Abstract
The mechanisms by which ethanol induces changes in behavior are not well understood. Here we show that C. elegans loss-of-function mutations in the synaptic vesicle-associated RAB-3 protein and its GTP exchange factor AEX-3 confer resistance to the acute locomotor effects of ethanol. Similarly, mice lacking one or both copies of Rab3A are resistant to the ataxic and sedative effects of ethanol, and Rab3A haploinsufficiency increases voluntary ethanol consumption. These data suggest a conserved role of RAB-3/RAB3A-regulated neurotransmitter release in ethanol-related behaviors.
Keywords: RAB-3/RAB3A, AEX-3, presynaptic, neurotransmitter release, ethanol resistance, ethanol consumption
INTRODUCTION
Ethanol has similar intoxicating effects in vertebrate and invertebrate systems. In humans, ethanol causes loss of both physical coordination and social inhibition at low doses, and incoherence and sedation at high doses. Similarly, in C. elegans and Drosophila, the same tissue concentrations of ethanol cause loss of coordination and sedation (Davies et al., 2003; Singh and Heberlein, 2000). Furthermore, mutational analysis has identified several genes as important for regulating the behavioral response to ethanol in multiple organisms. These include components of the cyclic AMP/PKA signaling pathway (Ferraro et al., 2006; Moore et al., 1998; Pandey et al., 2004; Tanaka et al., 2004; Thiele et al., 2000; Wand et al., 2001; Yang et al., 2003) and BK-type potassium channel (Cowmeadow et al., 2005, 2006; Davies et al., 2003). Taken together, these data suggest that the mechanisms of ethanol’s action may be conserved across species.
Although some molecular targets of ethanol have been identified, the mechanisms contributing to the behavioral effects of ethanol are poorly understood. Considerable evidence has established a role for ethanol at the level of the synapse. To date, biochemical and genetic studies have primarily demonstrated an effect of ethanol on postsynaptic targets, including alterations in glutamatergic and GABAergic receptors, scaffolding proteins, and intracellular signal transduction pathways (for review see Chandler, 2003). Evidence for presynaptic effects of ethanol has been less forthcoming, although Nie et al. (1997) have reported that blocking presynaptic metabotropic GABAB receptors in the rat brain enhances evoked transmitter release upon acute ethanol administration. Consistent with these observations, acute ethanol treatment increases GABA release in mouse VTA neurons and chronic ethanol treatment increases GABA release in rat central amygdala (Melis et al., 2002; Roberto et al., 2004). In Drosophila, RNA interference of dGABABR1 and treatment with the GABAB antagonist CGP 54626 attenuates ethanol-induced motor impairment (Dzitoyeva et al., 2003). However, it is unclear whether these effects are regulated pre- or postsynaptically.
RAB-3/RAB3A is a small G-protein expressed almost exclusively in neurons (Fischer von Mollard et al., 1990) and interacts directly with synaptic vesicles to regulate their release (Geppert et al., 1994, Iwasaki et al., 1997, Nonet et al., 1997). To determine if genetic disruption of this presynaptic protein alters the behavioral response to ethanol, we have analyzed rab-3/Rab3A loss-of-function mutants in C. elegans and the mouse for ethanol-related behaviors.
METHODS
C. elegans methods
Nematode culture and strains
Nematodes were cultured using standard methods (Brenner, 1974). The strains used in this study were: N2 var. Bristol, rab-3(js49), rab-3(y250), aex-3(y255), aex-3(sa5) and aex-3(n2166), and cab-1(tg46). The rab-3, aex-3 and cab-1 mutants are all strong loss-of-function alleles for the respective genes. In all analyses, first day adult animals of each strain were used.
Dispersal assays
Dispersal assays were performed as described (Crowder et al., 1996) with modifications: Standard large (10 cm) NGM plates were seeded with a ring of E. coli bacteria (OP50) near the edge of the plate which was allowed to grow for 2 hours at 37°C. A 2 mm diameter copper wire was melted into the surface of the plate to divide the plate into two semicircles. A representative plate was melted to determine the volume, and 100% ethanol was added to test plates to an appropriate final concentration (0mM, 200mM or 400mM). 100% ethanol will kill bacteria and render it unattractive to the worms, so care was taken to place the ethanol away from the food. The plate was sealed with Parafilm and the ethanol was allowed to equilibrate in the agar overnight at room temperature. On the day of the assay, approximately 100 animals of each strain were incubated in M9 buffer and ethanol [(22mM KH2PO4, 22mM Na2HPO4, 85mM NaCl, 1mM MgSO4) and the appropriate concentration of ethanol (0mM, 200mM or 400mM)] for 20 minutes. Worms were spun down, and the pellet was pipetted to a central spot near the dividing bar, and the worms were allowed to move toward the food. Mutant and control strains were treated in parallel and placed on the plate in opposite semicircles at the same time. Worms were counted at 10-minute intervals. A dispersal index (DI) was calculated: DI= worms in food/total worms in assay. In this and the analysis of locomotion experiments, we have chosen to treat the animals with an exogenous dose of 400mM ethanol for three reasons. First, treatment with 400mM ethanol generates an internal ethanol concentration that is consistent with a concentration that would intoxicate humans. Second, 400mM is sufficient to induce significant reversible behavioral inhibition in C. elegans. Third, we have used a 400mM ethanol dose in our previous acute locomotion studies (Davies et al. 2003) and have maintained this consistency to allow a comparison of results across experiments.
Analysis of locomotion
The effects of ethanol on C. elegans locomotion rate were assayed as described (Davies et al., 2003). NGM plates were used without a bacterial lawn. The assay plates were dried at 37°C for 2 hours, copper rings were melted into the surface and ethanol was added to a final concentration of 0mM or 400mM. The plates were sealed with Parafilm and left for 2 hours at room temperature. Ten animals were moved to a plate without bacteria for 30 minutes prior to the assay then placed on the assay plate and their movement recorded at 20 minutes. Subjects were video recorded for 2 minutes using a CCD camera (1 frame/second). The time-lapse recordings were analyzed using DIAS software (Solltech, Inc., Atlanta, GA, US). To minimize the effects of differences in the basal speed of each strain we calculated a relative speed for each strain (treated average speed/untreated average speed × 100, n ≥ 3). To eliminate possible plate-to-plate variation in treatment conditions, the relative locomotion of each mutant strain was compared to the locomotion of controls treated on the same ethanol (or no ethanol) plates as the experimental group.
Internal ethanol concentration measurements
Internal ethanol concentrations following exposure to ethanol were determined as follows. In order to determine the internal concentration of ethanol during the period quantified during the analysis of locomotion, ethanol treatment plates were prepared exactly as described for the analysis of locomotion. 300–600 worms were placed on dried (37°C for 2 hours) plates without bacteria that had ethanol added to a final concentration of 0mM or 400mM. The animals were left on the plates for 20 minutes before they were washed from the plate with ice-cold dH20. The animals were briefly centrifuged at 4°C to form a pellet and the supernatant was removed. The volume of the worm-containing pellet was determined and ice-cold dH20 was added to a final volume of 30 μl. The worms were frozen at −85°C for 30 min before homogenization on ice. The concentration of ethanol in the homogenate was determined according to the manufacturer’s instructions using an Alcohol Reagent Kit (Pointe Scientific, Canton, MI, US). Strain comparisons were performed using a two-tailed t-test.
Mouse methods
Subjects
Rab3Atm1Sud mice (Jackson Laboratories, Bar Harbor, ME, US) maintained on a mixed C57BL/6J X 129/SvJ background were backcrossed to wild-type C57BL/6J mice for 6 generations prior to intercrossing of Rab3Atm1Sud/+ heterozygous mice to generate progeny for behavioral testing. Male mice were housed 3–5 per cage and were 2–4 months of age at onset of behavioral testing and 4–6 months of age upon completion of testing. All animal protocols were approved by the EGCRC institutional animal care and use committee.
Behavioral Testing
Each mouse was subjected to the following series of behavioral assays: week1: stationary dowel test; weeks 2–4: loss-of righting reflex assay testing response to single 3.2, 3.6, and 4.0 doses EtOH presented weekly in pseudorandom order; weeks 5–8: 2-bottle choice test for EtOH consumption/preference; week 9: 2-bottle choice test for saccharin and quinine taste preference. A separate, drug-naïve cohort of subjects was used to assess ethanol metabolism and clearance.
Stationary dowel assay
A horizontal wooden dowel (1.5 cm diameter, 30 cm long) was suspended between two Plexiglas walls 50 cm above a cushioned surface. Mice were trained to remain on the stationary dowel for 5 min. Each mouse was then given an IP injection of 1.5 g/kg of ethanol (10% w/v in saline) and placed on the dowel. At this dose, these animals were unable to balance on the dowel. Five minutes after the mouse fell off of the dowel, it was tested (and every 5 min thereafter) until it was able to remain on the dowel for >1 min. with recovery time recorded as time from loss of balance to time of balance recovery.
Loss-of righting reflex assay
Ethanol (10% w/v in saline) was administered IP in different volumes to obtain three test doses (3.2, 3.6 or 4.0 g/kg). After injection, mice were placed on their backs and tested for loss of righting reflex. The mouse was judged to have lost the righting reflex at the time when it could not right itself three times within 30 sec. When the animal was able to right itself three times within 30 sec. it was deemed to have recovered. The duration of the loss of the righting reflex was calculated as the difference between when the reflex was lost and when it was recovered. Mice were tested at all three doses (3.2, 3.6 and 4.0 g/kg) of ethanol using a random order balanced design, with a week interval between each test session.
Ethanol metabolism and clearance
A separate group of ethanol-naïve mice were injected with 4g/kg of ethanol and tail blood samples (10 μl) obtained at 30, 60 and 120 minutes to measure blood alcohol levels. Blood ethanol content was assessed from serum using the Analox AM1 Analyzer (Analox Instruments, North Yorkshire, UK).
Two-bottle choice assay
Oral alcohol self-administration and preference were examined using a two-bottle choice protocol. Water intake (ml) and body weight (g) were recorded on alternate days for 4 days to test potential differences in fluid consumption. During this period, water was the only fluid available, but from 2 separate bottles. The animals were then given a choice between ethanol (3% w/v) and water. Two-bottle drinking sessions were conducted 23 h per day, 7 days per week. During the course of the exposure period, ethanol concentration was increased from 3% to 20% (3, 6, 10, 14, 20%) with four days access at each concentration. On alternate days, the mice were weighed and placed in individual holding chambers while the fluids were replaced in the home cage. The position (left or right) of each solution was alternated every two days to control for side preferences. One week after the ethanol self-administration procedure, the same mice were tested for saccharin (sweet) and quinine (bitter) intake and preference also using a two-bottle choice protocol. Each tastant was provided in water at 2 concentrations (saccharin: 0.03% and 0.06% w/v; quinine: 0.015mM and 0.03mM) and tested at each concentration for 2 days.
Statistical Analysis
C. elegans data were analyzed using Graphpad Prism software with the following statistical tests performed: dispersal data: 2-way ANOVA followed by Bonferroni posttests, where appropriate; locomotion and internal ethanol data: paired two-tailed t-test.
Statistical analysis of mouse behavioral data was performed using SigmaStat 3.1, with appropriate post-hoc comparisons performed as indicated by SigmaStat. Statistical tests were performed as follows: loss of righting reflex assay: 2-way ANOVA for genotype x dose, followed by Newman-Keuls posthoc comparison; ethanol metabolism and clearance: 2-way repeated measures ANOVA for genotype x time; stationary dowel assay: 1-way ANOVA for genotype; 2-bottle choice assay: 2-way repeated measures ANOVA for genotype x ethanol concentration, followed by Tukey posthoc comparison. Ethanol preference data were further analyzed within genotype using 1-way repeated measures ANOVA for ethanol concentration.
RESULTS
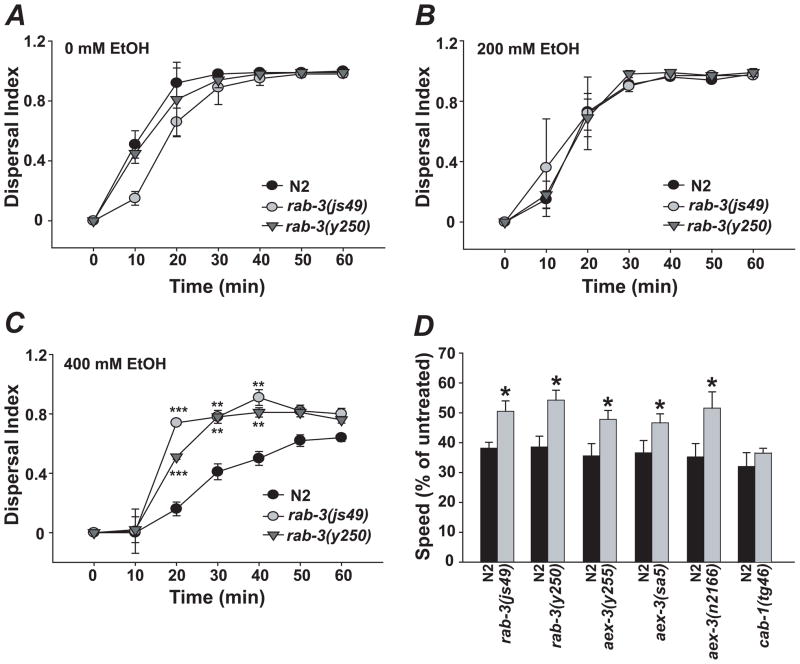
We tested existing C. elegans mutants with synaptic defects for altered responses to ethanol using a locomotor dispersal assay, which quantifies the ability of worms to move toward a bacterial food source on an agar plate (Crowder et al., 1996). We observed that rab-3 mutants exhibit significant resistance to ethanol in the dispersal assay when treated with 400 mM exogenous ethanol while dispersing normally in the absence of ethanol (Fig. 1A–C). This was observed with two different null alleles, js49 and y250).
Figure 1.
rab-3 mutants are resistant to locomotor depressant effects of ethanol. A–C, Dispersal indices (D.I.s) of wild type (N2) control, rab-3(js49) and rab-3(y250) mutants on 0, 200, and 400mM ethanol. D, rab-3(js49 and y250) and aex-3(y255, sa5 and n2166) mutants move significantly faster than wild-type (N2) controls when treated with 400mM ethanol. In contrast, cab-1(tg46) mutants have wild-type ethanol sensitivity. Error bars are mean +/− SEM. Asterisks in C indicate levels of statistical significance (*, P < 0.05; **, P < 0.01; *** P < 0.001).
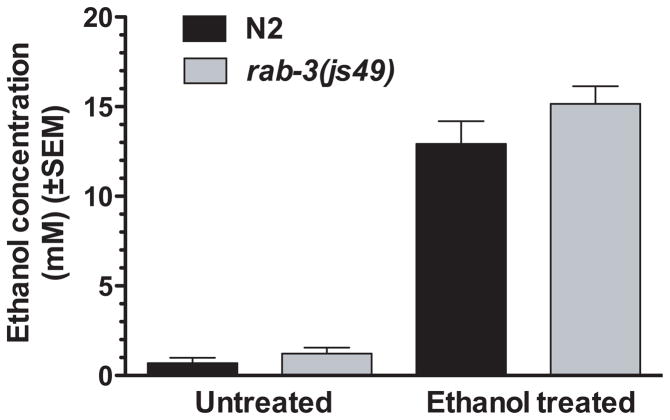
Although the dispersal assay provides a means of rapidly assessing the ethanol sensitivity of different strains, changes in the dispersal assay could arise through effects of ethanol on behaviors other than locomotion. For instance, movement of animals to bacteria is in part mediated by chemosensory neurons that are sensitive to odorants in the environment. An effect of ethanol on the activity of these neurons could influence the dispersal of animals in these assays. To more directly analyze the effects of ethanol specifically on the locomotor behavior of rab-3 mutants, we also quantified the speed of movement of animals using image analysis software. The software allows simultaneous tracking of multiple animals on the same test plate. The locomotion rates of wild-type and rab-3 animals were compared on the same plate (in the absence of food) using metal rings to isolate the groups of animals. In this assay, rab-3(js49) and rab-3(y250) showed significant resistance to ethanol (Fig. 1D, P < 0.05). We also analyzed rab-3(js49) and wild-type control animals treated with 400mM ethanol for internal ethanol concentration and found no strain effect, indicating that ethanol absorption/metabolism is normal in rab-3 mutants. (Fig. 2).
Figure 2.
rab-3 mutations do not alter ethanol pharmacokinetics in C. elegans. The internal ethanol concentration of worms was analyzed at 20 minutes of exposure to an exogenous ethanol dose of either 0mM or 400mM. Internal ethanol concentrations are substantially lower than exogenous ethanol concentrations. Internal ethanol concentrations of N2 and rab-3(js49) are not significantly different from each other at either 0mM or 400mM as tested by paired two-tailed t-test (t3 = 1.71, P = 0.19).
RAB-3 regulates synaptic vesicle trafficking in its active, GTP-bound form (Geppert et al., 1994). Although the process by which this occurs is poorly understood, several RAB-3-interacting proteins have been identified. One of these, AEX-3, is a RAB-3 guanine nucleotide exchange factor (Iwasaki et al., 1997). Loss of AEX-3 is expected to shift the equilibrium from active, GTP-bound RAB-3 to inactive, GDP-bound RAB-3, and should therefore mimic the effect of loss of RAB-3 function. We tested three aex-3 loss-of-function alleles (y255, sa5 and n2166) for sensitivity to the sedative effects of ethanol on locomotion. As predicted, disruption of aex-3 in C. elegans phenocopied the resistance to ethanol observed in rab-3 mutants (Fig. 1D, P < 0.05).
We more fully characterized ethanol’s mechanism of action in the rab-3 and aex-3 pathway. We tested the specificity of the ethanol resistance generated by loss of function in rab-3 and aex-3 by examining loss of function in another gene (cab-1) that functions in neurons with aex-3 but independently of rab-3. cab-1 encodes a protein that binds AEX-3 and has been shown to partially mediate aspects of the phenotype associated with mutations in aex-3 (Iwasaki and Toyonaga, 2000). Comparison of the ethanol sensitivity of cab-1(tg46) mutant animals with wild-type animals revealed no difference in the effect of ethanol on speed of locomotion (t4 = 1.14, P = 0.32) (Fig. 1D). The lack of ethanol resistance in the cab-1 mutant animals suggests that the resistance associated with aex-3 is due to its functional interaction with rab-3.
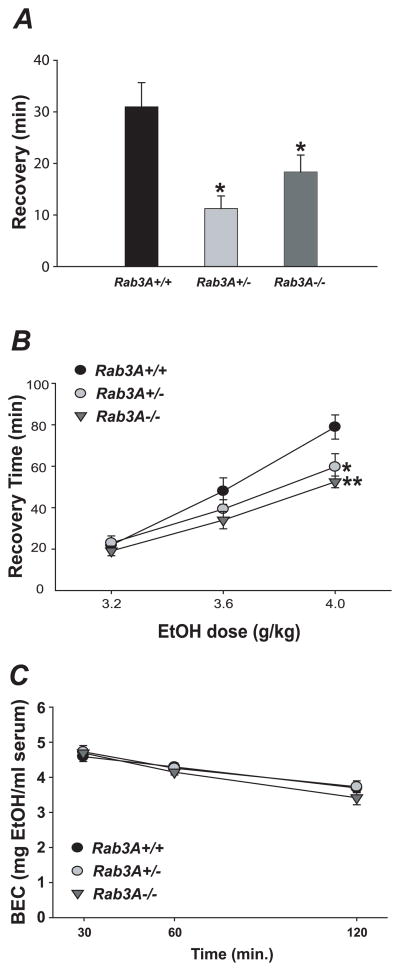
To determine if the role of rab-3 in the behavioral response to ethanol is conserved in mammals, we assayed mice carrying a previously described Rab3A null allele (Geppert et al, 1994) for altered sensitivity to the acute ataxia-inducing effects of a low, 1.5mg/ml dose ethanol. We observed a significantly shortened recovery time in both Rab3A−/− and Rab3A+/− mice relative to Rab3A+/+ controls (n = 12–14; P < 0.01) (Fig. 3A). Although not significant, we observed a trend among Rab3A+/− and Rab3A−/− mice toward increased latency to initial loss of balance in this assay, also indicative of ethanol resistance (data not shown). Mice were also tested for sensitivity to the acute sedative/hypnotic effects of ethanol in the loss-of-righting reflex assay. We observed a significant reduction in recovery time for both Rab3A−/− and Rab3A+/− mice relative to Rab3A+/+ controls at the 4g/kg dose (n = 9–14; Rab3A+/+ vs. Rab3A+/−, P < 0.01; Rab3A+/+ vs. Rab3A−/−, P < 0.001) (Fig. 3B). A drug naïve cohort of subjects was used to assess ethanol metabolism/clearance of a 4g/kg IP injection of ethanol. We failed to detect a significant main effect for genotype (F[2, 15] = 0.41, P = 0.67), indicating that Rab3A mutant mice metabolize ethanol normally (Fig. 3C). Thus, loss of either one or two copies of Rab3A results in resistance to the acute ataxic and sedative effects of ethanol.
Figure 3.
Disruption of Rab3A in the mouse results in altered ethanol-dependent behaviors. A, Rab3A−/− and Rab3A+/− mice are resistant to the ataxic effects of 1.5g/kg ethanol in the stationary dowel test. B, Rab3A−/− and Rab3A+/− mice are resistant to the sedative effects of 4g/kg ethanol in the LORR assay. C, Normal ethanol metabolism of 4g/kg IP dose is observed in Rab3A mutant mice; BEC = “blood alcohol content”. Error bars are mean +/− SEM. Asterisks in A and B indicate levels of statistical significance (*, P < 0.01; **, P < 0.001).
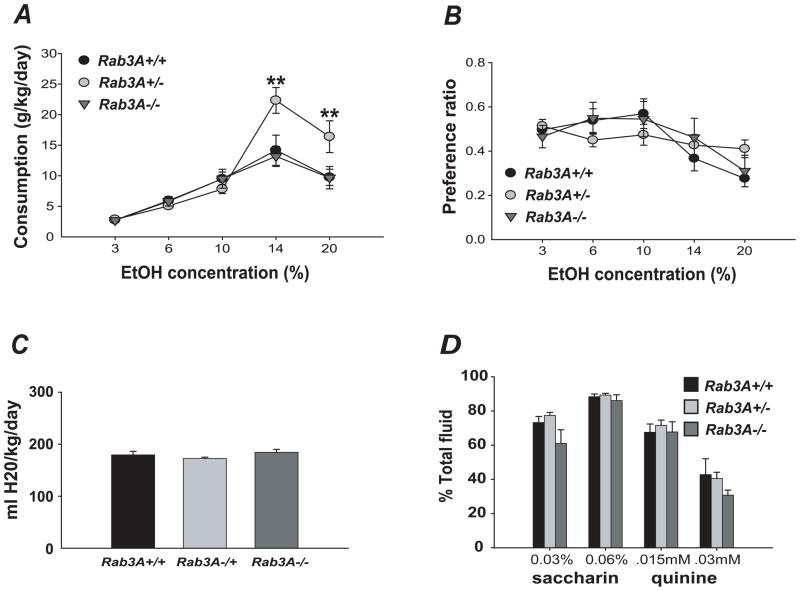
Studies in humans and several mouse genetic models have established a positive correlation between resistance to the acute effects of ethanol and the propensity for alcohol consumption/abuse (Schuckit, 1994). We therefore assessed voluntary ethanol consumption in the Rab3A mutant mice using the two-bottle choice assay. Interestingly, we observed overdominant effects in the heterozygotes: Rab3A+/− mice showed increased consumption of 14% and 20% ethanol solutions (n = 10–13, P < 0.001 for 14% ethanol, P < 0.005 for 20% ethanol). Across all ethanol concentrations, a significant genotype x solution interaction was observed (F[8, 272] = 5.16, P < 0.001; posthoc comparisons of genotype within 14%: P < 0.001 and within 20%: P < 0.005) (Fig. 4A). Although we failed to detect a significant strain effect for ethanol preference, we observed a significant genotype x concentration interaction (F[8, 272] = 2.26, P < 0.05, Fig. 4B). Further analysis revealed that ethanol preference in Rab3A+/− mice was not dependent upon ethanol concentration (n = 10, P = 0.20) in contrast to Rab3A+/+ and Rab3A−/− mice, which showed a significant concentration-dependent effect on preference (n = 11–13, P < 0.001 and P < 0.005, respectively). We observed no strain effect for consumption or preference for water, saccharin solution, or quinine solution, indicating that the increased ethanol consumption and altered preference were not due to altered taste preference or liquid consumption (Fig. 4C, D).
Figure 4.
Altered ethanol consumption and preference in Rab3A+/− mice. A, Rab3A haploinsufficiency results in increased voluntary ethanol intake. B, Ethanol preference of Rab3A+/− mice is not dependent on ethanol concentration, in contrast to Rab3A+/+ and Rab3A−/− mice. C, Normal water consumption is seen in Rab3A mutant mice. D, Normal saccharin and quinine preference in Rab3A mutant mice. Error bars are mean +/− SEM. Asterisks in A indicate levels of statistical significance (**, P < 0.005).
DISCUSSION
The data presented here show that loss-of function mutations in rab-3 and the rab-3 exchange factor aex-3 confer resistance to the locomotor effects of ethanol in C. elegans. Furthermore, we demonstrate that disruption of the cab-1 gene, which encodes a presynaptic protein that associates with AEX-3 (Iwasaki and Toyonaga, 2000), fails to phenocopy the ethanol resistance of rab-3 and aex-3 mutants, suggesting that the behavioral effect is specific to RAB-3 function. The lack of cab-1-associated ethanol resistance also minimizes the possibility that the resistance we observe with rab-3 and aex-3 mutants is due to a general effect on synaptic transmission; cab-1 mutants, like rab-3 and aex-3 mutants, display increased resistance to the acetylcholinesterase inhibitor aldicarb, a phenotype that is indicative of decreased levels of synaptic transmission (Iwasaki et al., 1997; Iwasaki and Toyonaga, 2000; Nonet et al., 1997). If a decrease in levels of synaptic transmission were a general mechanism for ethanol resistance, then we would expect all three mutants to show resistance. That is not the case; in fact, we have shown previously that mutations in the slo-1 gene, which increase synaptic transmission, also result in ethanol resistance (Davies et al., 2003). This suggests that the ethanol phenotype does not simply reflect a nonspecific alteration in synaptic transmission.
A potential mechanism for nonspecific resistance to the behavioral effects of ethanol is a change in the penetration of ethanol into the animal or altered metabolism of ethanol. Such a mechanism does not appear to contribute to the resistance of rab-3 mutants because we found that tissue concentrations of ethanol in rab-3 mutant animals are equivalent to those observed in wild-type animals when the strains are exposed to the same exogenous dose of ethanol under the same conditions in which they were tested for locomotion. It is worth noting that internal tissue concentrations of ethanol are substantially lower than exogenous ethanol concentrations (also see Davies et al., 2003, 2004). The lower tissue concentrations resulting from these high exogenous doses reflects the lack of permeability of C. elegans and has also been observed in studies of other neuroactive compounds (Rand and Johnson, 1995). These tissue concentrations of ethanol are the same as those resulting in intoxication in vertebrate systems, including humans (Diamond and McIntire, 2002).
Consistent with our behavioral data in C. elegans, we show that Rab3A+/− and Rab3A−/− mice are acutely resistant to the ataxic and sedative effects of ethanol. These data do not address whether the resistance to ethanol in both species is the result of altered acute sensitivity or abnormal development of tolerance. It may be noted that prior to the sedation and ethanol consumption assays, our subjects were not ethanol naïve (ie, mice had previously been exposed to low doses of ethanol for the ataxia assay), so it is possible that the altered sedation and/or consumption phenotypes we observe have been affected by RAB3A-dependent differences in tolerance. It will be important for future studies to investigate what role, if any, RAB3A has in the development of ethanol tolerance. Interestingly, we failed to observe a strain effect for the loss-of righting reflex test at a low ethanol dose (3.2g/kg), possibly reflecting a floor effect of recovery time on sedation at this dose in these animals (i.e., all genotypes showed a minimal recovery time of approximately 20 minutes at this dose), whereas a trend toward reduced recovery time was observed in mutants at the 3.6g/kg dose. One might predict a more robust shift in the dose response curve in Rab3A mutant mice on a more sensitive genetic background.
Previous studies in C. elegans have shown that synaptic transmission is impaired in rab-3 (js49 and y250) mutants, an observation attributed to alterations in either RAB-3 regulated recruitment of synaptic vesicles to the active zone and/or their sequestration near release sites (Nonet et al., 1997). Electrophysiological studies of Rab3A null mice have established a regulatory role for RAB3A in Ca2+–dependent synaptic vesicle release (Geppert et al., 1994, 1997), although its precise function(s) remains unclear. In mammals, four related RAB3 proteins have been shown to be partially functionally redundant, complicating the analysis of RAB3A function (Schluter et al., 2004). Interestingly, RAB3 proteins in the mouse, in contrast to in C. elegans, do not appear to be required for synaptic vesicle trafficking to the active zone per se, but rather may affect neurotransmitter release by increasing the release probability of a subset of primed vesicles (Nonet et al., 1997; Schluter et al., 2006). Additional evidence suggests that mammalian RAB3A may also be involved in regulating trafficking of recycled synaptic vesicles (Star et al., 2005).
Although small GTP binding (RAB) proteins have not previously been implicated in behavioral sensitivity to ethanol, there is evidence that RAB proteins regulate vesicular trafficking in hepatocytes in response to ethanol treatment (Larkin et al., 1996). Moreover, ethanol-induced disruption of the secretory machinery has been reported to alter RAB protein levels in astrocytes and the pituitary (Ren et al., 2005; Tomas et al., 2005). Ethanol may similarly affect vesicular trafficking or exocytosis in neurons through a RAB-3/RAB3A dependent mechanism, although we have no direct evidence to support this.
Ethanol has been shown to indirectly inhibit the release of neurotransmitter through modulation of neural activity. Ethanol activates presynaptic BK potassium channels in C. elegans and vertebrates (Cowmeadow et al., 2005, 2006; Davies et al., 2003; Gruss et al., 2001), inhibiting neurotransmitter release. Similarly, inhibition of presynaptic N/P/Q-voltage gated calcium channels by ethanol reduces vesicular release (Maldve et al., 2004). One might expect that disruption of RAB-3/RAB3A function in the mutants would impair neurotransmitter release further, potentially resulting in an enhanced response to ethanol or hypersensitivity. However, the unexpected resistance of rab-3/Rab3A mutants to ethanol suggests that instead ethanol may exert additional inhibitory effects on RAB3-dependent neurotransmitter release, downstream of BK and Ca2+ channel function.
We observed that Rab3A+/− mice voluntarily consume significantly more ethanol than wild-type or Rab3A−/− mice and that ethanol preference is not dependent on ethanol concentration in Rab3A+/− mice. Based on human and rodent studies indicating that initial sensitivity predicts subsequent ethanol consumption (Boyce-Rustay et al., 2006; Crabbe et al., 1996; Fee et al., 2004; Naassila et al., 2002, 2004; Newton and Messing, 2007; Offenhauser et al., 2006; Palmer et al., 2004; Schuckit, 1994; Thiele et al., 2000), we would have expected Rab3A−/− mice to consume more ethanol than wild-type animals. Instead, it appears that ethanol sensitivity and consumption are dissociated in Rab3A−/− mice. Why we observe increased ethanol consumption in Rab3A+/− mice but not Rab3A−/− mice is unclear. One possible explanation is that in the absence of RAB3A protein, compensatory changes may occur in other RAB proteins that do not occur in the Rab3A heterozygotes. Some degree of functional redundancy among RAB3 genes has been observed with respect to embryonic viability (Schluter et al., 2004). Behaviorally, such an effect would appear to be specific to ethanol consumption, as we observe resistance to some of the acute effects of ethanol in Rab3A−/− mice. It may be noted that a similar overdominance effect on ethanol-related behaviors has been reported for other genes: dopamine transporter (DAT) haploinsufficiency in female mice results in increased voluntary ethanol consumption relative to wild-type and homozygous null mice (Savelieva et al., 2002). Gat1 (GABA transporter subtype 1) haploinsufficiency has also been reported to result in increased ethanol consumption in mice, whereas Gat1−/− mice consume normal amounts of ethanol (Cai et al., 2006). It is possible that the ethanol consumption phenotype of Rab3A+/− mutants reflects altered dopaminergic and/or GABAergic neurotransmission.
Behaviorally, Rab3A disruption in the mouse has been shown to affect circadian activity and sleep homeostasis (Kapfhamer et al., 2002) with conflicting reports of altered performance in certain learning and memory tasks (D’Adamo et al., 2004; Hensbroek et al., 2003; Yang et al., 2007). Our data extend the behavioral role of RAB3A to include the regulation of ethanol-related behaviors and suggest a possible novel mechanism underlying the behavioral response to ethanol.
Acknowledgments
The authors thank M. Ferwerda, M. Wallace and J. Connolly for technical assistance and members of the McIntire, Heberlein and Messing labs for helpful discussions. This work was supported by the State of California for medical research on alcohol and substance abuse through UCSF, and by the NIAAA, NIH (J.C.B., A.G.D., S.L.M., U.H.), ABMRF (A.G.D.) and by the Jeffress Memorial Trust (J.C.B.).
References
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, Cai GQ, Liu GX, Cai Q, Shi JH, Shi J, Ma SK, Sun X, Sheng ZJ, Mei ZT, Cui D, Guo L, Wang Z, Fei J. Mice with genetically altered GABA transporter subtype I (GAT1) expression show altered behavioral responses to ethanol. J Neurosci Res. 2006;84:255–67. doi: 10.1002/jnr.20884. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Ghezzi A, Al’Hasan YM, Wang YZ, Atkinson NS. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30:745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Crowder CM, Shebester LD, Schedl T. Behavioral effects of volatile anesthetics in Caenorhabditis elegans. Anesthesiology. 1996;85:901–912. doi: 10.1097/00000542-199610000-00027. [DOI] [PubMed] [Google Scholar]
- D’Adamo P, Wolfer DP, Kopp C, Tobler I, Toniolo D, Lipp HP. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. Eur J Neurosci. 2004;19:1895–1905. doi: 10.1111/j.1460-9568.2004.03270.x. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. Natural Variation in the npr-1 Gene Modified Ethanol Responses of Wild Strains of C. elegans. Neuron. 2004;42:731–743. doi: 10.1016/j.neuron.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Diamond IF, McIntire SL. Alcohol neurotoxicity. In: Asbury AK, McKhann GM, McDonald WI, Goadsby PJ, McArthur JC, editors. Diseases of the Nervous System: Clinical Neuroscience and Therapeutic Principles. Cambridge: Cambridge University Press; 2002. pp. 1814–1826. [Google Scholar]
- Dzitoyeva S, Dimitrijevic N, Manev H. Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci U S A. 2003;100:5485–5490. doi: 10.1073/pnas.0830111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–1468. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro FM, 3rd, Sparta DR, Knapp DJ, Breese GR, Thiele TE. Increased consumption but not operant self-administration of ethanol in mice lacking the RIIbeta subunit of protein kinase A. Alcohol Clin Exp Res. 2006;30:825–835. doi: 10.1111/j.1530-0277.2006.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Mignery GA, Baumert M, Perin MS, Hanson TJ, Burger PM, Jahn R, Sudhof TC. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990;87:1988–92. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Gruss M, Henrich M, Konig P, Hempelmann G, Vogel W, Scholz A. Ethanol reduces excitability in a subgroup of primary sensory neurons by activation of BK(Ca) channels. Eur J Neurosci. 2001;14:1246–1256. doi: 10.1046/j.0953-816x.2001.01754.x. [DOI] [PubMed] [Google Scholar]
- Hensbroek RA, Kamal A, Baars AM, Verhage M, Spruijt BM. Spatial, contextual and working memory are not affected by the absence of mossy fiber long-term potentiation and depression. Behav Brain Res. 2003;138:215–223. doi: 10.1016/s0166-4328(02)00243-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron. 1997;18:613–622. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Toyonaga R. The Rab3 GDP/GTP exchange factor homolog AEX-3 has a dual function in synaptic transmission. EMBO J. 2000;19:4806–4816. doi: 10.1093/emboj/19.17.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhamer D, Valladares O, Sun Y, Nolan PM, Rux JJ, Arnold SE, Veasey SC, Bucan M. Mutations in Rab3a alter circadian period and homeostatic response to sleep loss in the mouse. Nat Genet. 2002;32:290–295. doi: 10.1038/ng991. [DOI] [PubMed] [Google Scholar]
- Larkin JM, Oswald B, McNiven MA. Ethanol-induced retention of nascent proteins in rat hepatocytes is accompanied by altered distribution of the small GTP-binding protein rab2. J Clin Invest. 1996;98:2146–2157. doi: 10.1172/JCI119021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldve RE, Chen X, Zhang TA, Morrisett RA. Ethanol selectively inhibits enhanced vesicular release at excitatory synapses: real-time visualization in intact hippocampal slices. Alcohol Clin Exp Res. 2004;28:143–152. doi: 10.1097/01.ALC.0000106304.39174.AD. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Increased sensitivity to the aversive effects of ethanol in PKCepsilon null mice revealed by place conditioning. Behav Neurosci. 2007;121:439–442. doi: 10.1037/0735-7044.121.2.439. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances GABAergic transmission in nucleus accumbens: regulation by metabotropic mechanisms. Alcohol Clin Exp Res. 1997;21:74A. [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Sharpe AL, Burkhart-Kasch S, McKinnon CS, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology (Berl) 2004;176:386–397. doi: 10.1007/s00213-004-1896-5. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB, Johnson CD. Genetic pharmacology: interactions between drugs and gene products in Caenorhabditis elegans. Methods Cell Biol. 1995;48:187–204. doi: 10.1016/s0091-679x(08)61388-6. [DOI] [PubMed] [Google Scholar]
- Ren JC, Zhu Q, Lapaglia N, Emanuele NV, Emanuele MA. Ethanol-induced alterations in Rab proteins: possible implications for pituitary dysfunction. Alcohol. 2005;35:103–112. doi: 10.1016/j.alcohol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva KV, Caudle WM, Findlay GS, Caron MG, Miller GW. Decreased ethanol preference and consumption in dopamine transporter female knock-out mice. Alcohol Clin Exp Res. 2002;26:758–764. [PubMed] [Google Scholar]
- Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Basu J, Sudhof TC, Rosenmund C. Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2000;24:1127–1136. [PubMed] [Google Scholar]
- Star EN, Newton AJ, Murthy VN. Real-time imaging of Rab3a and Rab5a reveals differential roles in presynaptic function. J Physiol. 2005;569:103–117. doi: 10.1113/jphysiol.2005.092528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hashimoto H, Shintani N, Yamamoto A, Baba A. Reduced hypothermic and hypnotic responses to ethanol in PACAP-deficient mice. Regul Pept. 2004;123:95–98. doi: 10.1016/j.regpep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas M, Marin P, Megias L, Egea G, Renau-Piqueras J. Ethanol perturbs the secretory pathway in astrocytes. Neurobiol Dis. 2005;20:773–784. doi: 10.1016/j.nbd.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, Abel T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J Neurosci. 2001;21:5297–5303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Farias M, Kapfhamer D, Tobias J, Grant G, Abel T, Bucan M. Biochemical, molecular and behavioral phenotypes of Rab3A mutations in the mouse. Genes Brain Behav. 2007;6:77–96. doi: 10.1111/j.1601-183X.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Oswald L, Wand G. The cyclic AMP/protein kinase A signal transduction pathway modulates tolerance to sedative and hypothermic effects of ethanol. Alcohol Clin Exp Res. 2003;27:1220–1225. doi: 10.1097/01.ALC.0000081626.02910.19. [DOI] [PubMed] [Google Scholar]