Abstract
Leontopodium alpinum (‘Edelweiss’) was phytochemically investigated for constituents that might enhance cholinergic neurotransmission. The potency to increase synaptic availability of acetylcholine (ACh) in rat brain served as key property for the bioguided isolation of cholinergically active compounds using different chromatographic techniques. The dichlormethane (DCM) extract of the root, fractions and isolated constituents were injected i.c.v. and the effect on brain ACh was detected via the push–pull technique. The DCM extract enhanced extracellular ACh concentration in rat brain and inhibited acetylcholinesterase (AChE) in vitro. The extracellular level of brain ACh was significantly increased by the isolated sesquiterpenes, isocomene and 14-acetoxyisocomene, while silphiperfolene acetate and silphinene caused a small increasing tendency. Only silphiperfolene acetate showed in vitro AChE inhibitory activity, thus suggesting the other sesquiterpenes to stimulate cholinergic transmission by an alternative mechanism of action. Isocomene was further investigated with behavioural tasks in mice. It restored object recognition in scopolamine-impaired mice and showed nootropic effects in the T-maze alternation task in normal and scopolamine-treated mice. Additionally, this sesquiterpene reduced locomotor activity of untreated mice in the open field task, while the activity induced by scopolamine was abolished. The enhancement of synaptic availability of ACh, the promotion of alternation, and the amelioration of scopolamine-induced deficit are in accordance with a substance that amplifies cholinergic transmission. Whether the mechanism of action is inhibition of AChE or another pro-cholinergic property remains to be elucidated. Taken together, isocomene and related constituents of L. alpinum deserve further interest as potential antidementia agents in brain diseases associated with cholinergic deficits.
Keywords: Edelweiss, Isocomene, Acetylcholine release, T-maze, Object recognition
1. Introduction
Leontopodium alpinum Cass. (Asteraceae), widely known as ‘Edelweiss’, grows on rocky and grassy slopes, mostly on limestone, at altitudes from 2200 to 3140 m in the mountainous regions of Europe, especially in the Alps, Carpatians, the mountains of the Balkan peninsula, the Tatra and the Pyrennees. In alpine folk medicine extracts of this plant are used for the therapy of abdominal aches, tonsillitis, bronchitis, cancer, diarrhoea, dysentery and fever. Recent phytochemical analysis of the aerial parts and roots, and subsequent pharmacological investigations revealed constituents with anti-inflammatory, leukotriene-inhibitory and antimicrobial activity [1-4]. The plant has not yet been investigated for compounds with pro-cognitive, cholinergic transmission enhancing activity.
The world wide ageing of the population has increased the incidence of cognitive deficits, such as the age-associated memory impairment and senile dementias and Alzheimer’s disease, and the awareness of their disruptive impact on the life of the affected individuals. The “cholinergic hypothesis of learning” played a pivotal role in the development of drugs for Alzheimer’s disease and other types of dementia [5]. Consequently, most of the therapeutics developed for these diseases are agents that enhance cholinergic transmission. This property is shared by cholinesterase inhibitors, cholinergic precursors, nicotine receptor agonists and muscarinic M2 receptor antagonists. At present, cholinesterase inhibition is the mainstay of treatment for Alzheimer’s disease and serves as a promising strategy also for the treatment of senile dementia, ataxia, myasthenia gravis, and Parkinson’s disease. These drugs cause symptomatic improvement by inhibiting the breakdown of acetylcholine (ACh) to increase its synaptic availability. Recent studies suggest that these drugs are also anti-inflammatory and neuroprotective via cholinergic upregulation and influence on nicotinic ACh receptors and the immune system [6].
Despite the substantial progress in the field of cognitive impairments, the available AChE inhibitors and other cognition-enhancing agents available are still not satisfactory. The disadvantages of relatively small pro-cognitive effects in patients and frequent side effects are shared by all drugs on the market. The scientific society is still in search for effective drugs with fast penetration in the CNS, long duration of action and low toxicity. Aim of this study was to investigate whether L. alpinum contains substances which enhance cholinergic transmission. Extracts from the plant were subjected to bioguided fractionation based on in vivo ACh release in order to isolate cholinergically active compounds. The established AChE inhibitors galantamine and tacrine were used as positive control. Fractions and constituents of L. alpinum were also screened in vitro for AChE inhibitory properties. The most promising constituent, isocomene was investigated in behavioural tasks with mice for cognition-improving and cholinergic impairment ameliorating properties.
2. Materials and methods
2.1. Preparation of extracts and isolation of compounds
2.1.1. Plant material
Dried sub-aerial parts from cultivated L. alpinum were kindly provided by Pentapharm Ltd., Basel, Switzerland. A voucher specimen (CH 02-0603) was deposited at the Herbarium of the Institute of Pharmacy/Pharmacognosy, Leopold-Franzens-University of Innsbruck.
2.1.2. General
All reagents were of puriss or analytical quality and purchased from Merck (VWR Darmstadt, Germany) unless otherwise specified. Puriss grade solvents were distilled before use. NMR: 2-D and 1-D measured at a Bruker DRX-300 (Bruker Biospin Rheinstetten, Germany) at 300 MHz (1H) and 75 MHz (13C); spectra were recorded at 300 °K in deuterated chloroform containing 0.05% tetramethylsilan (Eurisotop, Gif-Sur-Yvette, France) as internal standard. GC: PerkinElmer – Auto system GC with FID-Detector (PerkinElmer, Norwalk, USA); Stationary phase: Permabond SE-54-DF-0.25; 50 m × 0.32 mm ID (Macherey-Nagel, Düren, Germany); starting temperature: 70 °C hold for 5.0 min; heating rate of 3 °C/min to 240 °C, hold for 20 min; carrier gas: helium, flow rate 1.0 ml/min; carrier pressure: 90 kPa; split-ratio: 1:7.5; injector temperature: 260 °C; FID: 280 °C; sample volume: 2.5 μl.
2.1.3. Semi preparative HPLC
Dionex system (Dionex Corporation, Sunnyvale, CA, USA) with a P580 pump, ASI-100 autosampler, UVD 170U detector and a Gilson 206 fraction collector (Gilson, Middleton, WI, USA).
2.1.4. Preparation and isolation procedures
Ground roots (1907.84 g) were exhaustively macerated with dichloromethane (12.5 l DCM, at RT, eight times). Extracts were evaporated to dryness yielding 43.0 g crude DCM extract. 40.0 g of the obtained extract were suspended in 100 ml MeOH and separated in a MeOH soluble and insoluble part. The soluble part was separated by Sephadex® LH 20 (Pharmacia Biotech, Sweden) column (90 cm × 3.5 cm) with MeOH as mobile phase. The eluate was collected in portions of 10 ml and compared by thin layer chromatography (TLC). Similar eluate-portions were combined to eight fractions. Fraction 5 (15.13 g; 320–410 ml elution volume) was rechromatographed by means of silica gel column chromatography (CC) (silica gel 60, 230–400 mesh; 180 g, 41 cm × 3.5 cm) using a petroleum ether (PE)-acetone gradient with an increasing amount of acetone. The eluate was collected in portions of 10 ml and compared by TLC. Comparable eluate-portions were combined to 40 fractions (A1-A40). A2 (311 mg; sesquiterpene mixture) was further purified by means of AgNO3-silica gel CC using hexane as mobile phase. TheAgNO3-silica gel column was prepared as follows: 40 g silica gel were mixed with 80 ml of a 10% (w/v) aqueous solution of AgNO3; this mixture was dried for 3 h at 110 °C, suspended in hexane and used as stationary phase after a rinsing step with 250 ml hexane to remove any excesses AgNO3. The eluate was collected in fractions of 2 ml and compared by TLC. The separation resulted in the isolation of four compounds: 170.0 mg isocomene (fraction 1–23); 57.2 mg modhephene (fraction 25–35), 7.7 mg silphinene (fraction 38–56), and 18.0 mg β-isocomene (fraction 61–110). Their structures were elucidated by mass spectrometry and NMR spectroscopy and in comparison of spectroscopic and physical data with literature [7-9]
A10 and A11 (2991.0 mg; sesquiterpene acetate fraction) were further purified by silica-gel CC (PE/diethyl ether gradient; 40 g, 42 cm × 2.0 cm). The eluate was collected in fractions of 5 ml and compared by TLC. Similar portions were combined affording 17 fractions (Aa1-Aa17). 80 mg of fraction Aa4 (758.4 mg) were further separated by means of semi preparative HPLC (Phenomenex Synergy Max-RP column (10 μm, 10 mm × 250 mm); mobile phase: 80% methanol/20% water, isocratic elution; flow: 3.50 ml/min; 25 °C; injection volume: 100 μl of a methanolic solution of 20 mg/ml of Aa4) yielding 27.2 mg silphiperfolene acetate and 10.5 mg 14-acetoxyisocomene. The identity of compounds was verified by a comparison of spectroscopic and physical data with literature data [2,7].
2.2. Brain ACh
All surgical and pharmacological techniques and behavioural procedures were approved by the committee on animal health of the government of Austria (Kommission für Tierversuchsangelegenheiten, Bundesministerium für Wissenschaft, Forschung und Kunst, Austria).
2.2.1. Surgery and push–pull technique
Sprague-Dawley rats (250–300 g; Research Institute for Laboratory Animal Breeding, University of Vienna, Himberg, Austria) were anaesthetized with urethane 1,2 g/kg, i.p. The head was fixed in a stereotaxic frame and a push–pull cannula (outer tubing: o.d. 0.75 mm, i.d. 0.41 mm; inner tubing: o.d. 0.26 mm, i.d. 0.13 mm) was mounted on a microdrive. The cannula was stereotaxically inserted through a hole in the skull into the nucleus accumbens (coordinates, mm from bregma: AP, +1.2; L, +2.5; V, −7.8; [10]. A second hole was drilled into the skull for the i.c.v. application of drugs (coordinates, mm from bregma: AP: −2.8; L: +1.5). The nucleus accumbens was superfused at 20 μl/min with artificial cerebrospinal fluid (CSF), pH 7.2. The CSF consisted of: NaCl, 140; KCl, 3; CaCl2, 1.2; MgCl2, 1; Na2HPO4, 1; NaH2PO4, 0.3; glucose 3.0 (mmol l−1), neostigmine 0.1 (μmol l−1; Sigma-Aldrich Chemie, Steinheim, Germany). After 1 h of the cannula insertion the samples were collected into tubes kept at −20 °C in time periods of 10 min and then stored at −80 °C until ACh was determined (11).
2.2.2. I.c.v. application of plant extracts and substances
Extracts were dried and redissolved in dimethylsulfoxide (DMSO) at a concentration of 2 μmol total sesquiterpenes per 20 μl of solution. Isolated substances were dissolved in 20 μl of DMSO:EtOH 1:1. Galantamine was dissolved in DMSO, tacrine in CSF (both: Tocris; Cookson Ltd., Avonmouth, UK, Bristol). Using a motorized microsyringe connected to a stereotaxic microdrive, the tip was introduced with an angle of 60° through the drug application-hole into the ipsilateral ventricle (coordinates mm from bregma: AP: −0.9; L: +1.5; V: −3.8). The drug solutions (20 μl/250 g body weight) were injected at 2 μl/min. The control rats were injected with the vehicles (DMSO, DMSO:EtOH 1:1 or CSF).
2.2.3. Histology
At the end of the experiment the rats were killed with an overdose of urethane, a solution of cresyl violet was injected through the push–pull and i.c.v. cannulas. The brains were removed and stored in buffered formaldehyde solution (7%). Serial coronal sections were cut at 50 μm intervals. Localisations of the push–pull cannula and i.c.v. microinjection were verified on serial sections by the trace of the cannula and staining of the ventricle. Experiments with unsatisfactory localisation were discarded.
2.2.4. Determination of ACh
ACh content in the samples was determined by HPLC with a cation-exchanging analytical column and a post-column reactor followed by electrochemical detection [11-13]. The system consisted of an HPLC-pump (JASCO PU-1580, Jasco Corporation, Tokyo, Japan) and an autosampler (AS-4000, Hitachi Ltd., Tokyo, Japan) with a 100-μL sample loop. The mobile phase was (mm): K2HPO4, 100; KCl, 5; tetramethylammonium hydroxide, 1; Na-EDTA, 0.1 and 0.5 ml/l Kathon® CG (Rohm & Haas, Frankfurt/Main, Germany), pH adjusted to 7.9 with H3PO4, pumped at a flow rate of 0.4 ml/min. ACh was separated on an analytical column (80 mm × 3 mm, Kromasil® 100–5 C18; MZ Analysentechnik, Mainz, Germany) loaded with laurylsulphate. At the postcolumn enzyme reactor (20 mm × 1 mm Kromasil® 100–10 NH2) to which AChE (EC 3.1.1.7; Sigma-Aldrich Chemie, Steinheim, Germany) and choline oxidase (EC 1.1.3.17; Sigma-Aldrich Chemie, Steinheim, Germany) were bound, ACh was hydrolized to acetate and choline. Subsequently, choline was oxidized to betaine and hydrogen peroxide. The peroxide was electrochemically detected by a UniJet platin electrode (3 mm) at +500 mV with an amperometric detector (BAS LC-4, Bioanalytical Systems, West Lafayette, IN, USA). The detection limit for ACh (signal-to-noise ratio = 3) was 10 fmol per sample. ACh was quantified with external standards that were injected at the beginning and the end of the analysis.
2.2.5. Statistical analysis
Statistical significance of drug effects on ACh release was calculated by Friedman’s analysis of variance, followed by Dunn’s test for multiple comparisons using the mean release rate before the administration of drugs as controls. Data are shown as means ± S.E.M. of relative values. The mean release rate before drug administration was taken as 1.0.
2.3. Microplate assay of AChE inhibitory activity
The AChE inhibitory activity was determined using a modified Ellman’s method [14,15] with acetylcholinesterase EC 3.1.1.7, acetylthiocholiniodide, and 5,5′-dithiobis-(2-nitrobenzoic acid): Sigma-Aldrich Chemie Gmbh, Steinheim, Germany; positive control: galantamine·HBr and tacrine·HCl·H2O in a 96-well microplate assay as previously described [16]. The percentage of the enzyme inhibition was calculated by determining the rate in presence of inhibitor and the vehicle (containing 1% DMSO) compared to the rate in the control sample (n = 4) and analyzed with Student’s t-test.
2.4. Behavioural study
2.4.1. Animals
C57BL/6N female, 3-month mice, weighing 22–27 g (Charles River Laboratories, Sulzfeld, Germany) were used in the present study. The animals were housed in groups of six in cages (40 cm (L) × 25 cm (W) × 15 cm (H)) placed in a room at constant temperature (22 ± 1 °C), 12 h-light/12 h-dark cycle (7:00–19:00, with food and water ad libitum. Experiments were conducted between 09:00 and 16:00 h.
2.4.2. Surgery
Mice were anaesthetized with pentobarbital sodium (60 mg/kg, i.p.) and placed in a stereotaxic frame. A guide cannula (gauge 25, o.d. 0.52 mm, i.d. 0.26 mm and 7 mm long) was stereotaxically implanted through a hole on the skull into the brain area 0.5 mm higher than the lateral cerebral ventricle (−0.1 mm posterior, 0.8 mm lateral to bregma, −2.2 mm ventral to the dura) [17]. Each animal recovered for 5 days prior to experimentation.
2.4.3. Drugs
Isocomene was dissolved in vehicle solution (DMSO:sterile water 3:7) at a concentration of 8.16 μg (42 nmol) in 1 μl, and scopolamine (scopolamine hydrobromide; Sigma, St. Louis, MO, USA) was dissolved in saline at 20 μg (50 nmol) in 1 μl.
2.4.4. Experimental design and microinjection
Animals were randomly divided into four experimental groups (8–9 mice per group) as follows: 1, vehicle; 2, isocomene 42 nmol; 3, scopolamine hydrobromide 50 nmol (Sigma, St. Louis, MO, USA), and scopolamine hydrobromide (50 nmol) and isocomene (42 nmol). A microinjector (connected with i.d. 0.28 mm polyethylene tubing to a 5 μl Hamilton syringe) was inserted through the guide cannula and the substances were administered in a volume of 1 or 2 μl during 1 min. The microinjector protruded 0.5 mm from the tip of the guide cannula to reach the cerebral ventricle and was left in place an extra 1 min for drug diffusion after injection. The vehicle or substances were injected 15 min before the test (T-maze and open field) or before the training trial T1 (object recognition test).
2.4.5. Histology
Localisations of i.c.v. microinjections in mouse brain were verified on serial sections (50 μm intervals) by the trace of the cannula and staining of the ventricle. Experiments with unsatisfactory localisation were discarded.
2.4.6. Object recognition task
The test arena consisted of a Plexiglas box (40 cm (L) × 25 cm (W) × 15 cm (H)), which was illuminated (100 lux) by a lamp above the box and surmounted by a video camera connected to a monitor and a video recorder. The objects to be discriminated were cubes or cylinders, 1.5 cm high in green or white colour. The test was performed as described by Ennaceur and Delacour [18] and modified to make the test suitable for mice. On the day before testing, the animals were allowed to explore the arena for 10 min (without objects). On the testing day a session of two 10-min trials was given. During the training trial (sample trial) (T1), two identical objects were placed in a symmetrical position (distance of 18 cm between objects and 6 cm from the rear wall). Mice were placed in the middle of apparatus and left to explore the two identical objects for 10 min. After T1, mice were returned to their home cage for either 3- or 18-h inter-trial intervals. Subsequently, the testing trial (choice trial) (T2), was performed for 10 min. During T2, two dissimilar objects (one with a familiar shape and the other a new one) were presented. When mice recognize the familiar object they spend more time on exploration of the novel one. All combinations and locations of objects were used in a balanced manner to reduce potential biases due to preferences for particular locations or objects. Olfactory trails were cleaned with water from objects and arena after each trial.
Exploration was defined as follows: directing the nose toward the object at a distance of less than 0.5 cm and/or touching the object with its forepaw or nose. Turning around or sitting on the object was not considered as exploratory behaviour. The times spent by mice in exploring each object during experiment were analyzed on videotapes. Then the total time spent in exploring the two identical objects in T1 and that spent in exploring the two different objects, familiar (F) and novel (N) in T2 was calculated. To eliminate differences in overall levels of exploration, a discrimination index (D) was calculated; D = N − F/N + F. D is the discrimination index and represents the difference in exploration time expressed as a proportion of the total time spent exploring the two objects in T2. The object exploration time ratio (E) was calculated as object exploration time with a novel object during testing divided by object exploration time with a familiar object during testing; E = N/F. Such ratio is considered to be the main measure of retention in this task.
2.4.7. T-maze spontaneous alternation task
The T-maze was constructed of grey perspex with three guillotine doors according to the measures provided by Gerlai [19]. The arms were 12 cm wide, the two arms aside from the left and right guillotine door were 31 cm long. The length of the central arm (stem of the T) was 76 cm including the compartment of 24 cm in front of the entrance door.
Spontaneous alternation is defined as a visit to the other of the two goal arms of the T-maze than that visited in the previous trial. Testing of a mouse consisted of one single session, which started with one forced-choice trial, followed by 14 free-choice trials.
In the first trial, the “forced-choice trial”, either the left or right goal arm was blocked by lowering the guillotine door. The mouse was released from the start arm, entered the open goal arm, and returned to the start position where it was confined for 5 s by lowering the guillotine door of the start arm.
During the subsequent 14 “free-choice” trials, all doors were open. The mouse could choose freely between the left and right goal arm. A session was terminated and the animal was removed from the maze as soon as 14 free-choice trials had been performed or 30 min had elapsed, whatever event occurred first. The alternation score for each mouse was calculated as the ratio of the actual number of alternations to the possible number (defined as the total number of arm entries minus two) multiplied by 100.
2.4.8. Open field experiments
The open field consisted of a transparent plastic box with white floor (41 cm × 41 cm × 41 cm). The illumination at floor level was 150 lux. Mice were individually placed into the centre of the open field and their behaviours were tracked with an automated activity monitoring system (True Scan, Coulbourn Instruments, Allentown, Pennsylvania, USA). The time of locomotor activity, the overall distance traveled by the mice and the vertical plane entries (rearings) were monitored for 10 min [20].
2.4.9. Statistical analysis
All data from behavioural studies are shown as mean ± S.E.M. Statistical analys is was performed using the analysis of variance (ANOVA), followed by post hoc multiple comparisons Tukey–Kramertest.All statistical analyses were carried out by using the Graphpad Prism Program, version 4.0, Graphpad software, Inc.
3. Results
3.1. Bioguided fractionation and isolation of constituents
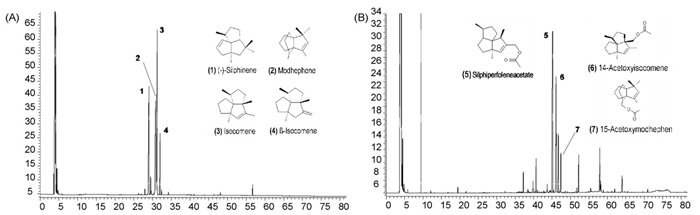
The effectiveness of extracts and fractions to increase synaptic availability of ACh in rat brain served as basis for the isolation of cholinergically active constituents. Extracts, fractions and substances were injected i.c.v. and extracellular ACh was monitored with the push–pull technique. Investigations started with the crude DCM extract of L. alpinum roots, which in the microtiter plate enzyme assay using Ellman’s spectrophotometric method exhibited an AChE inhibition of 78.79 ± 2.59% when tested at a concentration of 1 mg/ml. At the same concentration the methanol extract was less effective (51.81 ± 5.74%). Thus, the DCM extract was subjected to bioguided fractionation monitored by the above mentioned in vivo model. Scheme 1 and Fig. 1 show the purification procedures and the GC chromatograms of the two most potent fractions containing the subsequently purified constituents.
Scheme 1.
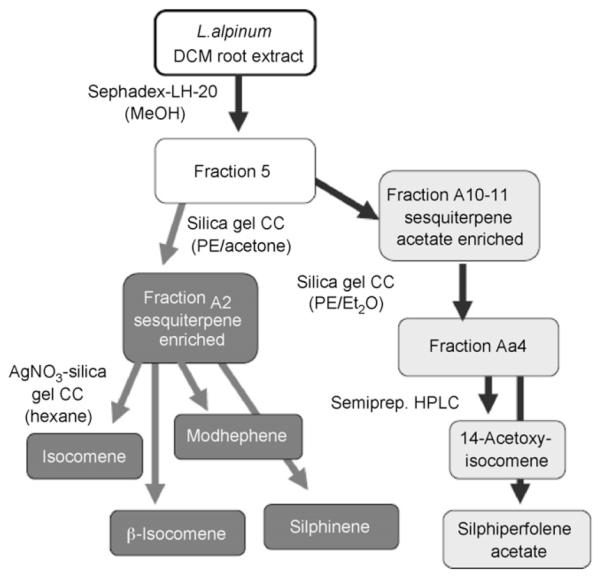
Bioguided fractionation of the DCM extract of L. alpinum. Isolated compounds were identified by comparison of spectroscopic data. See Materials and methods for detailed description of the isolation procedure.
Fig. 1.
Chromatographic analysis of (A) fraction A2 containing isocomene, β-isocomene, modhephene and silphinene; (B) fraction Aa4 containing 14-acetyxy-isocomene and silphiperfolene acetate. For details and analytical parameters see Section 2.1.4.
3.2. Brain ACh
The concentration of ACh in the samples from nucleus accumbens of urethane-anaesthetized rat was 775 ± 267 fmol/10 min (n = 61). 60 min after beginning of the superfusion the initially high concentration of ACh reached a stable basal level which did not change throughout the experiment. After four control samples the constituents were injected i.c.v. Injections of the pure vehicles without samples did not significantly influence extracellular ACh, as shown in Figs. 2 and 5A (DMSO), Fig. 3A (DMSO:EtOH 1:1) and Fig. 6A (CSF).
Fig. 2.
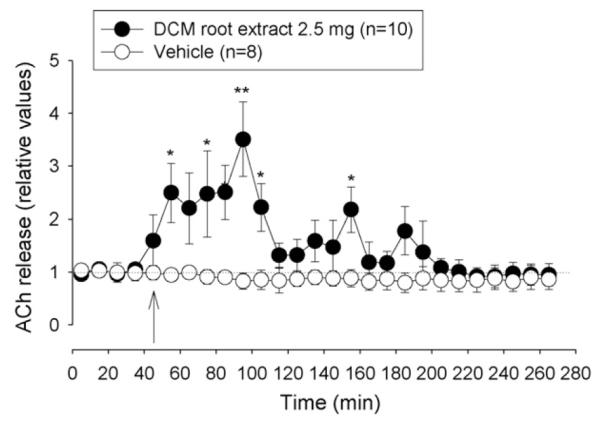
Effect of DCM root extract (2.5 mg) and vehicle (DMSO) on extracellular ACh concentration in the nucleus accumbens. Arrow indicates time when i.c.v. injection (20 μl at 2 μl/min) was started. Data are shown as relative values of basal release which is the mean of four preinjection samples. Basal release is taken as 1.0 and indicated by a dotted line. *p < 0.05, Friedman’s analysis of variance followed by Dunn’s multiple comparisons test using the pre-injection sample as control.
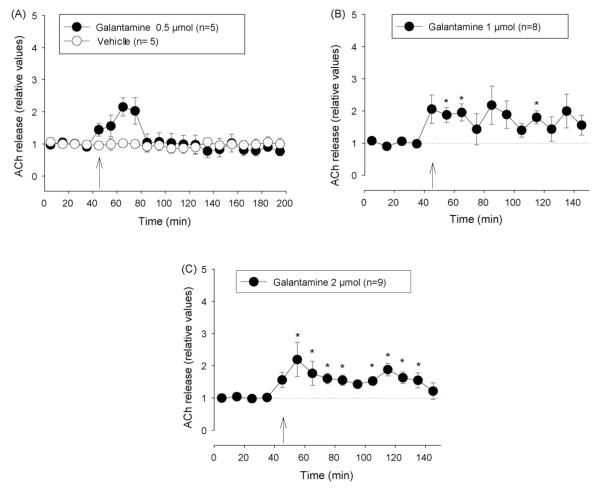
Fig. 5.
Effect of galantamine and vehicle (DMSO) on extracellular ACh concentration in the nucleus accumbens. (A) Vehicle and 0.5 μmol galantamine, (B) 1 μmol, (C) 2 μmol galantamine. Arrows indicate time when i.c.v. injection (20 μl at 2 μl/min) was started. Data are shown as relative values of basal release which is the mean of four pre-injection samples. Basal release is taken as 1.0 and indicated by a dotted line. *p < 0.05, Friedman’s analysis of variance followed by Dunn’s multiple comparisons test using the pre-injection sample as control.
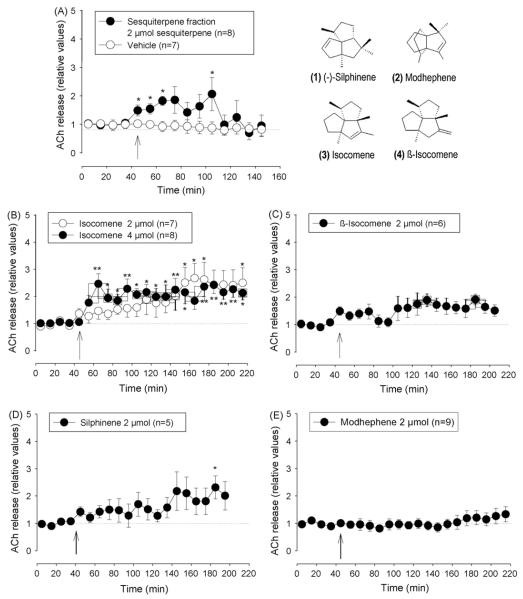
Fig. 3.
Effects of sesquiterpene fraction and the isolated sesquiterpenes on extracellular ACh concentrations in the nucleus accumbens. (A) Sesquiterpene fraction and vehicle, (B) isocomene, (C) β-isocomene, (D) modhephene, and (E) silphinene. Arrows indicate time when i.c.v. injection (20 μl at 2 μl/min) was started. Data are shown as relative values of basal release which is the mean of four pre-injection samples. Basal release is taken as 1.0 and indicated by a dotted line. *p < 0.05, **p < 0.01, Friedman’s analysis of variance followed by Dunn’s multiple comparisons test using the pre-injection sample as control.
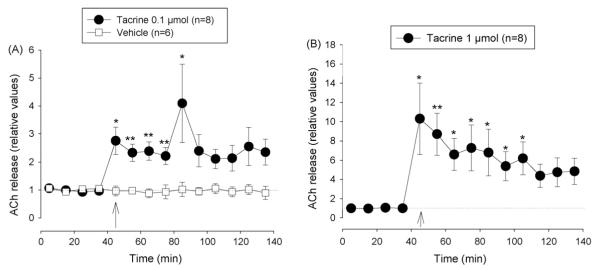
Fig. 6.
Effect of tacrine and vehicle (CSF) on extracellular ACh concentration in the nucleus accumbens. (A) Vehicle and 0.1 μmol tacrine, (B) 1 μmol tacrine. Arrows indicate time when i.c.v. injection (20 μl at 2 μl/min) was started. Data are shown as relative values of basal release which is the mean of four pre-injection samples. Basal release is taken as 1.0 and indicated by a dotted line. *p < 0.05, Friedman’s analysis of variance followed by Dunn’s multiple comparisons test using the pre-injection sample as control.
I.c.v. injection of the DCM root extract (2.5 mg corresponding to 2 μmol of total sesquiterpenes) caused a pronounced increase in extracellular ACh to about 2.5–3 times of basal level, lasting about 1 h followed by another hour of only slightly enhanced release (Fig. 2). Fractionation of the crude DCM extract was carried out by gel permeation chromatography over Sephadex LH-20. Eight fractions were collected and tested for their influence on the ACh release in the nucleus accumbens of rats. The ACh-enhancing effect (145 ± 29% increase, mean ± S.E.M., n = 2) was found in the fraction 5 while the other fractions did not raise ACh-levels in the superfusate (data not shown). The most promising fraction 5 containing mainly sesquiterpenes was selected for further purification and separated by silica gel chromatography into 40 subfractions (A1-A40). Because of the sesquiterpene content two subfractions were of major interest for further pharmacological investigations: A2 containing mainly sesquiterpenes and A10–11 with a high content of acetylated sesquiterpenes.
At a dose of 2 μmol fraction A2 (sesquiterpene mixture with identical molecular weight) caused an acute increase in extracellular ACh which lasted about 60 min after application. The average for 1 h after injection was elevated by a factor of 1.42 ± 0.42* (t-test, *p < 0.05 from basal release) as shown in Fig. 3A. Four sesquiterpenes were isolated from the fraction A2: isocomene, β-isocomene, silphinene and modhephene. I.c.v. injection of isocomene enhanced extracellular ACh in a dose dependent way. 2 μmol isocomene increased ACh gradually to a level of 1.8 times the control value. When increasing the dose to 4 μmol ACh rose to about two times the basal value and remained at this level (Fig. 3B). 2 μmol of the isomer β-isocomene and silphinene did not significantly increase ACh though both compounds tended to increase the concentration of ACh in the samples over time (Fig. 3C and D). The effect of modhephene at the dose of 2 μmol was comparable to that of the vehicle since it exhibited no influence extracellular ACh concentration (Fig. 3E).
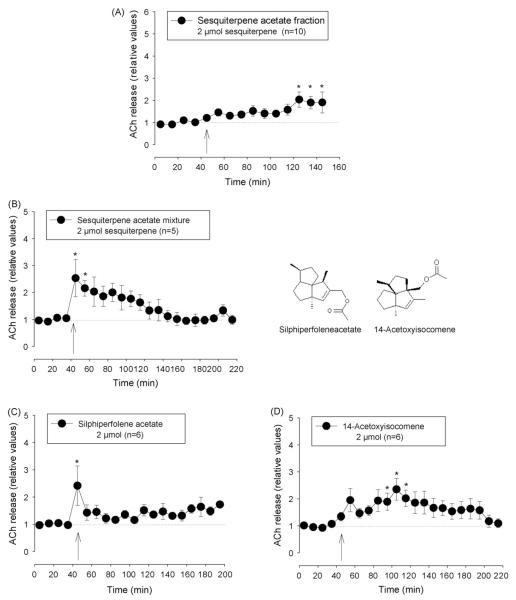
The sequiterpene acetate-enriched fraction A10–11 (2 μmol total sequiterpenes i.c.v.) enhanced ACh during the 100 min after application to 1.44 ± 0.26* (t-test, *p < 0.05 from basal release). The maximum level of about two times the basal level was achieved after a delay of 80 min (Fig. 4A). Silica gel column chromatography of this fraction yielded a mixture of two main compounds, silphiperfolene acetate and 14-acetoxy-isocomene. At a dose of 2 μmol this mixture caused an initial very pronounced increase in extracellular ACh followed by a gradual decline of the release rate to the basal value (Fig. 4B). Compared to the sesquiterpene acetate-enriched fraction A10–11 the effectiveness to enhance ACh was higher and the time course was different, most likely due to the exclusion of substances which inhibit the cholinergic system or modify the interaction of silphiperfolene acetate and 14-acetoxy-isocomene with their targets (Fig. 4B).
Fig. 4.
Effect of sesquiterpene acetate-enriched fraction and the constituents silphiperfolene acetate and 14-acetoxy-isocomene on extracellular ACh concentration in the nucleus accumbens. (A) Sesquiterpene acetate-enriched fraction, (B) mixture of silphiperfolene acetate and 14-acetoxy-isocomene, (C) silphiperfolene acetate, and (D) 14-acetoxy-isocomene. Arrows indicate time when i.c.v. injection (20 μl at 2 μl/min) was started. Data are shown as relative values of basal release which is the mean of four pre-injection samples. Basal release is taken as 1.0 and indicated by a dotted line. *p < 0.05, Friedman’s analysis of variance followed by Dunn’s multiple comparisons test using the pre-injection sample as control.
I.c.v. injection of pure silphiperfolene acetate and 14-acetoxy-isocomene alone showed that their effects can explain the influence of their mixture on extracellular concentration of ACh shown above. 2 μmol siphiperfolene acetate led to an initial peak of pronounced increase in ACh. Thereafter the release rate declined to a level of about 130–150% of the basal value (Fig. 4C). The rise in ACh by 14-acetoxy-isocomene was slower but reached a higher level of permanent elevation (Fig. 4D).
Administration of 0.5 μmol galantamine caused a short increase in extracellular ACh while 1 and 2 μmol caused a longer lasting increase of similar magnitude (means ± S.E.M. of post-injection samples: 1.78 ± 0.32 and 1.68 ± 0.20). However, the effect in the group treated with 1 μmol galantamine showed a higher variability (Fig. 5A-C). At the doses of 0.1 and 1 μmol tacrine caused long lasting rises in ACh level to about 2.5 and 8 times the basal values, respectively (Fig. 6A and B).
3.3. AChE microplate assay
To determine the mode of action the isolated pure compounds were studied for their ability to inhibit AChE in the microplate assay. Interestingly, only silphiperfolene acetate showed a weak effect, inhibiting AChE by 40.64 ± 7.09% (concentration: 200 μM). The positive control galantamine showed an IC50 of 3.2 μM and an inhibition rate of 89.30 ± 2.29% at 100 μM. Isocomene, β-isocomene, silphinene, modehephene and 14-acetoxy-comene, up to a concentration of 500 μM were ineffective.
3.4. Effects of isocomene on memory and behaviour
3.4.1. Object discrimination task
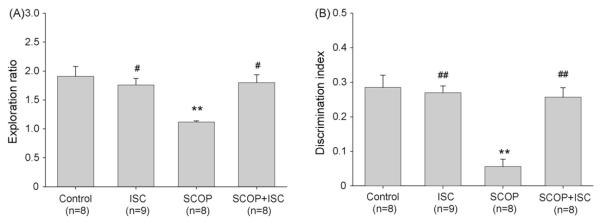
Mice spent an equal time exploring the two identical objects during learning trial (T1; not shown). Recognition of objects (T2) was tested after the interval of 3 h. Control mice spent more time exploring the novel object than the familiar object in T2 and discriminated well between familiar and novel objects. Isocomene (42 nmol) had no significant effect on object memory when applied to normal mice not treated with scopolamine (Fig. 7).
Fig. 7.
Effect of isocomene (ISC) on normal and scopolamine (SCOP)-impaired object recognition of mice. (A) Exploration ratio, (B) discrimination index. Isocomene (42 nmol) and scopolamine (50 nmol) were injected i.c.v. Data are shown as means ± S.E.M., analysis of variance ANOVA followed by multiple comparison Tukey–Kramer test. **p < 0.01, ***p < 0.001 compared to the control group; ##p < 0.01, ###p < 0.001 compared to scopolamine group.
Scopolamine (50 nmol) drastically impaired learning. The mice of this group did not discriminate at all between the novel object and the familiar object in the T2. The exploratory activity was not impaired since the exploration time showed no difference to that of the vehicle-treated control mice (not shown). Isocomene restored the scopolamine-impaired object recognition to a large extent since exploration ratio and discrimination index of these animals was not significantly different from control.
3.4.2. T-maze alternation test
Control mice selected the unfamiliar arm of the maze at a rate not significantly different from chance (53 ± 1.5% mean ± S.E.M.). However, mice injected with 42 nmol isocomene showed an increased alternation rate of 69 ± 6% which indicates a preference for entering the unfamiliar maze arm. In contrast to isocomene, the anticholinergic drug scopolamine (50 nmol) induced a pronounced decrease in alternation rate. Isocomene abolished this effect of scopolamine (Fig. 8).
Fig. 8.
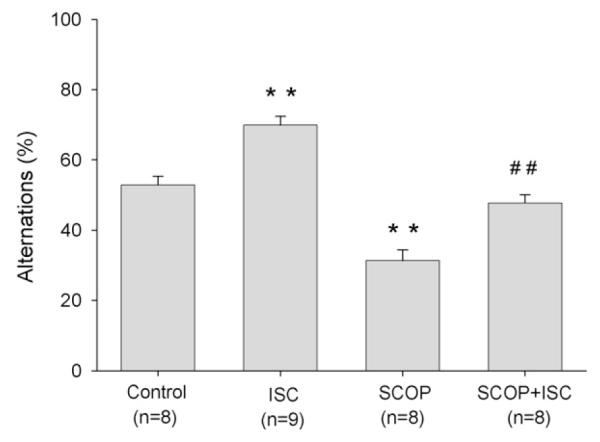
Effect of isocomene (ISC) on normal and scopolamine (SCOP)-impaired alternation behaviour of mice in T-maze. Isocomene (42 nmol) and scopolamine (50 nmol) were injected i.c.v. Data are shown as means ± S.E.M., analysis of variance ANOVA followed by multiple comparison Tukey–Kramer test. **p < 0.01 compared to the control group; ##p < 0.01 compared to scopolamine group.
3.4.3. Locomotor activity in the open field
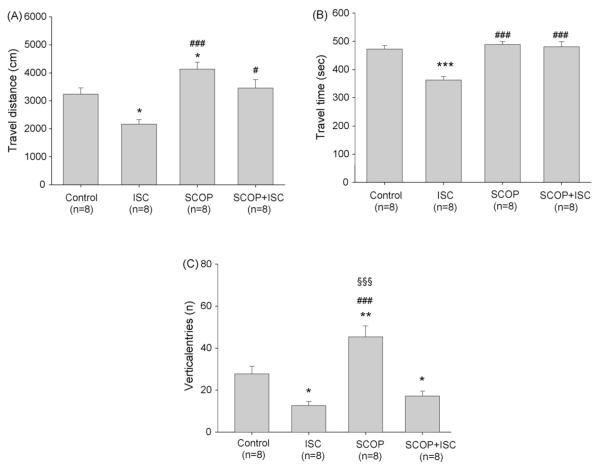
Isocomene (42 nmol) reduced the locomotor activity in the open field as shown by a decrease in the locomotion indexes travel distance, travel time and number of rearings (Fig. 9A-C). In contrast to isocomene the anticholinergic scopolamine (50 nmol) enhanced travel distance and number of rearings while travel time was not influenced. When injected with scopolamine isocomene did not influence travel time. However, it abolished the scopolamine-induced increase in travel distance and reduced the frequency of rearings to a value not significantly different from that of isocomene alone, indicating a more potent influence of isocomene on this parameter than scopolamine.
Fig. 9.
Effect of isocomene (ISC) on locomotion of mice in the open field test. (A) Travel distance, (B) travel time, (C) vertical entries. Isocomene (42 nmol) and scopolamine (50 nmol) were injected i.c.v. Data are shown as means ± S.E.M., analysis of variance ANOVA followed by multiple comparison Tukey–Kramer test. *p < 0.05, **p < 0.01, ***p < 0.001 compared to the control group; #p < 0.01, ###p < 0.001 compared to ISC group, §§§p < 0.001 compared to SCOP + ISC group.
4. Discussion
4.1. Bioguided fractionation and effects on brain ACh
In search for constituents of L. alpinum which enhance cholinergic transmission compounds in various stages of purity were injected i.c.v. in rats. To detect effects on cholinergic transmission we monitored the extracellular concentration of brain ACh with the push–pull technique [21]. The ACh content of the superfusate is a measure of synaptic availability and reflects the concentration of the neurotransmitter in the synaptic cleft [22].
DCM root extract from L. alpinum enhanced extracellular ACh in rat brain in vivo revealing the presence of constituents which enhance cholinergic transmission. Bioguided fractionation resulted in isolation and identification of single compounds which exhibited potencies which might explain the in vivo effects of the DCM extract. Our attention was drawn to isocomene since this compound produced a remarkably long-lasting elevation of extracellular ACh with potency similar to galantamine. The tricyclic sesquiterpene was isolated already in 1977 [23,24] and reported to possess antimicrobial and leukotriene synthesis inhibitory activity[3,4]. It has not yet been investigated whether isocomene can inhibit inflammatory processes in brain. Such a property, in addition to the nootropic effect, would be a benefit in the therapy of neurodegenerative diseases.
The 14-acetoxy derivate also enhanced extracellular ACh but compared to isocomene the effect declined relatively fast. Potentially, the acetoxy moiety changes pharmacokinetic and pharmacodynamic properties of the sesquiterpene but there are no data reported. The time course of effects after i.c.v. application might reflect a faster metabolism of the acetoxy compound in brain tissue and/or transport and diffusion to the periphery. On the other hand the long duration of the effect of isocomene might be due a higher intracellular distribution and binding to targets within or at cholinergic neurons.
It is evident that the anaesthesia and the neostigmine in the perfusion fluid may reduce the sensitivity of the extracellular ACh level to modulatory influences of cholinergic enhancers, particularly of AChE inhibitors. Hence tacrine and galantamine were included in our study, the latter because of its relatively low potency. The i.c.v. injection of these two AChE inhibitors enhanced the concentration of ACh in the superfusate in correlation with dose and potency showing the usefulness of our screening method in spite of the neostigmine present in the perfusion buffer. A similar effectiveness of tacrine on ACh levels has been shown with other methods [25]. The quantity of ACh in the superfusate reflects the concentration of the neurotransmitter at the synapse which is a function of release, metabolism by AChE to choline and acetate and uptake of choline. Whether inhibition of metabolism is the mode of action of the isolated sesquiterpenes is not definitely clear. Only silphiperfolene acetate showed in vitro a weak inhibition of AChE (40.64 ± 7.09% at 200 μM). In comparison, the positive control galantamine showed an IC50 of 3.2 μM and an inhibition rate of 89.30 ± 2.29% at 100 μM. For tacrine an IC50 of 0.13 μM was reported [25]. The correlation between these IC50 values of AChE inhibitors with their potency to increase ACh in the superfusate might suggest that an inhibitor with an IC50 of 500 μM or higher will hardly cause a significant ACh-enhancing effect. Isocomene, β-isocomene, silphinene and modehephene as well as 14-acetoxy-comene, up to a concentration of 500 μM did not show any inhibitory effects against AChE. On the other hand, the i.c.v. dose is relatively high for a definite conclusion on the role of AChE in the mode of action of isocomene and 14-acetoxyisocomene on cholinergic transmission.
4.2. Effects of isocomene on memory and behaviour
The object discrimination task, the T-maze alternation task and the locomotor activity in the open field are well suited to reveal the effects of cholinergic enhancers in the CNS. A substance which increases synaptic concentration of ACh in the brain exerts a characteristic pattern of effects on rodent behaviour in these tasks, as described below.
4.2.1. Object discrimination task
Isocomene abolished the amnesic effect of scopolamine in the object discrimination task. Treatment with isocomene enabled the scopolamine-injected mice to perform as good as the control group. The object discrimination paradigm reveals the ability of the animals to recognize the objects they have seen only once during the training trial. This kind of object recognition is based on episodic memory, a form of memory that is primarily affected in senile dementia and age-associated impairment and the Alzheimer type of dementia[26,27]. Also in mice and rats, object discrimination appears to depend on the integrity of the cholinergic system. AChE inhibitors such as tacrine improve performance of the animals in the object memory task [28,29]. In our study, isocomene did not improve object memory of vehicle-treated control mice though it abolished the amnesic effect of scopolamine treatment. However, the control group showed a 3 h-recognition index of about 0.3 which is excellent for mice of this strain and age [30]. For these animals the cognitive challenge might be too low to be susceptible to improvement by nootropic agents.
4.2.2. T-maze alternation
Isocomene increased the alternation rate of mice in the T-maze and abolished the decreasing effect of scopolamine on this parameter. These effects of isocomene correspond to the expected profile of enhancers of cholinergic activity. When placed in a T-maze, the tendency of mice and rats to alternate the arm choices on successive trials depends on working memory and spatial memory, the propensity to explore unfamiliar territory and emotional factors such as newness-induced anxiety. This task is sensitive to the consequences of normal and pathological aging. Neurochemical pathways using ACh, gamma-amino-butyric acid, and dopamine in the septum and hippocampus have been implicated in the exploration of novel maze arms. Spontaneous alternation rates have been shown to be decreased by drugs which impair cholinergic transmission [31,32]. In contrast, AChE inhibitors increase alternation rate in untreated and pirenzepine-impaired animals [33-36]. The impairment of working memory by scopolamine might cause the mice to choose always the same arm because of a preference to turn in a certain direction. Alternatively, the preference for the familiar arm might be the expression of a neophobic behaviour. It is known that anticholinergics enhance anxiety-related behaviour while AChE inhibitors exert an anxiolytic-like effect[37,38]. Thus, reduction of anxiety-level and/or improvement of working memory may be involved in the facilitation of alternation by AChE inhibitors. It should also be mentioned that the increase in alternation by isocomene cannot be just a function of enhanced locomotor activity, because the drug effects on alternation score and locomotor activity were negatively correlated.
4.2.3. Locomotor activity in the open field
It has been shown that manipulations of the cholinergic brain systems affect open field behaviour. At higher doses, anti-cholinergic drugs increase locomotor activity and cause hyperlocomotion in the open field. In contrast, AChE inhibitors have been reported to reduce spontaneous locomotor activity and counteract the stimulatory effect of antagonists such as scopolamine [39-41]. The reduction of locomotor activity by isocomene is in good accordance with the expression of a stimulatory effect on the cholinergic system.
The dose for the behaviour studies with isocomene was found by pilot experiments in which doses of 8 nmol and below were ineffective while doses of 200 nmol impaired normal behaviour. The low doses which improve cognition of mice were not tested for effectiveness on extracellular ACh in the rat because only significantly higher doses are able to induce a marked increase in ACh monitored with the push–pull technique. Reasons are the anaesthesia which suppresses cholinergic activity and the presence of 0.1 μM neostigmine in the superfusion fluid. The presence of the inhibitor is necessary to obtain detectable quantities of ACh in the superfusate. Nevertheless, the increase in extracellular ACh in the anaesthetized rat and the pattern of effects in the behaviour tasks are most likely due to the same cholinergic activity-enhancing mechanism of action.
Taken together the effects of isocomene in the three behaviour tasks on scopolamine-impaired and normal mice correspond to the expected properties of an enhancer of cholinergic activity. Isocomene increased learning and memory in the cholinergically impaired mice but exerted no effect in normal mice in the object memory task. Further, isocomene enhanced alternation rate of normal and scopolamine-treated mice in the T-maze. This parameter is reduced by cholinergic deficits and enhanced in normal and impaired rodents by AChE inhibitors. The alternation rate in this test represents a measure of the performance in working memory and of the balance between exploratory activity and neophobia [39]. The third indication of pro-cholinergic action was the effect in the open field task. The reduction of locomotor activity in untreated mice corresponds to the reported effect of cholinergic drugs such as AChE inhibitors [40-42]. The antagonism of isocomene on scopolamine-induced increase in locomotion suggests an enhancement of cholinergic transmission as mechanism of action.
In conclusion, we showed that sesquiterpene constituents of L. alpinum enhance synaptic availability of ACh in rat brain. Very likely these compounds cross readily the blood brain barrier due to their lipophilic properties. The constituent isocomene induces a long-lasting increase in extracellular ACh, improves learning and memory in normal and cholinergically impaired mice and influences behaviour in a way that is consistent with action as an enhancer of cholinergic activity. Isocomene and related molecules may have potential as antidementia agents in brain diseases associated with cholinergic deficits.
Acknowledgement
This work was supported by the Austrian Science Fund (FWF No. P18379).
References
- [1].Stuppner H, Ellmerer EP, Ongania KH, Dobner M. Bisabolane derivatives from Leontopodium alpinum. Helv Chim Acta. 2002;85:2982–9. [Google Scholar]
- [2].Dobner MJ, Ellmerer EP, Schwaiger S, Batsugkh O, Narantuya S, Stütz M, et al. New lignan, benzofuran, and sesquiterpene derivatives from the roots of Leontopodium alpinum and L. leontopodioides. Helv Chim Acta. 2003;86:733–8. [Google Scholar]
- [3].Dobner MJ, Schwaiger S, Jenewein IH, Stuppner H. Antibacterial activity of Leontopodium alpinum (Edelweiss) J Ethnopharm. 2003;89:301–3. doi: 10.1016/j.jep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [4].Schwaiger S, Adams M, Seger C, Ellmerer EP, Bauer R, Stuppner H. New constituents of Leontopodium alpinum and their in vitro leukotriene biosynthesis inhibitory activity. Planta Med. 2004;70:978–85. doi: 10.1055/s-2004-832625. [DOI] [PubMed] [Google Scholar]
- [5].Bartus RT, Dean RL, Beer B, Lipps AS. The cholinergic hypothesis of geriatric memory dysfunction: a critical review. Science. 1982;217:408–17. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- [6].Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T. Anti-inflammatory properties of cholinergic upregulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology. 2006;50:540–7. doi: 10.1016/j.neuropharm.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [7].Grey AI, Hook IL, James P, Sheridan H. Sesquiterpenes from Leontopodium alpinum. Phytochemistry. 1999;50:1057–60. Corrigendum: Phytochemistry 2000; 54:551. [Google Scholar]
- [8].Bohlmann F, Le Van G, Pham TVC, Jacupovic J, Schuster A, Zabel V, et al. β-Isocomene, ein neues Sesquiterpen aus Berkheya-Arten. Phytochemistry. 1979;18:1831–4. [Google Scholar]
- [9].Bohlmann F, Jacupovic J. Neue Sesquiterpen-Kohlen was serstoffe mit anomalen Kohlenstoffgerüst aus Silphium-arten. Phytochemistry. 1980;19:259–65. [Google Scholar]
- [10].Paxinos G, Watson H. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; Sidney: 1998. [Google Scholar]
- [11].Kraus MM, Prast H. The nitric oxide system modulates in vivo release of acetylcholine in the nucleus accumbens induced by stimulation of the hippocampal fornix/fimbria projection. Eur J Neurosci. 2001;14:1105–12. doi: 10.1046/j.0953-816x.2001.01735.x. [DOI] [PubMed] [Google Scholar]
- [12].Damsma G, Westerink BHC, de Boer P, de Vries JB, Horn AS. Determination of basal acetylcholine release in freely moving rats by transstriatal dialysis coupled to on-line HPLC analysis: pharmacological aspects. Life Sci. 1988;43:1161–8. doi: 10.1016/0024-3205(88)90475-4. [DOI] [PubMed] [Google Scholar]
- [13].Prast H, Tran MH, Fischer H, Philippu A. Nitric oxide-induced release of acetylcholine in the nucleus accumbens: role of cyclic GMP, glutamate, and GABA. J Neurochem. 1998;71:266–73. doi: 10.1046/j.1471-4159.1998.71010266.x. [DOI] [PubMed] [Google Scholar]
- [14].Ellman GL, Courtney D, Andres V, Featherstone RM. A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- [15].Ingkaninan K, de Best CM, van der Heijden R, Hofte AJP, Karabatak B, Irth H, et al. High-performance liquid chromatography with on-line coupled UV, mass spectrometric and biochemical detection for identification of acetylcholinesterase inhibitors from natural products. J Chromatogr A. 2000;872:61–73. doi: 10.1016/s0021-9673(99)01292-3. [DOI] [PubMed] [Google Scholar]
- [16].Rollinger JM, Hornick A, Langer T, Stuppner H, Prast H. Acetylcholinesterase inhibitory activity of scopolin andscopoletin discovered by virtual screening of natural products. J Med Chem. 2004;47:6248–54. doi: 10.1021/jm049655r. [DOI] [PubMed] [Google Scholar]
- [17].Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- [18].Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioural data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- [19].Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav Brain Res. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- [20].Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviours: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- [21].Kraus MM, Prast H. Involvement of nitric oxide, cyclic GMP and phosphodiesterase 5 in excitatory amino acid and GABA release in the nucleus accumbens evoked by activation of the hippocampal fimbria. Neuroscience. 2002;112:331–43. doi: 10.1016/s0306-4522(02)00079-9. [DOI] [PubMed] [Google Scholar]
- [22].Philippu A, Prast H, Singewald N. Identification and dynamics of neuronal modulation and function in brain structures and nuclei by continuous determination of transmitter release rates using the push–pull superfusion technique: a compelling approach to in vivo brain research. Sci Pharm. 1996;64:609–18. [Google Scholar]
- [23].Zalkow LH, Harris RN, Van Derweer D, Bertrand JA. Isocomene: a novel sesquiterpene from Isocoma Wrightii. X-Ray crystal structure of the corresponding diol. J Chem Soc Chem Commun. 1977;13:456–7. [Google Scholar]
- [24].Bohlmann F, Le Van N, Pickardt J. Natürlich vorkommende Terpen derivate. J Chem Ber. 1977;110:3777–81. [Google Scholar]
- [25].Snape MF, Misra A, Murray TK, De Souza RJ, Williams JL, Cross AJ, et al. A comparative study in rats of the in vitro and in vivo pharmacology of the acetylcholinesterase inhibitors tacrine, donezepil and NXX-066. Neuropharmacology. 1999;38:181–93. doi: 10.1016/s0028-3908(98)00164-6. [DOI] [PubMed] [Google Scholar]
- [26].Flicker C, Ferris SH, Crrok T, Bartus RT, Reisberg B. Cognitive function in normal aging and early dementia. In: Traber J, Gispen WH, editors. Senile dementia of the Alzheimer type. Springer; Berlin, Heidelberg, New York: 1985. pp. 2–17. [Google Scholar]
- [27].Lee AC, Rahman S, Hodges JR, Sahakian BJ, Graham KS. Associative and recognition memory for novel objects in dementia: implications for diagnosis. Eur J Neurosci. 2003;18:1660–70. doi: 10.1046/j.1460-9568.2003.02883.x. [DOI] [PubMed] [Google Scholar]
- [28].Scali C, Giovannini MG, Prosperi C, Bartolini L, Pepeu G. Tacrine administration enhances extracellular acetylcholine in vivo and restores the cognitive impairment in aged rats. Pharmacol Res. 1997;36:463–9. doi: 10.1006/phrs.1997.0252. [DOI] [PubMed] [Google Scholar]
- [29].Prickaerts J, Sik A, van der Staay FJ, de Vente J, Blokland A. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology. 2005;177:381–90. doi: 10.1007/s00213-004-1967-7. [DOI] [PubMed] [Google Scholar]
- [30].Sik A, Van Nieuwenhuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- [31].Meyers B, Domino EF. The effect of cholinergic drugs on spontaneous alternation in rats. Arch Int Pharmacodyn. 1964;150:3–4. [PubMed] [Google Scholar]
- [32].Kokkinidis L, Anisman H. Interaction between cholinergic and catecholaminergic agents in a spontaneous alternation task. Psychopharmacology. 1976;48:261–70. doi: 10.1007/BF00496859. [DOI] [PubMed] [Google Scholar]
- [33].Egger GJ, Livesey PJ, Dawson RG. Ontogenic aspects of central cholinergic involvement in spontaneous alternation behavior. Dev Psychobiol. 1973;6:289–99. doi: 10.1002/dev.420060402. [DOI] [PubMed] [Google Scholar]
- [34].Delatour B, Gisquet-Verrier P. Prelimbic cortex specific lesions disrupt delayed-variable response tasks in the rat. Behav Neurosci. 1996;110:1282–98. doi: 10.1037//0735-7044.110.6.1282. [DOI] [PubMed] [Google Scholar]
- [35].Ukai M, Shinkai N, Kameyama T. Cholinergic receptor antagonists inhibit pirenzepine-induced dysfunction of spontaneous alternation performance in the mouse. Gen Pharmacol. 1995;26:1529–32. doi: 10.1016/0306-3623(95)00038-0. [DOI] [PubMed] [Google Scholar]
- [36].Spowart-Manning L, van der Staay FJ. The T-maze continuous alternation task for assessing the effects of putative cognition enhancers in the mouse. Behav Brain Res. 2004;151:37–46. doi: 10.1016/j.bbr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- [37].Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer’s disease: the cholinergic hypothesis revisited. Neurology. 1996;47:876–83. doi: 10.1212/wnl.47.4.876. [DOI] [PubMed] [Google Scholar]
- [38].Sinkiewicz-Jarosz H, Czlonkowska A, Siemiatkowski M, Maciejak P, Szyndler J, Plaznik A. The effects of physostigmine and cholinergic receptor ligands on novelty-induced neophobia. J Neural Transm. 2000;107:1403–12. doi: 10.1007/s007020070004. [DOI] [PubMed] [Google Scholar]
- [39].Carlsson M, Carlsson A. Marked locomotor stimulation in monoamine-depleted mice following treatment with atropine in combination with clonidine. J Neural Transm. 1989;1:317–22. doi: 10.1007/BF02263486. [DOI] [PubMed] [Google Scholar]
- [40].Capone F, Oliverio A, Pomponi M, Marta M, Gatta F, Pavone F. Effects of the novel acetylcholinesterase inhibitor N-octyl-1,2,3, 4-tetrahydro-9-aminoacridine on locomotor activity and avoidance learning in mice. Neurobiol Learn Mem. 1999;71:301–7. doi: 10.1006/nlme.1998.3883. [DOI] [PubMed] [Google Scholar]
- [41].Mach M, Grubbs RD, Price WA, Paton SJ, Lucot JB. Behavioral changes after acetylcholinesterase inhibition with physostigmine in mice. Pharmacol Biochem Behav. 2004;79:533–40. doi: 10.1016/j.pbb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [42].Csernanski JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H. Cholinesterase inhibitors ameliorate behavioural deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30:2135–43. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]