAP symptom score (based on 3 items from the MAP questionnaire). However, the NPV of 75% was too low for the scoring symptom to be clinically useful.
Dixon et al. developed a prediction model for OSA using sex, age, BMI, observed sleep apnea, hemoglobin A1c, fasting plasma insulin, and neck circumference.30 A score ≥ 3 provided a sensitivity and specificity of 89% and 81%, respectively, for an AHI ≥ 15 in patients undergoing laparoscopic adjustable gastric band surgery who were suspected to have OSA (n = 99). However, the sensitivity and specificity was much lower at 75% and 57%, respectively, when Kolotkin et al. tested this model in a study sample of gastric bypass patients (n = 310) with no pre-selection for OSA symptoms.38 Kolotkin et al. also developed an alternate prediction model for OSA with 10 unique predictors: neck circumference, systolic blood pressure, waist/hip ratio, waist circumference, glucose, loud snoring, age, frequent snoring, BMI, and male gender. The presence of ≥ 5 of these predictors improved the sensitivity (77%) and specificity (77%) in predicting an AHI ≥ 15/h.38 Still, the authors concluded that the sensitivity and specificity of their model was not accurate enough to suggest that routine PSG testing could be eliminated.
Our study has several limitations. We were unable to report confirmed OSA prevalence in the entire LABS-2 cohort. While the prevalence of OSA among participants with a past-year PSG and available AHI was 81%, using self-report data for those without confirmed OSA, the prevalence of OSA in the entire cohort (n = 2,458) was much lower (53%). It is unclear whether this discrepancy is due to undiagnosed OSA in the group which did not report having undergone a past-year PSG (OSA prevalence of 45%), or actual differences in OSA prevalence between those who did and did not undergo testing. Given the possibility of undiagnosed OSA in the untested group, BQ analysis was limited to study participants with a known past-year AHI. Another important limitation of this study is that the majority of participants with confirmed OSA reported PAP use, which might have decreased their OSA symptoms, thus affecting their BQ responses. In particular, more participants with OSA might have been determined to be high risk according to the various BQ scoring schemes if not treated by PAP. However, there was no significant relationship between PAP use and severity of OSA as determined by AHI. Nor was there a significant relationship between PAP use and high risk status for the first three scoring algorithms, while a greater percentage of participants reporting PAP use were actually high risk with the fourth algorithm, due to a greater percentage reporting pauses in breathing. Still, it is possible that more participants with PAP use would have been categorized as high risk if their BQ responses were based on their untreated symptoms. Therefore, we did not test the predictive ability of the BQ alone for OSA, but rather determined if responses to individuals items and risk groups differed by OSA and PAP status. In addition, we repeated the analysis to identify factors independently associated with OSA status, excluding participants who reported PAP use, and found that results were very similar. Of note, we did not assess whether those self-reporting PAP use were compliant with their physician's suggested use (e.g., > 4 h, ≥ 70% of the time39), and it is not possible for us to confirm if subjects reporting PAP use were adequately treated for their OSA. Although a majority of treated participants still reported symptoms indicative of OSA, most of their responses did not significantly differ from those without OSA. Finally, self-reported OSA has been shown to be independently related to greater risk of short-term postoperative complications following bariatric surgery.2 Although peri- and postoperative complications were measured in the LABS-2 cohort, given the sample size of those with past-year AHI (n = 509), the high prevalence of OSA, and the low incidence of short-term postoperative complications, we were unable to test whether (1) confirmed OSA status, (2) severity of OSA, or (3) CPAP/BPAP use among those with OSA, were related to complications. A retrospective analysis from the Mayo clinic reported that severity of OSA was not associated with perioperative complications in patients treated adequately for OSA with CPAP.40 However, more work is needed to make a strong case that testing and adequate treatment of OSA prior to bariatric surgery specifically reduces surgical complications.
In summary, in this large cohort study of adults undergoing bariatric surgery from ten hospitals throughout the United States, less than one-third of participants underwent preoperative diagnostic testing for OSA within 12 months prior to surgery. The discrepancy between documented and self-reported OSA prevalence suggests that prevalence estimates of OSA may be greatly affected by testing practices and data collection methods, and that PSG testing may be underutilized in this population. Finally, our results suggest that although items from the BQ and participant characteristics are significantly related to OSA, given the high prevalence of OSA in this population, neither may be clinically useful in selecting which patients can safely forgo PSG prior to bariatric surgery. Future studies are needed to determine whether treating OSA prior to surgery reduces perioperative risk and if bariatric surgery leads to complete resolution of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Akram Khan has received grant support from United Therapeutics. Anita Courcoulas has received research grants from Allergan, Pfizer, Covidien, and EndoGastric Solutions, and is both a consultant for and on the Scientific Advisory Board of Ethicon J – J Healthcare System. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: Data Coordinating Center - U01 DK066557; Columbia-Presbyterian - U01-DK66667; University of Washington - U01-DK66568 (in collaboration with GCRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center - U01-DK66585; Oregon Health – Science University - U01-DK66555. This paper was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
REFERENCES
- 1.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200:378–85. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009 Jul 30;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YH, Johan A, Wong KK, Edwards N, Sullivan C. Prevalence and risk factors for obstructive sleep apnea in a multiethnic population of patients presenting for bariatric surgery in Singapore. Sleep Med. 2009;10:226–32. doi: 10.1016/j.sleep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–6. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 5.Sharkey KM, Machan JT, Tosi C, Roye GD, Harrington D, Millman RP. Predicting obstructive sleep apnea among women candidates for bariatric surgery. J Womens Health (Larchmt) 2010;19:1833–41. doi: 10.1089/jwh.2009.1859. [DOI] [PubMed] [Google Scholar]
- 6.Valencia-Flores M, Orea A, Castano VA, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res. 2000;8:262–9. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 7.Yeh PS, Lee YC, Lee WJ, et al. Clinical predictors of obstructive sleep apnea in Asian bariatric patients. Obes Surg. 2010;20:30–5. doi: 10.1007/s11695-009-9854-2. [DOI] [PubMed] [Google Scholar]
- 8.Sareli AE, Cantor CR, Williams NN, et al. Obstructive sleep apnea in patients undergoing bariatric surgery-a tertiary center experience. Obes Surg. 2011;21:316–27. doi: 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 9.Grunstein RR, Stenlof K, Hedner JA, Sjostrom L. Impact of self-reported sleep-breathing disturbances on psychosocial performance in the Swedish Obese Subjects (SOS) Study. Sleep. 1995;18:635–43. doi: 10.1093/sleep/18.8.635. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 12.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. quiz 117-8. [DOI] [PubMed] [Google Scholar]
- 13.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 14.Stierer TL, Wright C, George A, Thompson RE, Wu CL, Collop N. Risk assessment of obstructive sleep apnea in a population of patients undergoing ambulatory surgery. J Clin Sleep Med. 2010;6:467–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Gali B, Whalen FX, Schroeder DR, Gay PC, Plevak DJ. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110:869–77. doi: 10.1097/ALN.0b013e31819b5d70. [DOI] [PubMed] [Google Scholar]
- 16.Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16:118–22. [PubMed] [Google Scholar]
- 17.Viner S, Szalai JP, Hoffstein V. Are history and physical examination a good screening test for sleep apnea? Ann Intern Med. 1991;115:356–9. doi: 10.7326/0003-4819-115-5-356. [DOI] [PubMed] [Google Scholar]
- 18.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141:354–8. doi: 10.1016/j.surg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Varela JE, Hinojosa MW, Nguyen NT. Resolution of obstructive sleep apnea after laparoscopic gastric bypass. Obes Surg. 2007;17:1279–82. doi: 10.1007/s11695-007-9228-6. [DOI] [PubMed] [Google Scholar]
- 20.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13:58–61. doi: 10.1381/096089203321136593. [DOI] [PubMed] [Google Scholar]
- 21.Higa K, Ho T, Tercero F, Yunus T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7:516–25. doi: 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Jensen C, Tejirian T, Lewis C, Yadegar J, Dutson E, Mehran A. Postoperative CPAP and BiPAP use can be safely omitted after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:512–4. doi: 10.1016/j.soard.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 24.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–38. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadi N, Chung SA, Gibbs A, Shapiro CM. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:39–45. doi: 10.1007/s11325-007-0125-y. [DOI] [PubMed] [Google Scholar]
- 27.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 28.Gus M, Goncalves SC, Martinez D, et al. Risk for obstructive sleep apnea by Berlin Questionnaire, but not daytime sleepiness, is associated with resistant hypertension: a case-control study. Am J Hypertens. 2008;21:832–5. doi: 10.1038/ajh.2008.184. [DOI] [PubMed] [Google Scholar]
- 29.Serafini F, Anderson W, Rosemurgy A, Srait T, Murr M. Clinical predictors of sleep apnea in patients undergoing bariatric surgery. Obes Surg. 2001;11:28–31. doi: 10.1381/096089201321454079. [DOI] [PubMed] [Google Scholar]
- 30.Dixon JB, Schachter LM, O'Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese: indicators for polysomnography. Chest. 2003;123:1134–41. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 31.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belle SH, Chapman W, Courcoulas AP, et al. Relationship of body mass index with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS) Surg Obes Relat Dis. 2008;4:474–80. doi: 10.1016/j.soard.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 34.Stepnowsky CJ, Jr, Moore PJ. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003;54:599–605. doi: 10.1016/s0022-3999(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 35.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15:365–9. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest. 1993;103:1675–80. doi: 10.1378/chest.103.6.1675. [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 38.Kolotkin RL, LaMonte MJ, Walker JM, Cloward TV, Davidson LE, Crosby RD. Predicting sleep apnea in bariatric surgery patients. Surg Obes Relat Dis. 2011;7:605–10. doi: 10.1016/j.soard.2011.04.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 40.Weingarten TN, Flores AS, McKenzie JA, et al. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106:131–9. doi: 10.1093/bja/aeq290. [DOI] [PubMed] [Google Scholar]



