Abstract
The end-stage immunopathology of type 1 diabetes resulting in β-cell destruction appears to be strongly dominated by cytotoxic CD8 T lymphocytes (CD8 T cells). However, the mechanism of cytotoxicity used by autoreactive CD8 T cells in the human setting remains unknown. Using type 1 diabetes patient–derived preproinsulin-specific CD8 T-cell clones recognizing either an HLA-A2 (A*0201) or HLA-A24 (A*2402)-restricted epitope (peptide of preproinsulin [PPI]15–24, ALWGPDPAAA; or PPI3–11, LWMRLLPLL), we assessed the use of conventional mediators of cytotoxicity in the destruction of human β-cells in vitro compared with virus-specific cytotoxic CD8 T-cell clones. We show that PPI-specific CD8 T-cell clones are mainly reliant upon cytotoxic degranulation for inducing β-cell death. Furthermore, we find that in comparison with virus-specific CD8 T cells, there are differences in the killing potency of PPI-specific CD8 T cells that are not due to cell-intrinsic differences, but rather are mediated by differences in strength of signaling by peptide–HLA ligands. The study highlights the regulation of β-cell killing as a potential point for therapeutic control, including the possibility of blocking autoreactive CD8 T-cell function without impacting upon general immune competence.
The impaired glucose homeostasis that is characteristic of type 1 diabetes results from the selective destruction of insulin producing β-cells within pancreatic islets. Cytotoxic CD8 T lymphocytes (CD8 T cells) have been implicated as major mediators of β-cell damage on the basis of multiple strands of evidence including CD8 T-cell dominance of the islet infiltrate (1,2); single nucleotide polymorphism studies (3) showing that HLA class I genes predispose to type 1 diabetes; and numerous studies in animal models that support a CD8-centric view of β-cell killing (4,5), including, more recently, direct visualization (6). Evidence that CD8 T cells recognizing β-cell autoantigens have a direct role in β-cell death in human disease was obtained recently following the establishment of CD8 T-cell clones specific for an epitope from the signal peptide of preproinsulin (PPI) from the blood of a newly diagnosed type 1 diabetes patient (7). These CD8 T cells recognize residues 15–24 of PPI presented by HLA-A*0201 and selectively kill HLA-A2+ human β-cells in vitro (7). Using both enzyme-linked immunospot assays and PPI15–24 tetramer staining, it was subsequently shown that CD8 T cells recognizing PPI15–24 are enriched in the peripheral blood of patients near to disease onset (7,8). More recently, in situ PPI15–24 tetramer staining has shown that cells of this specificity are present in insulitic lesions characteristic of the disease (9).
CD8 T cells deliver cytolytic effector functions against target cells via contact-dependent mechanisms that use two discrete pathways: cytotoxic degranulation or interaction with tumor necrosis factor (TNF) family-related death receptors. Cytotoxic degranulation involves the release of perforin, which facilitates entry of coreleased granzymes with serine protease activity into cells, resulting in rapid cell death (10). Fas ligand (FasL) is the best-characterized TNF family-related death receptor, binding to Fas expressed on the target cell surface and initiating a series of intracellular pathways resulting in apoptosis (11). Although cytotoxic degranulation is recognized as the main, very potent lytic process used in the clearance of bacterial and viral pathogens, as well as being implicated in the killing of tumor cells (10,12), it is also known that this pathway can act in concert with FasL for pathogen removal (13).
Given the persuasive evidence that CD8 T cells contribute to β-cell death, the identity of the cytotoxic mechanisms that are used in this selective, destructive process represent an important knowledge gap. Studies that have previously attempted to address this question have used the nonobese diabetic (NOD) mouse model or human islets pulsed to present viral peptide to cognate antiviral CD8 T-cell clones (14). The results of these studies highlight the importance of granzyme and perforin in the murine model of type 1 diabetes and in virus-specific CD8 T-cell killing of virus peptide-pulsed islets, but raise the important question of the role of these mediators in the human autoimmune setting (15), in which CD8 T-cell biology may be different (16).
Novel insights into this process in a relevant human model could potentially enable the development of immune-based therapeutic approaches that specifically target pathways used by β-cell–specific CD8 T cells, but leave antiviral and antitumor killing intact (16). The current study was therefore designed to elucidate the mechanism(s) of CD8 T-cell–mediated β-cell death in human type 1 diabetes using autoreactive CD8 T-cell clones isolated from patients with new-onset disease. We obtained CD8 T cells from two donors with specificity for different PPI epitopes, restricted by two different HLA-A alleles, as well as virus-specific CD8 T cells as a well-established reference point. The roles of TNF-related death receptor ligands, together with degranulation, were assessed in coculture experiments using HLA-matched human islet cells and antigen-pulsed target cell lines in the presence and absence of relevant cytotoxic pathway inhibitors. In addition, we examined whether any differences in the mechanism and potency of killing by autoreactive and antiviral CD8 T cells could be attributable to cell-intrinsic factors or extrinsic effects such as the strength of peptide–HLA/T-cell receptor (TCR) interaction.
RESEARCH DESIGN AND METHODS
Generation and maintenance of CD8 T-cell clones and cell lines.
PPI signal peptide (SP) epitope-specific CD8 T-cell clones were isolated from patients with new-onset type 1 diabetes as previously described (7,17). The HLA-A2–restricted A2-PPI15–24 CD8 T-cell clone recognizes residues 15–24 of PPI SP (ALWGPDPAAA), and the HLA-A24–restricted A24-PPI3–11 CD8 T-cell clone recognizes a novel naturally β-cell–processed and HLA-A24–presented epitope of PPI SP represented by amino acids 3–11 (LWMRLLPLL) (17). Viral CD8 T-cell clones specific for cytomegalovirus (CMV) antigens and restricted by either HLA-A2 or HLA-A24 peptide were isolated alongside using a similar approach. The HLA-A2–restricted CD8 T-cell clone A2-CMVpp65495–503 is specific for residues 495–503 of CMV protein pp65 (NLVPMVATV). The HLA-A24–restricted CD8 T-cell clone A24-CMV-IE1248–257 recognizes an epitope from CMV 1E-1 (248–257; AYAQKIFKIL). Target cell lines C1R and K562 transfected with HLA-A*0201 were cultured in RPMI 1640 (Gibco) supplemented with 10% FCS (Gibco) containing 100 IU/ml penicillin and 100 μg/mL streptomycin and pulsed with peptide (10 μg/mL) for 1 h (37°C) followed by washing.
Islet-cell cytotoxicity assays.
Human islet isolations were performed as previously described (18) using pancreata retrieved with the consent of donors’ relatives and permission of the Ethical Review Committee of King’s College Hospital. Islet cells in monolayer cultures were cultured for 16–24 h in medium containing 16 mmol glucose and cytokines interleukin (IL)-1β (50 IU/mL; Strathmann Biotec), TNF-α (2500 IU/mL; Mitenyi Biotec), interferon (IFN)-γ (500 IU/mL; Miltenyi Biotec), and IFN-α (1000 IU/mL; Roche Laboratories) to increase class I expression and provide a greater dynamic range for analyzing the effect of inhibitory reagents on pathways of cytotoxicity (19). Cytotoxicity was analyzed as described (7,20) and specific cytotoxicity calculated using the formula: percent specific release = (experimental − spontaneous release) × 100/(maximum − spontaneous release). In a modified cytotoxicity assay conducted over 24 h to examine the role of Fas–FasL interactions, RNA was isolated from islet cell/CD8 T-cell clone cocultures using the RNeasy Mini Kit (Qiagen) and cDNA generated (High-Capacity cDNA Reverse-Transcription Kit; Applied Biosystems) before quantitative PCR was performed on an Applied Biosystems 7900HT Fast Real-Time PCR System with TaqMan Gene Expression Master Mix and TaqMan Gene Expression Assays for Insulin (Hs02741908-m1) and GAPDH (4326317E; Applied Biosystems). Cytotoxicity was measured as the percentage loss of insulin mRNA expression in test cocultures compared with islets cultured with an irrelevant T-cell clone.
Inhibitory reagents.
To inhibit perforin activity, CD8 T cells were cultured for 1 h prior to assay in medium containing concanamycin A (CMA, 100 nmol; Sigma-Aldrich) or diluent (DMSO). Blocking antibodies (or isotype-matched irrelevant controls) were present throughout assays for FasL (5 μg/mL clone NOK-1; BioLegend), TNF-related apoptosis-inducing ligand (TRAIL; 5 μg/mL clone RIK-1; BioLegend), and TNF-α (12.5 μg/mL; provided by Dr. Tim Bourne, UCB Celltech).
Carboxyfluorescein succinimidyl ester–based cytotoxicity assay.
Peripheral blood mononuclear cells (PBMCs) (106) from HLA-A2+ healthy donors were incubated with high or low concentrations of carboxyfluorescein succinimidyl ester (CFSE; 1.25 and 0.1 μmol, respectively) for 15 min at room temperature (RT). Labeling was quenched using 1 mL FCS (Invitrogen) and CFSE-low–labeled PBMCs were peptide-pulsed, whereas CFSE-high–labeled cells were pulsed with peptide diluent. PBMCs were then washed thoroughly, combined at a 1:1 ratio, and cocultured with T-cell clones for 4 or 8 h before staining with anti-CD14 antibody (BD Biosciences). Assessment of CD14+ CFSELOW and CFSEHIGH cell percentages was performed by flow cytometry and analyzed using FlowJo software (Tree Star, Ashland, OR).
Assessing intracellular protein and CD107a surface expression.
Anti-CD107a (fluorescein isothiocyanate; clone H4A3; BD Biosciences) was added to 5 × 105 cloned CD8 T cells in FACS tubes containing X-Vivo 15/5% AB serum/IL-7 (10 ng/mL), IL-15 (0.1 ng/mL), and 2.5% Cellkine (ZeptoMetrix Corporation). Islet or nonislet target cell preparations were added to tubes (5 × 105) for 1 h at 37°C (5% CO2) before 0.7 μg/mL monensin (GolgiStop; BD Biosciences) and 1 μg/mL brefeldin A (GolgiPlug; BD Biosciences) were added to cocultures. After 4 h incubation, cells were washed twice and resuspended in PBS containing the amine reactive dye Violet Viable Dye (ViViD; Invitrogen/Molecular Probes) for exclusion of dead cells. Cells were incubated at 4°C, washed in PBS containing 2% FCS, and stained with anti-CD8 antibody (BD Pharmingen). After 25 min at 4°C, cells were washed and resuspended in 300 μL BD-Cytofix/Cytoperm solution (BD Biosciences) and incubated at RT for 15 min. Cells were washed and resuspended in 500 μL BD-Fixation/Permeabilization solution for 10 min (RT) before being centrifuged at 400 × g for 5 min and stained with antibodies for granzyme B (clone GB11; BD Biosciences) and TNF-α (clone MAb11; BD Pharmingen). Cells were incubated with antibody for 25 min at 4°C, washed, and analyzed by flow cytometry.
Measuring cytokines in coculture supernatant.
Cloned CD8 T cells (2 × 104) were seeded in 96-well plates containing 2 × 104 target cells and cultured for 18 h (37°C/5% CO2) in medium containing X-Vivo 15/5% AB serum/IL-7 (10 ng/mL), IL-15 (0.1 ng/mL), and 2.5% Cellkine and cytokines measured by Luminex technology (Milliplex Mag Kit; Millipore).
Surface plasmon resonance analysis.
Binding analysis was performed using a BIAcore T100 equipped with a CM5 sensor chip (BIAcore) as reported previously (21).
RESULTS
PPI-specific CD8 T-cell clones kill human islets primarily through cytotoxic degranulation.
For these studies, we focused on two CD8 T-cell clones that recognize PPI and kill β-cells: those specific for PPI15–24 (ALWGPDPAAA) presented by HLA-A2 (A2-PPI15–24 CD8 T cells) (7) and for PPI3–11 (LWMRLLPLL; A24-PPI3–11 CD8 T cells) (17). In a replication of our original report (7), A2-PPI15–24 CD8 T-cell clone 3F2 kills human HLA-matched islets (different donor than that used in the original studies) (Supplementary Fig. 1A). As a comparator, we used a CD8 T-cell clone recognizing the dominant pp65 epitope of CMV (pp65495–503; NLVPMVATV) presented by HLA-A2 that does not kill human islets in the absence of pulsing with cognate peptide (Supplementary Fig. 1A). During the interaction with HLA-A2+ human islets, the A2-PPI15–24 CD8 T-cell clones secrete IFN-γ and macrophage inflammatory protein-1β (Supplementary Fig. 1B and C) and are also known to secrete TNF-α (7). It is noteworthy that under conditions using islets prepulsed with cognate peptide, levels of killing and cytokine secretion are generally higher with the antiviral CD8 T cell (see below). Similar results for human islet killing were obtained using the A24-PPI3–11 CD8 T cell (17) and an HLA-A24–restricted CMV-specific comparator clone (A24-CMV-IE1248–257; AYAQKIFKIL) (22,23).
We next examined the effects of degranulation inhibition on this killing process. CMA degrades perforin within cytotoxic granules of CD8 T cells and has little impact upon the degranulation process (24). Pretreatment of CD8 T-cell clones with CMA 60 min before human islet coculture resulted in significant reduction in islet killing by A2-PPI15–24 CD8 T cells (P = 0.002; Fig. 1A). In complementary studies, a similar reduction in A24-PPI3–11 CD8 T-cell cytotoxicity against A24+ human islet cells was also observed (Fig. 1A). As a comparison, CMA pretreatment of A2-CMVpp65495–503CD8 T-cell clones also inhibited killing of pp65495–503 peptide-pulsed HLA-A2+ human islet cells (P < 0.0001; Fig. 1A). The observed reduction in cytotoxicity was not due to toxic effects of CMA, as demonstrated by assessing viability of A2-PPI15–24 CD8 T-cell clone (Fig. 1B), nor did it impede release of TNF-α (Fig. 1C). These data implied usage of perforin for the targeted cellular entry of granule contents as a major mechanism of autoreactive CD8 T-cell killing of islet cells.
FIG. 1.
Effect of blockade of cytotoxic degranulation on PPI-specific CD8 T-cell–induced islet cell death. Panel A shows cytotoxicity assays performed using A2-PPI15–24 CD8 T-cell (bars represent means for three independent assays; error bars are SEM) and A24-PPI3–11 CD8 T-cell clones (bars represent means from a single assay due to limited donor islet material) in presence or absence of pretreatment for 60 min with 100 nmol CMA. Statistically significant reductions in mean A2-PPI15–24 CD8 T-cell cytotoxicity were found following clone pretreatment with CMA (**P = 0.002; assigned using a two-tailed paired t test). For comparison, a similar experiment has been performed using A2-CMVpp65495–503 CD8 T cells with human islet cells pulsed with cognate CMVpp65 peptide as target cells (n = 3 independent experiments; ***P < 0.0001 calculated using a two-tailed paired t test). B: CMA pretreatment of A2-PPI15–24 CD8 T-cell clone demonstrated no toxicity as shown by ViViD incorporation after 5-h culture. Panels show flow cytometry histograms of ViViD staining, and numbers on each panel indicate percentage of cells positive for ViViD staining. Concentration of CMA used in each experiment (nM) is shown above panels. C: CMA pretreatment of A2-PPI15–24 CD8 T-cell clone did not affect levels of TNF-α released after coculture with K562 target cells transfected to express HLA-A2 and cultured with cognate peptide. D: Recapitulation of the inhibitory effect upon cytotoxicity induced by CMA pretreatment was found for both A2-PPI15–24 CD8 T-cell and CMV-specific CD8 T-cell A2-CMVpp65495–503 when cocultured with HLA-A2–expressing K562 target cells presenting cognate peptide (n = 4 independent experiments; ***P < 0.0001). Cytotoxicity assays were performed at effector/target ratios of 25:1; cells were incubated for 4 h before assessment of target cell lysis. Bars represent means of triplicate assay wells and error bars the SEM. CTL, cytotoxic T lymphocyte.
These effects could be recapitulated using the K562 myeloid cell line transfected with HLA-A2 (A*0201) (K562-A2) (7). Pretreatment of A2-PPI15–24 CD8 T cells with CMA reduced killing of K562-A2 cells pulsed with cognate PPI15–24 peptide (P < 0.0001; Fig. 1D). A similar reliance upon perforin was observed for A2-CMVpp65495–503 CD8 T cells when cocultured with target K562-A2 cells pulsed with CMVpp65495–503 (P < 0.0001; Fig. 1D). As further evidence that granule content release is an important sequel of activation of these CD8 T cells, we showed a modest reduction in intracellular granzyme B following engagement with human islets (example shown for A2-PPI15–24 CD8 T cell) (Supplementary Fig. 1D). Taken together, these results indicate that autoreactive CD8 T cells specific for PPI targets presented by human islet cells release cytotoxic granule contents as their predominant mechanism of target cell killing.
The role of Fas–FasL interactions.
Using flow cytometry, we observed that all PPI- and CMV-specific CD8 T cells have intracellular stores of TRAIL and FasL (Supplementary Fig. 2A). We have shown that pretreatment of human islets with proinflammatory cytokines increases surface expression of HLA class I and enhances their killing by A2-PPI15–24 CD8 T cells (19); phenotypic analysis of cytokine pretreated islets from three donors revealed increased surface expression of Fas (P = 0.013; Fig. 2A) with maintenance of islet cell viability (Supplementary Fig. 2B). Because not all CD8 T-cell–mediated islet cell death could be accounted for by degranulation (Fig. 1A), we investigated whether Fas engagement contributes to islet cell death.
FIG. 2.
Assessing Fas-induced cell death of peptide-pulsed monocytes and human islet target cells by PPI-specific CD8 T-cell clone. Panel A shows mean levels of Fas (CD95) expression on islets from three donors using flow cytometry analysis. Fas expression increases when islet cells are pretreated with proinflammatory cytokines (*P = 0.013). B and C: To assess potential FasL usage in killing, PBMCs were labeled with two different CFSE concentrations and pulsed with wild-type peptide (CFSELOW) or DMSO diluent (CFSEHIGH) and cocultured together with CD8 T-cell clone in the presence or absence of anti-FasL antibody. The Fas-expressing CD14+ population was assessed for CFSE loss. A2-PPI15–24 CD8 T cell showed reduced cytotoxicity of Fas+ peptide-pulsed monocytes after 8 h with FasL blockade. Example staining is shown in panel B and accumulated data from three independent experiments in panel C (8-h time point; **P = 0.0095). D: A2-PPI15–24 and A24-PPI3–11 CD8 T cell showed no inhibition of cytotoxicity when cultured with islet targets in the presence of monoclonal anti-FasL antibody (5 μg/mL) or isotype control. Cytotoxicity assays were performed for 4 h at an effector/target ratio of 25:1. Bars represent means of triplicate assay wells and error bars SEMs. E: Blocking FasL on A2-PPI15–24 CD8 T cells in coculture with islet cells does not preserve β-cell function, as measured by insulin mRNA levels. Bars represent mean insulin mRNA levels within islet coculture conditions, relative to insulin mRNA in cocultures with control clone A2-CMVpp65495–503. CD8 T-cell clones were incubated with islet cells at a ratio of 25:1 for 24 h to maximize visualization of the involvement by FasL on β-cell killing. Blocking FasL antibody remained present within cultures throughout assays. CTL, cytotoxic T lymphocyte.
To examine the potential of A2-PPI15–24-specific CD8 T cells to mediate FasL–Fas killing, we initially used monocyte targets (because they express Fas constitutively; data not shown). Neither A2-PPI15–24 nor A2-CMVpp65495–503 CD8 T-cell killing was affected significantly by FasL inhibition in short (4-h) assays (Fig. 2B). However, in 8-h assays, Fas+ monocytes presenting PPI15–24 exhibited reduced cell death when FasL–Fas interaction was blocked (P = 0.0095; Fig. 2C), whereas A2-CMVpp65495–503 CD8 T cells killed without requirement for intact FasL–Fas signaling (Fig. 2C). In conventional 4-h cytotoxicity assays using islet-cell targets, FasL blockade had no effect on cytotoxicity of either PPI-specific CD8 T-cell clone (Fig. 2D). Similarly, when investigating the contribution of FasL to islet cell death by A2-PPI15–24 CD8 T-cell clones over a longer time period (in a modified cytotoxicity assay measuring insulin mRNA), we could not detect a role for FasL (Fig. 2E). Taken together, these data show the potential for the autoreactive CD8 T-cell clone to use FasL–Fas for inducing target cell death, but illustrate that it does not appear to play a role in β-cell killing by A2-PPI15–24-specific clones in vitro.
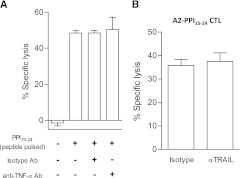
Effects of TNF-α and its related apoptosis inducing ligand (TRAIL) on target cell cytotoxicity.
Activated A2-PPI15–24 CD8 T cells secrete copious quantities of TNF-α (7), a cytokine known to have cytotoxic properties. TNF-α–blocking humanized monoclonal antibody (Supplementary Fig. 3) had no effect on A2-PPI15–24 CD8 T-cell killing of peptide-pulsed K562-A2 cells (Fig. 3A). Blockade of TRAIL had no inhibitory effect on CD8 T-cell–mediated islet cell cytotoxicity (Fig. 3B). Thus, it would appear that apart from TNF-α activity as a part of a cytokine cocktail that renders human islets more susceptible to CD8 T-cell killing (19), TNF-α and its related receptors do not have any major, direct role in islet-cell death.
FIG. 3.
Assessing the effect of TNF-α and TRAIL on A2-PPI15–24 CD8 T-cell cytotoxicity. A: A humanized anti–TNF-α antibody was used in cytotoxicity assays to block TNF-α produced by A2-PPI15–24 CD8 T-cell clones in coculture with K562-A2 target cells presenting cognate PPI15–24 peptide. No inhibition of target cell lysis by the A2-PPI15–24 CD8 T-cell clone was detected with blockade of soluble TNF-α. B: Using blocking anti-TRAIL antibody in cytotoxicity assays does not inhibit A2-PPI15–24 CD8 T-cell cytotoxicity. Bars represent means from triplicate assay wells and error bars SEM. Cytotoxicity assays were performed at an effector/target ratio of 25:1 for 4 h. CTL, cytotoxic T lymphocyte.
Maximal killing by autoreactive CD8 T cells is dependent upon the avidity of the T-cell interaction.
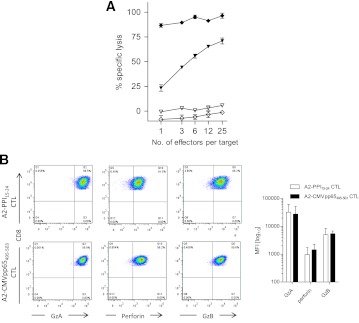
We noted that even under maximal killing conditions (i.e., when islet cells were prepulsed with cognate peptide) A2-PPI15–24 CD8 T cells were less potent than comparator A2-CMVpp65495–503 CD8 T cells (mean percentage killing 62 ± 7.6% vs. 86 ± 2.8% [SEM], respectively) (Supplementary Fig. 1A). Similar results were obtained using HLA-A*0201–transfected K562 cells pulsed with cognate peptide as targets, which should render all target cells in the cultures maximally susceptible to killing. In these assays, A2-PPI15–24 CD8 T-cell killing of peptide-pulsed K562-A2 cells was lower at all effector-to-target ratios compared with A2-CMVpp65495–503 CD8 T cells (Fig. 4A). This difference was not due to differences in binding of PPI and CMV peptides to HLA-A2 (Supplementary Fig. 4). We hypothesized that there could be two possible underlying mechanisms for a lower level of maximal killing by A2-PPI15–24 CD8 T cells. On the one hand, it is conceivable that there are cell-intrinsic properties in autoreactive CD8 T cells that explain their different effector functions from those of antiviral CD8 T cells. On the other hand, cell-extrinsic properties, for example reflecting the known ultra-low affinity of the TCR of A2-PPI15–24 CD8 T cells for cognate peptide–HLA (pHLA) could account for different killing properties (19,25) through reduced overall avidity of the interaction with target cells.
FIG. 4.
Comparison of A2-CMVpp65495–503 and A2-PPI15–24 CD8 T-cell clones killing of cognate peptide-pulsed targets and granule content. A: Cytotoxicity assays were performed with A2-PPI15–24 CD8 T cells (triangles) and A2-CMVpp65495–503 CD8 T cells (diamonds) using K562-A2 target cells pulsed with 10 μg/mL cognate peptide (filled symbols) or DMSO diluent (open symbols). Even at low effector/target ratios, the CMV clone causes maximal killing, whereas even at high ratios, the autoreactive clone achieves submaximal cytotoxicity. B: Example staining (left panel) and accumulated results from three experiments (right panel) showing perforin and granzyme A (GzA) and B (GzB) levels in A2-PPI15–24 CD8 T-cell clones (open bars) and A2-CMVpp65495–503 CD8 T-cell clones (shaded bars) are shown. Effector CD8 T-cell and target cells were cocultured at a ratio of 25:1 for 4 h in cytotoxicity assays. Bars represent the mean of triplicate assay wells; error bars indicate the SEM. Staining is expressed as the median fluorescence intensity (MFI) of three compiled experiments. CTL, cytotoxic T lymphocyte. (A high-quality color representation of this figure is available in the online issue.)
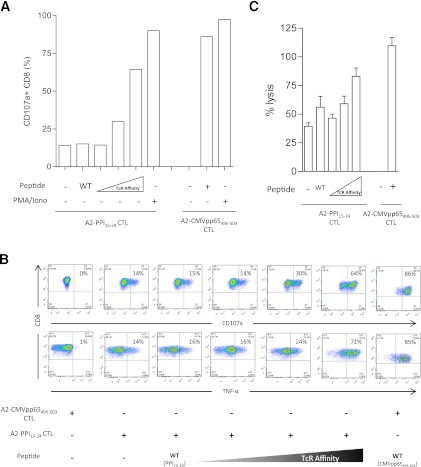
First, we examined whether differences in killing peptide-pulsed targets could be cell-intrinsic by assessing whether autoreactive and antiviral clones have comparable cytotoxic potential. Both autoreactive and antiviral clones have similar amounts of cytotoxic granule components (Fig. 4B) and in response to maximal stimulation with phorbol ester and ionomycin degranulate to a similar degree (Fig. 5A), indicating no defect in signal transduction or granule exocytosis. Experiments were then designed to examine the effect on killing of changes in cell-extrinsic properties, namely the avidity of the interaction between target cells and A2-PPI15–24 CD8 T cells. For this purpose, we used a series of heteroclitic peptides based upon the PPI15–24 (ALWGPDPAAA) index sequence but that stimulate A2-PPI15–24 CD8 T cells as well as, or better than, the wild-type sequence, this superagonist effect being achieved through superior affinity of these peptides for the A2-PPI15–24 CD8 T-cell TCR (Supplementary Fig. 5) (25). We selected three heteroclitic peptides designated as having low, medium, or high affinity for the A2-PPI15–24-specific TCR (Table 1), and A2-PPI15–24 CD8 T cells were cocultured with human islet cells prepulsed with each. We observed that CD8 T-cell secretion of TNF-α and degranulation both increased, whereas granzyme B levels decreased in accordance with the affinity of peptide for TCR (Fig. 5A and B and Supplementary Fig. 1D). In keeping with this, the death of islet cells was determined by the TCR affinity of the heteroclitic peptide with which they were pulsed (Fig. 5C). Similar results were obtained when the same heteroclitic peptides were pulsed onto nonislet target cells (Supplementary Fig. 6). These results suggest a cell-extrinsic explanation for reduced killing by A2-PPI15–24 CD8 T cells compared with their antiviral counterparts.
FIG. 5.
Reduced A2-PPI15–24 CD8 T-cell cytotoxicity is due to reduced activation when recognizing wild-type peptide. A and B: A2-PPI15–24 CD8 T-cell clones were incubated with either wild-type peptide-pulsed islet cells or islet cells presenting heteroclitic peptides with low, medium, or high affinity for PPI15–24-TCR (see Table 1 for details). As pHLA affinity for TCR increases, so does intracellular TNF-α production and expression of degranulation marker CD107a by the clone (representative of n = 2). C: This finding also correlates with the percentage of specific lysis; high TCR affinity peptide-pulsed islet target cells are lysed to a level comparable to target cells pulsed with pp65495–503 peptide in the presence of A2-CMVpp65495–503 CD8 T-cell clone. Cytotoxicity assay bars represent means of triplicate assay wells; error bars show SEM. CTL, cytotoxic T lymphocyte; WT, wild type. (A high-quality color representation of this figure is available in the online issue.)
TABLE 1.
Biophysical binding affinity and kinetic measurements (using surface plasmon resonance) of A2-PPI15–24 CD8 T-cell TCR interaction with wild-type and heteroclitic PPI peptides bound to HLA-A2

DISCUSSION
This study is the first to investigate the cytotoxic mechanisms used by autoreactive CD8 T cells that target human β-cells in type 1 diabetes. Our results indicate that the predominant killing mechanism requires cytotoxic granule components to be released onto the islet cell during a cognate interaction that is dependent upon CD8 T-cell recognition of a β-cell specific target; in this case, an epitope of the signal peptide of PPI. Previous studies designed to address the mechanisms of cytotoxicity used by human CD8 T cells to kill β-cells were limited by the use of virus-specific CD8 T cells and virus peptide-pulsed islet cells as targets (14). This approach can indicate the susceptibility of islet cells to classic types of effector functions used to combat viruses, but has the disadvantage that it takes no account of the possibility that autoreactive CD8 T cells may behave differently from pathogen-specific CD8 T cells. We took advantage of having well-characterized CD8 T-cell clones derived from patients with type 1 diabetes with known specificity for PPI epitopes that are frequently targeted in the disease process (7,8,17) and therefore likely to have pathogenic relevance. Our major finding is that autoreactive CD8 T cells exert their killing effect on human islet cells via release of granule contents. This provides strong evidence that following TCR-mediated recognition of autoantigenic peptide–HLA targets on the β-cell surface, a cytolytic process ensues that involves CD8 T-cell degranulation and perforin release to facilitate entry of granzymes, which then initiate proapoptotic cascades.
Although we have discovered that the main CD8 T-cell–mediated pathway of β-cell death operates via cytotoxic granules, we also demonstrate the potential for deployment of additional pathways. Whereas CMV-specific CD8 T-cell killing could be almost entirely inhibited by blocking degranulation, we were unable to fully account for PPI-specific killing in the same way. This finding is reminiscent of observations in the perforin gene knockout NOD mouse (26), which, although demonstrating a dramatic reduction in diabetes development, retains a significant low level of disease, again implying that killing mechanisms that are not degranulation-dependent can be operative. We therefore examined other possible killing mechanisms. We provide evidence that PPI-specific autoreactive CD8 T cells have the potential to mediate target cell death via the FasL–Fas pathway, revealed using non–β-cell targets. However, blockade of FasL appeared to have no inhibitory effect on A2-PPI15–24 CTL killing, suggesting that, in vitro at least, Fas–FasL pathways are not operative. Similarly, our studies did not find a direct cytotoxic role for TNF-α or related receptor systems. This suggests that any beneficial clinical effect that may be obtained using TNF-α blockade with monoclonal antibodies or soluble receptors administered at type 1 diabetes diagnosis, as was reported recently (27), may not be achieved by prevention of TNF-mediated β-cell death pathways induced by CD8 T cells.
In part, our study was conceived with the aim of defining differential mechanisms of target cell killing between auto- and pathogen-reactive CD8 T cells, in the hope that these could be exploited therapeutically. Although the CD8 T-cell systems we studied are representative rather than comprehensive, there is a strong theme in our findings that the major mechanism used by both auto- and pathogen-reactive CD8 T cells is similar and requires cytotoxic degranulation. However, we made a novel observation in relation to the potency of the killing. Despite T cells harboring comparable levels of granzymes and perforin, the PPI-specific CD8 T cells displayed a reduced level of maximal killing and degranulation in comparison with the CMV-specific CD8 T-cell clone when stimulated with cognate peptide. Using heteroclitic peptides that we recently identified as being superagonists for PPI-specific clones (25), we were able to demonstrate that a full level of degranulation and killing capacity can be achieved when the autoreactive CD8 T-cell clones are maximally stimulated with these reagents. This indicates that an underlying difference in avidity between virus-specific and autoreactive CD8 T cells for their respective targets can result in reduced killing potency. This may well be a factor that contributes to the long preclinical prodrome in type 1 diabetes, which typically lasts for months or years and implies that β-cell destruction sufficient to precipitate clinical disease is not a rapid process.
Studies such as ours may open up future therapeutic avenues targeting granule components and their release. As a potential example, the alkalinizing reagent chloroquine inhibits lysosomal release and hence degranulation by CD8 T cells (28) and significantly reduces NOD mouse diabetes incidence (29). In conclusion, we show that killing by autoreactive CD8 T cells is mediated by a qualitatively similar process to that used by antiviral CD8 T cells. However, we observed that under conventional conditions, without manipulation of the target cell specificity, autoreactive CD8 T cells are less potent at killing targets than their antiviral counterparts. We provide evidence that this important difference is cell-extrinsic and possibly attributable to the low-affinity TCR interaction with self-pHLA that we have observed for one autoreactive PPI-specific CD8 T-cell specificity (19), but which needs to be confirmed as a model in future studies. Should this prove the case, it is in juxtaposition to CMV-specific CD8 T-cell clonotypes, which have been selected from the high-avidity clones that dominate the cytotoxic response (30). As a result, differences in the quality of antipathogen and antiself killing may present a therapeutic window within which blockade of autoreactive CD8 T-cell function can be effective in preventing autoimmune damage, without impacting negatively on antipathogen immune competence.
ACKNOWLEDGMENTS
R.R.K. is in receipt of a PhD studentship from Diabetes UK. D.K., M.P., and A.S. received support from the United Kingdom Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St. Thomas’ National Health Service Foundation Trust in partnership with King’s College London, which also funds D.K. via a PhD studentship. M.P. receives funding via the European Union’s Seventh Framework Programme EU Framework 7 Large-scale Focused Collaborative Research Project on Natural Immunomodulators as Novel Immunotherapies for Type 1 Diabetes. D.K.C. is a Wellcome Trust Research Career Development Fellow (WT095767).
No potential conflicts of interest relevant to this article were reported.
R.R.K., D.K., M.E., and A.S. designed and performed experiments and with M.P. analyzed data and wrote the manuscript. R.R.K., M.P., and A.S. conceived ideas and oversaw research. M.Z. and G.C.H. performed human islet experiments. A.B., L.W., D.K.C., and A.K.S. identified and characterized the superagonist peptides. M.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 12th International Congress of the Immunology of Diabetes Society, Victoria, Vancouver, Canada, 15–19 June 2012.
The authors thank Prof. Tom Kay and Dr. Pete Campbell (St. Vincent’s Institute, Melbourne, Australia) for constructive experimental advice and helpful critique of the manuscript. The humanized anti–TNF-α reagent was provided by Dr. Tim Bourne of UCB Celltech.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0315/-/DC1.
REFERENCES
- 1.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 [DOI] [PubMed] [Google Scholar]
- 2.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nejentsev S, Howson JM, Walker NM, et al. Wellcome Trust Case Control Consortium Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007;450:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wicker LS, Leiter EH, Todd JA, et al. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes 1994;43:500–504 [DOI] [PubMed] [Google Scholar]
- 5.Liblau RS, Wong FS, Mars LT, Santamaria P. Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity 2002;17:1–6 [DOI] [PubMed] [Google Scholar]
- 6.Coppieters K, Amirian N, von Herrath M. Intravital imaging of CTLs killing islet cells in diabetic mice. J Clin Invest 2012;122:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skowera A, Ellis RJ, Varela-Calviño R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest 2008;118:3390–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velthuis JH, Unger WW, Abreu JR, et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes 2010;59:1721–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 2003;3:361–370 [DOI] [PubMed] [Google Scholar]
- 11.Itoh N, Imagawa A, Hanafusa T, et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med 1997;186:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kägi D, Ledermann B, Bürki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol 1996;14:207–232 [DOI] [PubMed] [Google Scholar]
- 13.Shrestha B, Diamond MS. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol 2007;81:11749–11757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell PD, Estella E, Dudek NL, et al. Cytotoxic T-lymphocyte-mediated killing of human pancreatic islet cells in vitro. Hum Immunol 2008;69:543–551 [DOI] [PubMed] [Google Scholar]
- 15.Thomas HE, Trapani JA, Kay TW. The role of perforin and granzymes in diabetes. Cell Death Differ 2010;17:577–585 [DOI] [PubMed] [Google Scholar]
- 16.Roep BO, Peakman M. Diabetogenic T lymphocytes in human Type 1 diabetes. Curr Opin Immunol 2011;23:746–753 [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg D, Knight RR, Estorninho M, et al. Circulating Preproinsulin Signal Peptide-Specific CD8 T Cells Restricted by the Susceptibility Molecule HLA-A24 Are Expanded at Onset of Type 1 Diabetes and Kill β-Cells. Diabetes 2012;61:1752–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang GC, Zhao M, Jones P, et al. The development of new density gradient media for purifying human islets and islet-quality assessments. Transplantation 2004;77:143–145 [DOI] [PubMed] [Google Scholar]
- 19.Bulek AM, Cole DK, Skowera A, et al. Structural basis for the killing of human beta cells by CD8(+) T cells in type 1 diabetes. Nat Immunol 2012;13:283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomberg K, Hautala R, Lövgren J, Mukkala VM, Lindqvist C, Akerman K. Time-resolved fluorometric assay for natural killer activity using target cells labelled with a fluorescence enhancing ligand. J Immunol Methods 1996;193:199–206 [DOI] [PubMed] [Google Scholar]
- 21.Cole DK, Dunn SM, Sami M, Boulter JM, Jakobsen BK, Sewell AK. T cell receptor engagement of peptide-major histocompatibility complex class I does not modify CD8 binding. Mol Immunol 2008;45:2700–2709 [DOI] [PubMed] [Google Scholar]
- 22.Kuzushima K, Hayashi N, Kimura H, Tsurumi T. Efficient identification of HLA-A*2402-restricted cytomegalovirus-specific CD8(+) T-cell epitopes by a computer algorithm and an enzyme-linked immunospot assay. Blood 2001;98:1872–1881 [DOI] [PubMed] [Google Scholar]
- 23.Burrows SR, Elkington RA, Miles JJ, et al. Promiscuous CTL recognition of viral epitopes on multiple human leukocyte antigens: biological validation of the proposed HLA A24 supertype. J Immunol 2003;171:1407–1412 [DOI] [PubMed] [Google Scholar]
- 24.Kataoka T, Takaku K, Magae J, et al. Acidification is essential for maintaining the structure and function of lytic granules of CTL. Effect of concanamycin A, an inhibitor of vacuolar type H(+)-ATPase, on CTL-mediated cytotoxicity. J Immunol 1994;153:3938–3947 [PubMed] [Google Scholar]
- 25.Wooldridge L, Ekeruche-Makinde J, van den Berg HA, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem 2012;287:1168–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kägi D, Odermatt B, Seiler P, Zinkernagel RM, Mak TW, Hengartner H. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J Exp Med 1997;186:989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 2009;32:1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klempner MS, Styrt B. Alkalinizing the intralysosomal pH inhibits degranulation of human neutrophils. J Clin Invest 1983;72:1793–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Lee AS, Shameli A, et al. TLR9 blockade inhibits activation of diabetogenic CD8+ T cells and delays autoimmune diabetes. J Immunol 2010;184:5645–5653 [DOI] [PubMed] [Google Scholar]
- 30.Day EK, Carmichael AJ, ten Berge IJ, Waller EC, Sissons JG, Wills MR. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J Immunol 2007;179:3203–3213 [DOI] [PubMed] [Google Scholar]