Abstract
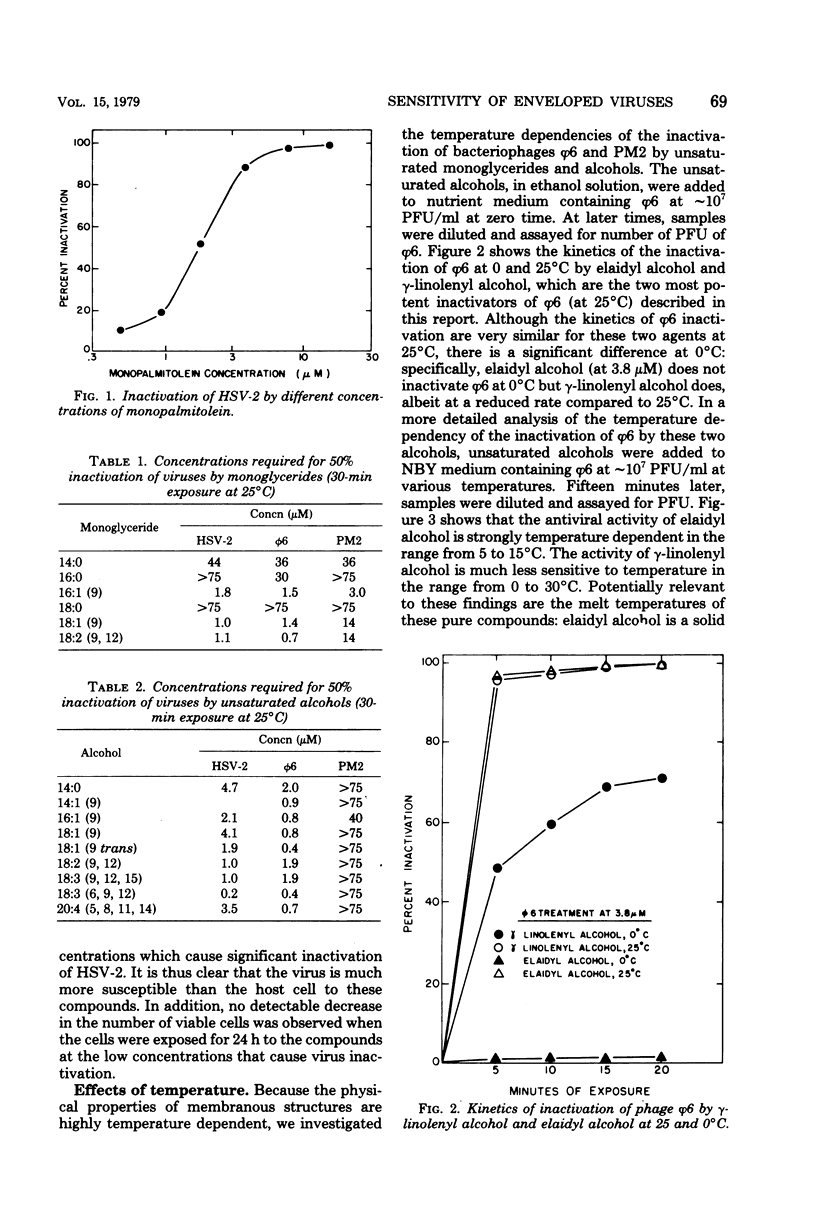
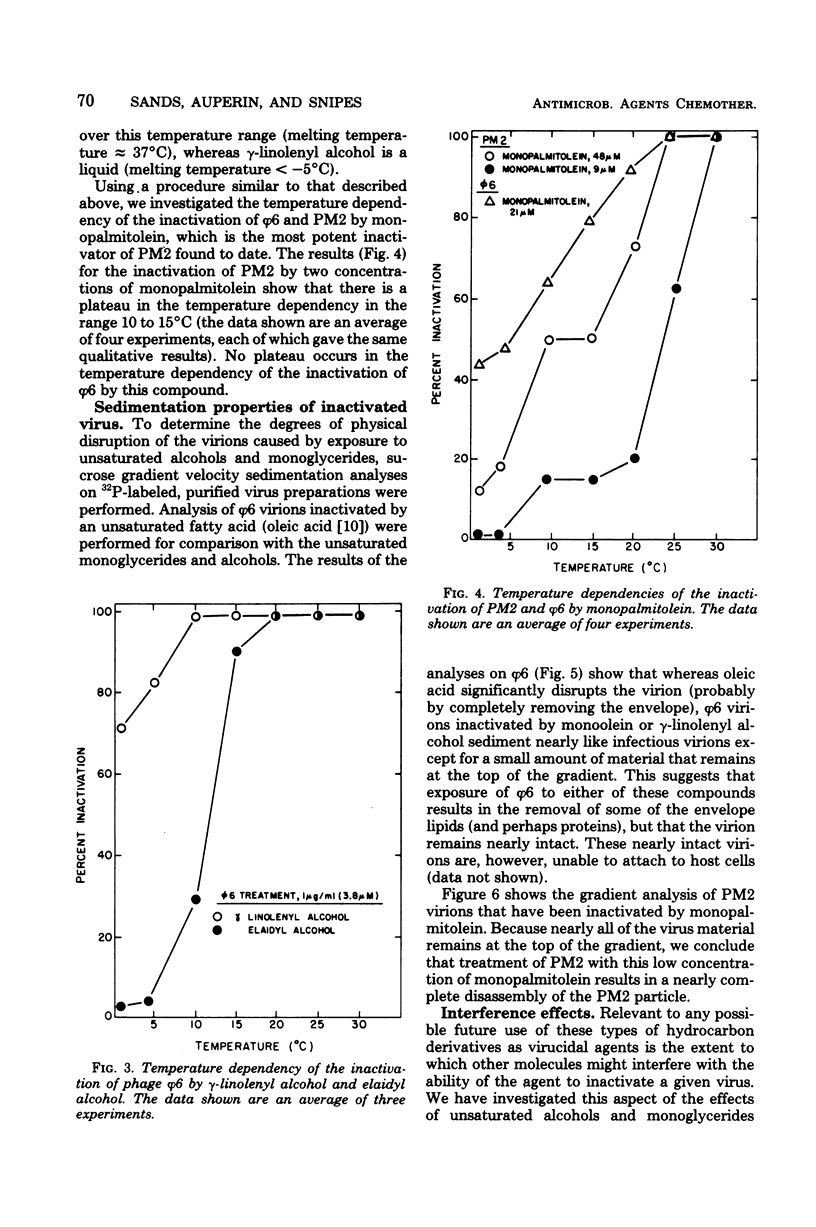
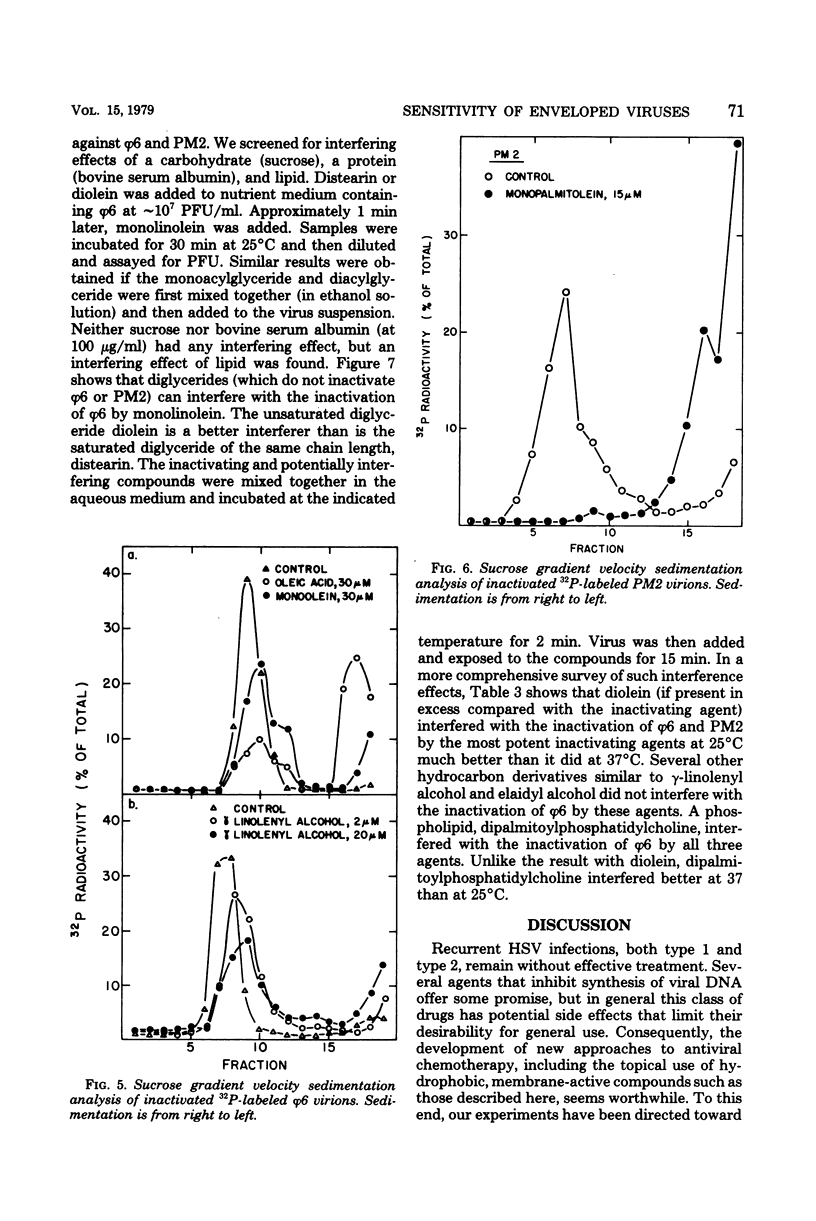
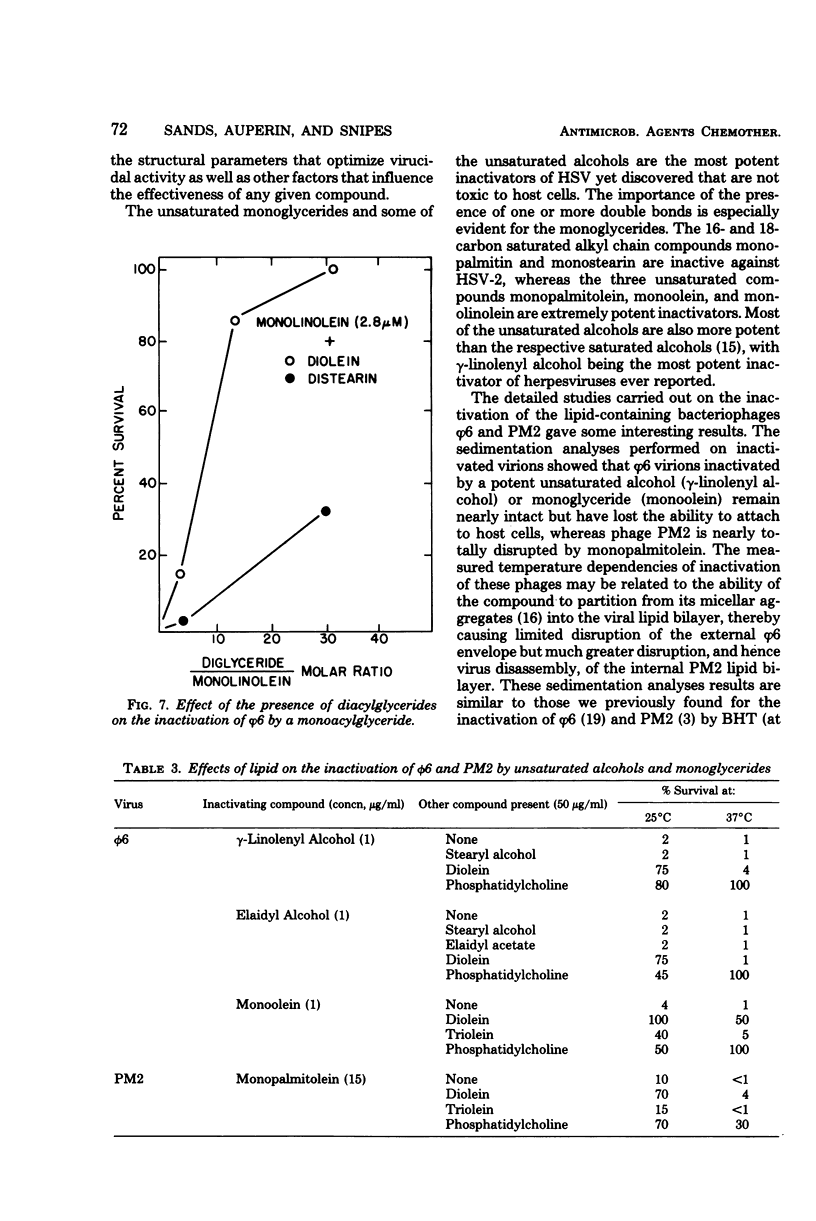
Unsaturated monoglycerides and alcohols of chain lengths of 16 or 18 carbons were found to be extremely potent inactivators of two enveloped viruses, herpes simplex virus type 2 and bacteriophage φ6. The lipid-containing bacteriophage PM2 was also inactivated by some of these amphiphilic molecules. Treatment of herpes simplex virus type 2 with these compounds at concentrations as low as 0.2 μM reduced virus survival to 50% in 30 min, making these agents the most potent inactivators of herpes simplex viruses discovered that are not cytotoxic to mammalian cells. Detailed characterizations of the effects of unsaturated monoglycerides and alcohols on bacteriophages φ6 and PM2 showed that the inactivated φ6 virion remained nearly intact but that PM2 was almost completely disrupted by the inactivating treatment. Some of the compounds inactivate the viruses even at low temperature (0°C). Excess amounts of diglycerides and phospholipids interfere with the inactivating abilities of some of the unsaturated monoglycerides and alcohols against φ6 and PM2. Our findings suggest that the unsaturated monoglycerides and some of the unsaturated alcohols should be further studied as potential antiviral agents, particularly for application to herpesvirus-infected areas of the skin and accessible epithelium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford D. H., Palva E. T., Lounatmaa K. Ultrastructure and life cycle of the lipid-containing bacteriophage phi 6. J Gen Virol. 1976 Aug;32(2):249–259. doi: 10.1099/0022-1317-32-2-249. [DOI] [PubMed] [Google Scholar]
- Brugh M., Jr Butylated hydroxytoluene protects chickens exposed to Newcastle disease virus. Science. 1977 Sep 23;197(4310):1291–1292. doi: 10.1126/science.897670. [DOI] [PubMed] [Google Scholar]
- Cupp J., Wanda P., Keith A., Snipes W. Inactivation of the lipid-containing bacteriophage PM2 by butylate hydroxytoluene. Antimicrob Agents Chemother. 1975 Dec;8(6):698–706. doi: 10.1128/aac.8.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Structure and synthesis of bacteriophage PM2, with particular emphasis on the viral lipid bilayer. Curr Top Microbiol Immunol. 1974;(68):107–159. doi: 10.1007/978-3-642-66044-3_5. [DOI] [PubMed] [Google Scholar]
- Jordan G. W., Seet E. C. Antiviral effects of amphotericin B methyl ester. Antimicrob Agents Chemother. 1978 Feb;13(2):199–204. doi: 10.1128/aac.13.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara J. J., Swieczkowski D. M., Conley A. J., Truant J. P. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972 Jul;2(1):23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt A., Cadden S., Sands J. A. Inhibitory effect of fatty acids on the entry of the lipid-containing bacteriophage PR4 into Escherichia coli. J Virol. 1978 Feb;25(2):479–485. doi: 10.1128/jvi.25.2.479-485.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A., Cupp J., Keith A., Snipes W. Temperature sensitivity of the assembly process of the enveloped bacteriophage phi6. Biochim Biophys Acta. 1974 Dec 10;373(2):277–285. doi: 10.1016/0005-2736(74)90151-5. [DOI] [PubMed] [Google Scholar]
- Sands J. A. Inactivation and inhibition of replication of the enveloped bacteriophage phi6 by fatty acids. Antimicrob Agents Chemother. 1977 Oct;12(4):523–528. doi: 10.1128/aac.12.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A. The phospholipid composition of bacteriophage phi6. Biochem Biophys Res Commun. 1973 Nov 1;55(1):111–116. doi: 10.1016/s0006-291x(73)80066-x. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Tzagoloff A., Levine D., Mindich L. Proteins of bacteriophage phi6. J Virol. 1975 Sep;16(3):685–695. doi: 10.1128/jvi.16.3.685-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes W., Cupp J., Sands J. A., Keith A., Davis A. Calcium requirement for assemby of the lipid-containing bacteriophage PM2. Biochim Biophys Acta. 1974 Mar 29;339(3):311–322. doi: 10.1016/0005-2736(74)90158-8. [DOI] [PubMed] [Google Scholar]
- Snipes W., Person S., Keith A., Cupp J. Butylated hydroxytoluene inactivated lipid-containing viruses. Science. 1975 Apr 4;188(4183):64–66. doi: 10.1126/science.163494. [DOI] [PubMed] [Google Scholar]
- Snipes W., Person S., Keller G., Taylor W., Keith A. Inactivation of lipid-containing viruses by long-chain alcohols. Antimicrob Agents Chemother. 1977 Jan;11(1):98–104. doi: 10.1128/aac.11.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanda P., Cupp J., Snipes W., Deith A., Rucinsky T., Polish L., Sands J. Inactivation of the enveloped bacteriophage phi6 by butylated hydroxytoluene and butylated hydroxyanisole. Antimicrob Agents Chemother. 1976 Jul;10(1):96–101. doi: 10.1128/aac.10.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]


