Abstract
Dipeptidyl peptidase-4 (DDP4) inhibitors target the enzymatic degradation of incretin peptides and represent a major advance in the treatment of type 2 diabetes. DPP4 has a number of nonenzymatic functions that involve its interaction with adenosine deaminase (ADA) and other extracellular matrix proteins. Here, we assessed the nonenzymatic role of DPP4 in regulating dendritic cell (DC)/macrophage–mediated adipose inflammation in obesity. Both obese humans and rodents demonstrated increased levels of DPP4 expression in DC/macrophage cell populations from visceral adipose tissue (VAT). The DPP4 expression increased during monocyte differentiation to DC/macrophages and with lipopolysaccharide (LPS)-induced activation of DC/macrophages. The DPP4 colocalized with membrane-bound ADA on human DCs and enhanced the ability of the latter to stimulate T-cell proliferation. The DPP4 interaction with ADA in human DC/macrophages was competitively inhibited by the addition of exogenous soluble DPP4. Knockdown of DPP4 in human DCs, but not pharmacologic inhibition of their enzymatic function, significantly attenuated the ability to activate T cells without influencing its capacity to secrete proinflammatory cytokines. The nonenzymatic function of DPP4 on DC may play a role in potentiation of inflammation in obesity by interacting with ADA. These findings suggest a novel role for the paracrine regulation of inflammation in adipose tissue by DPP4.
Also known as CD26, dipeptidyl peptidase-4 (DDP4) is widely recognized for its role in enzymatic degradation of incretin peptides, including glucagon-like peptide-1, glucagon-like peptide-2, and glucose-dependent insulinotropic peptide (1). Current pharmacologic approaches to inhibit DPP4 rely exclusively on inhibition of its catalytic function. In addition to preservation of glucose homeostasis by its enzymatic activity, DPP4 may have broad functional roles in other processes as evidenced by its widespread expression in other cell types (2). Despite the well-documented role of DPP4 in modulating incretin function, its nonenzymatic function in processes such as inflammation, especially in the context of diabetes and obesity, remains poorly characterized (3). Although early work on DPP4 focused on its role in T-cell function (4,5), the role of DPP4 in dendritic cells (DC) and macrophages has not received much attention (6–8). In this study, we investigated the functional role of DPP4 in visceral adipose tissue (VAT) after initially observing a significantly higher expression of DPP4 on DCs and macrophages in VAT compared with peripheral blood. Our study suggests that membrane-bound DPP4 on macrophages and DCs, via its interaction with adenosine deaminase (ADA), may be responsible for creating a microenvironment that facilitates T-cell proliferation. The elucidation of enzymatic versus nonenzymatic effects of DPP4 and their contribution to inflammation and metabolism may be of importance in obesity/type 2 diabetes.
RESEARCH DESIGN AND METHODS
Animal models, human tissues, and ethical approval.
Human visceral adipose tissue was harvested from the greater omentum during endoscopic repair of hernias from lean control subjects and during the performance of bariatric surgery in obese subjects. Human peripheral blood was collected from healthy volunteer donors. C57BL/6 and ob/ob mice were purchased from Jackson Laboratory (Jax Laboratories, Bar Harbor, ME). All procedures of this study were approved by the Committees on Use and Care of Animals and the Office of Responsible Research Practices, Human Institutional Review Board of the Ohio State University, under Ohio State University protocol 2008H0177. Human informed consent was obtained in writing and a copy was inserted in the medical records of the patients.
Peripheral blood mononuclear cell isolation and cell culture.
Peripheral blood mononuclear cells (PBMCs) were isolated from human peripheral blood by the density gradient separation. Briefly, K2 EDTA-anticoagulated blood was diluted 1:1 with sterile PBS and layered on Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). Samples were centrifuged for 30 min at 500g without applying a brake. The PBMC interface was carefully removed by pipetting and washed twice with PBS. Cells were then collected for indicated experiments.
For monocyte-derived DC (MDDC) induction, PBMCs were cultured in CO2 cell culture incubator for 2 h, and then adherent cells (monocytes) were cultured in the presence of 20 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (R&D, Minneapolis, MN) and 10 ng/mL interleukin (IL)-4 (R&D). Media were replaced every 2 days. Seven days later, suspension cells were collected for experiment.
Bone marrow-derived DCs were produced as previously described (9). In brief, mouse bone marrow cells were harvested by flushing the femur and tibia with PBS containing 5% FBS. The cells were cultured in DC growth media (RPMI-1640 suspended with 10% FBS, 20 ng/mL recombinant mouse granulocyte-macrophage colony-stimulating factor and 10 ng/mL recombinant mouse IL-4). Media were replaced every 2 days. Suspension of cells was collected for experiments after 7 days in culture.
Intraperitoneal glucose tolerance test.
The C57BL/6 mice were fed a high-fat diet (HFD) or normal chow at the age of 6 weeks. The intraperitoneal glucose tolerance test was performed after 12 weeks of diet, feeding as detailed previously (10). After fasting overnight (16–18 h) with free access to water, mice were weighed and baseline blood glucose was determined. Animals were then intraperitoneally injected with 2 g/kg d-glucose. Blood glucose was detected 30, 60, 90, and 120 min after the glucose injection by using Contour blood glucose meter (Bayer, Pittsburgh, PA).
siRNA transfection.
The small interfering RNA (siRNA) transfection was performed as described (11). Briefly, cells were seeded in a 12-well plate the day before transfection. On the second day, 100 nmol/L of DPP4-specific or control siRNA (Santa Cruz, CA) was transfected into the cells using the Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA) as instructed. Cells were used for experiment or fluorescence-activated cell sorter detection 24 h after transfection.
Human and mouse stromal vascular fraction isolation.
The stromal vascular fraction (SVF) was isolated from visceral adipose tissue by digesting it with collagenase type 2 from Clostridium histolyticum (Sigma, St. Louis, MO) (12). Human or mouse visceral adipose tissues were extensively rinsed in PBS, minced, and then digested with 1 mg/mL collagenase type II at 37°C and 140 rpm for 45 min. The digesta was then filtered through a 100-µm nylon cell strainer, followed by centrifugation at 300g for 5 min. The resulting pellet was lysed with 1× red blood cell lysing buffer (Sigma) to get rid of contaminating erythrocytes. After wash with PBS, the pellet (SVF) was resuspended with PBS and ready for use.
Flow cytometry.
All antibodies used in fluorescence-activated cell sorter were purchased from BioLegend (San Diego, CA) or BD (San Jose, CA); 1 × 106 cells were resuspended in 100 µL PBS containing 1% FBS and blocked with human Fc receptor blocking solution (BioLegend, San Diego, CA) or anti-mouse CD16/32 Fcγ III/II receptor (BD). Cells were then stained with indicated antibodies at 4°C for 30 min. After wash with PBS, cells were then analyzed on BD LSRII or FACSCalibur flow cytometer (BD).
Enzyme-linked immunosorbent assay.
The day after siRNA transfection, MDDCs were either unstimulated or stimulated with lipopolysaccharide for 24 h. Culture supernatant was then collected and subjected to the determination of tumor necrosis factor-α (TNF-α) and IL-6. The amount of TNF-α and IL-6 in the culture media was determined using the sandwich enzyme-linked immunosorbent assay kits (Biolegend, San Diego, CA) as instructed by the manufacturer.
T-cell proliferation assay.
PBMCs were cultured in a CO2 cell culture incubator for 2 h, and suspension cells (lymphocytes) were harvested for T-cell proliferation assay. T lymphocytes were then purified by using human T-cell enrichment columns (R&D, Minneapolis, MN) according to the manufacturer’s instruction. T cells were labeled with 5µM CFSE using CellTrace CFSE Cell Proliferation Kit (Invitrogen, Carlsbad, CA) as instructed. In the presence of 0.2 µg/mL anti-CD3 (Biolegend), CFSE-labeled T cells were then cultured with prepared MDDCs at the ratio of T:DC = 4:1. After 3 or 5 days of culture, cells were run on a flow cytometer, and proliferation indexes were calculated according to the equation outlined by Wallace (13).
Confocal microscopy and competitive binding assay.
PBMCs isolated from peripheral blood were incubated with 1µM bovine ADA (Roche, Branchburg, NJ) for 1 h. After an extensive wash with PBS, cells were stained with biotin-labeled anti-human ADA (Abcam, Cambridge, MA) and mouse anti-human DPP4 Ab, followed by staining with fluorescein isothiocyanate (FITC)-labeled avidin and Texas Red-labeled chicken anti-mouse secondary Ab. After washing with PBS, the cells were spread on microscopic slides and analyzed under Zeiss LSM 510 Confocal (Carl Zeiss). For the competitive binding assay, the cells were incubated with 50 ng/mL ADA (Roche) for 1 h in the presence or absence of 10 µg/mL soluble DPP4 (R&D, Minneapolis, MN). After a wash, cells were stained with PE-labeled DPP4 and biotin-labeled anti-ADA, followed by staining with FITC-labeled avidin, and then run on BD LSRII.
Statistical analysis.
All data are expressed as mean ± SEM unless otherwise specified. P < 0.05 was considered statistically significant. Graphpad Prism 4 was used for statistical analysis using Student t test or one-way ANOVA and Boneferroni post hoc test when appropriate.
RESULTS
Expression profile of DPP4 in DCs and macrophages.
DPP4 is ubiquitously expressed in many organs and has been previously demonstrated to be expressed on CD4+ T lymphocytes, where it plays a role in functional activation (2,14). To investigate its role in DCs/macrophages in VAT, we contrasted the expression of DPP4 in peripheral blood with adipose inflammatory cells derived from the stromal vascular fraction of VAT. Similar to previous reports (15), CD4+ T cells in peripheral blood highly express DPP4 (52.6 ± 5%; Supplementary Fig. 1A). This was similar to the expression in VAT, in which 56.1 ± 9.2% of CD4+ T cells express DPP4 (Fig. 1A). Although a similar percentage of DPP4+ CD4+ T cells was detected, the fluorescence intensity was higher in the CD4+ T cell of adipose tissue when compared with that of peripheral blood (Supplementary Fig. 2). Compared with CD4+ T cells, the expression of DPP4 on CD8+ T cells and innate immune cells (including CD14+ monocytes, CD11c+ DCs, and CD11b+ macrophages) was lower (Supplementary Fig. 1B). Approximately 5.3% and 4.1% of DCs and macrophages, respectively, in peripheral blood expressed DPP4. DPP4 expression was higher on DCs and macrophages of VAT compared with that of peripheral blood (16.9% and 19.4%, respectively; Fig. 1A). We also investigated expression of DPP4 as a function of differentiation in DCs. DPP4 expression on human peripheral blood--derived monocytes increased with functional differentiation to DC or macrophage in vitro (Fig. 1B). These results were identical when the differentiation was examined in mouse bone marrow-derived DCs (Supplementary Fig. 3).
FIG. 1.
DPP4 expression is increased in immune cells in VAT. A: DPP4 expression was detected on the CD4+ T cells, DCs, and macrophages in human peripheral blood, as well as in human VAT by flow cytometry. B: DPP4 expression on fresh-isolated monocytes, in vitro differentiated MDDC, and monocyte-derived macrophages by flow cytometry. Monocytes were isolated from human peripheral blood for the detection of DPP4 expression. MDDC was induced by culturing human monocytes with 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 10 ng/mL IL-4 for 7 days. Cells were then harvested for the detection of DPP4 expression. Monocyte-derived macrophages were induced by culturing human monocytes in the presence of GM-CSF for 7 days and then used for the detection of DPP4 expression. (A high-quality color representation of this figure is available in the online issue.)
DPP4 expression on DC and adipose tissue macrophages is elevated in obesity.
Adipose inflammation is a sine qua non for insulin resistance (16,17). To evaluate the relevance of DPP4 expression in adipose inflammatory cells from VAT and its relationship to insulin resistance, adipose tissue was collected prospectively from obese patients undergoing bariatric surgery and lean control subjects undergoing inguinal hernia repair. Table 1 depicts the metabolic profile of obese patients and lean control subjects recruited into the study. As depicted in Fig. 2A, obese patients had a higher percentage of DPP4+ macrophages in visceral adipose tissue. And the expression of DPP4 was positively correlated with fasting insulin (R2 = 0.456, P = 0.026; Supplementary Fig. 4) and homeostasis model assessment of insulin resistance (HOMA-IR) (R2 = 0.404, P = 0.016; Fig. 2B). We detected the expression of DPP4 in the adipose tissue of both HFD-induced (C57BL/6) and genetically obese (ob/ob) mice (12 weeks). After 12 weeks of HFD feeding, blood glucose was measured before and after an intraperitoneal glucose challenge in C57BL/6 mice. Mice fed an HFD were significantly glucose-intolerant compared with the mice fed a normal diet (Supplementary Fig. 5A), and percentage of classically activated macrophages (M1) was higher in the adipose tissue of mice fed an HFD (Supplementary Fig. 5B). Consistent with observations in humans, the expression of DPP4 on macrophages and DCs was higher in HFD-induced and ob/ob mice compared with controls (Fig. 2C and D and Supplementary Fig. 5C). We additionally confirmed cell surface detection of DPP4 in DCs derived from human visceral adipose tissue (Supplementary Fig. 6).
TABLE 1.
Metabolic profile of human subjects
FIG. 2.
Increased DPP4 expression on inflammatory DCs/macrophages in obese humans and ob/ob mice. A and B: Stromal vascular fraction was harvested from VAT by digesting it with collagenase II. DPP4 expression in SVF was detected by flow cytometry. Percentage of DPP4+ macrophages in VAT of obese (n = 6) and lean human subjects (n = 6) (A) and correlation between HOMA-IR and percentage of DPP4+ macrophages (B) are shown. *P < 0.05 when compared with lean group. C and D: SVF was isolated from epididymal fat of ob/ob or wild-type mice. A representative flow-contour plot showing increased expression of DPP4 detected on macrophages (C) and DCs (D) is depicted. HOMA-IR, homeostasis model assessment of insulin resistance; SVF, stromal vascular fraction. (A high-quality color representation of this figure is available in the online issue.)
Increased T-cell activation in adipose tissue of obese subjects.
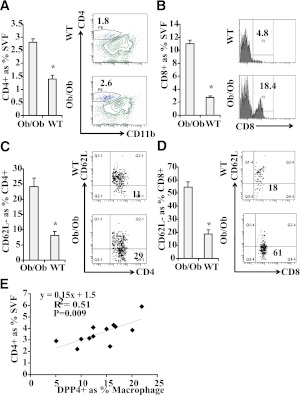
Because we detected relatively high levels of DPP4 on adipose DCs/macrophages in both obese mice and humans, we evaluated its functional effects on T-cell proliferation and activation by analyzing SVF from epididymal fat of ob/ob and control mice. An increase of both CD4+ and CD8+ T-cell populations was observed in the VAT of ob/ob mice compared with control mice (Fig. 3A and B). Furthermore, the percentage of CD4+ and CD8+ T-cells with CD62L− phenotype was higher in the VAT of ob/ob mice, suggesting there were more activated T cells (Fig. 3C and D), which is consistent with our previous finding in human VAT (12). The number of CD4+ T cells strongly correlated with DPP4 expression in macrophages (Fig. 3E). These results suggested a role for DPP4 in T-cell inflammation in obesity and raised the possibility that DPP4 may directly mediate adipose tissue inflammation in obesity/insulin resistance.
FIG. 3.
Increased T-cell proliferation and activation in ob/ob mice and obese human subjects. A: VAT (epididymal) was collected from ob/ob (n = 8) or wild-type (WT) mice (n = 6) and used for SVF isolation. Isolated SVF cells were then stained with indicated Ab. The percentage of CD4+ T cells in SVF was shown (left). A representative flow cytometric contour plot is depicted (right). *P < 0.05 when compared with WT group. B: The percentage of CD8+ T cells in SVF is shown (left). A representative flow cytometric plot is depicted (right). *P < 0.05 when compared with WT group. C: The percentage of activated CD4+ T cells (CD62L-CD4+) in CD4+ T cells is shown (left). A representative flow cytometric plot (right) is shown. *P < 0.05 when compared with WT group. D: The percentage of activated CD8+ T cells (CD62L-CD8+) in CD8+ T cells is shown (left). A representative flow cytometric plot is depicted (right). *P < 0.05 when compared with WT group. E: SVF isolated from human VAT was stained with CD4, CD11b, and DPP4 Abs, and then analyzed using flow cytometry. Correlation plot between CD4+ T-cell percentage and DPP4 expression is depicted. SVF, stromal vascular fraction. (A high-quality color representation of this figure is available in the online issue.)
DPP4 on DC and macrophages increases on activation.
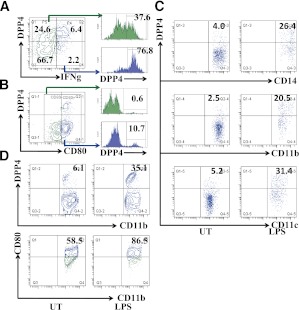
Previous studies have shown that DPP4 is highly expressed on activated T cells and serves as a marker for T-cell activation (18). However, the functional relevance of DPP4 expression on DC and macrophages and its relationship to activation status has not been investigated. We initially evaluated expression of DPP4 in T cells during activation. T cells isolated from human blood were stimulated with anti-CD3 and anti-CD28 antibody and detected the levels of DPP4. Activated T cells (interferon-gamma [IFN-γ]--positive) expressed significantly higher levels of DPP4 compared with IFN-γ−negative T cells (Fig. 4A). To investigate the potential involvement of DPP4 in the activation of macrophages, we tested the expression of DPP4 on mature and immature macrophages. Almost all DPP4+ macrophages expressed CD80, a maturation marker, indicating only mature macrophages express DPP4 (Fig. 4B). To further confirm this result, DPP4 expression levels were detected on untreated and lipopolysaccharide (LPS)-stimulated monocytes, DCs, and macrophages. As shown in Fig. 4C, DPP4 level increased 5- to 10-fold after 24h LPS stimulation. Peritoneal macrophages isolated from mice with LPS stimulation displayed a more mature phenotype and significantly higher expression of DPP4 compared with controls (6.4 ± 2.1% vs. 37.2 ± 5.7%; Fig. 3D).
FIG. 4.
DPP4 expression is increased on activation of inflammatory cells. A: T cells isolated from human peripheral blood were activated with anti-CD3 plus anti-CD28 mAb and then stained with IFN-γ and DPP4 Abs. IFN-γ--producing cells expressed higher levels of DPP4. Coexpression of DPP4 with IFN-γ (left) and DPP4 expression on IFN-γ--positive and IFN-γ--negative populations (right) are depicted. B: Peritoneal macrophages were collected from C57BL/6 mice and stained with CD11b, CD80, and DPP4 Abs. Activated macrophages (CD11b+ CD80+) expressed higher levels of DPP4. Coexpression of DPP4 with CD80 (left) on macrophages and the expression of DPP4 on CD80-positive and CD80-negative populations (right) are shown. C: Human peripheral blood mononuclear cells were stimulated with LPS (1μg/mL) for 24 h. DPP4 expression and activation were evaluated on CD14, CD11b, and CD11c populations. D: Peritoneal macrophages were isolated by injecting LPS (25 mg/kg body weight) intraperitoneally in C57BL/6 mice. Peritoneal macrophages were collected and DPP4 expression was evaluated on these cells. A higher maturation (bottom) and higher expression of DPP4 (top) was observed. (A high-quality color representation of this figure is available in the online issue.)
Binding of DPP4 to ADA.
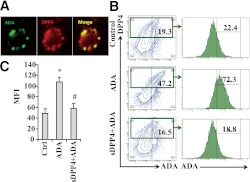
Previous studies have shown that ADA binds T-cell--expressing DPP4 and regulates T-cell activation (7,8). However, the involvement of ADA in DC-mediated immune response is not fully understood. To investigate the mechanism by which DC-expressing and macrophage-expressing DPP4 may modulate adipose inflammation, we first detected the binding of DPP4 to ADA. PBMCs were incubated with ADA for 1 h, followed by staining with ADA and DPP4 antibody and corresponding fluorescent secondary antibody. As shown in Fig. 5A, ADA colocalized with DPP4 on the cell membrane. Human VAT sections (Supplementary Fig. 7) demonstrated numerous cells expressing DPP4 that colocalized with ADA. DPP4 expression occurred in CD11c+ DCs, as shown in Supplementary Fig. 6. DPP4+, but not DPP4−, PBMCs showed a higher fluorescent intensity of ADA after incubation with ADA, suggesting DPP4 can directly bind to ADA. Preincubation with soluble DPP4 competitively inhibited the binding of DPP4 with ADA (Fig. 5B and C).
FIG. 5.
ADA binds to cell surface DPP4 in human DC/macrophages. Human PBMCs were cultured on six-well plates for 4 h and were incubated with ADA (50 ng/mL) for 30 min, following which the cells were washed and stained with anti-ADA and DPP4 Abs. A: Representative confocal micrograph showing expression of DPP4, ADA, and their colocalization. B: The binding of ADA to DPP4 was investigated in cultured PBMCs by competition assays in which in exogenous ADA was added, followed by the competitive inhibition of ADA binding by exogenously added soluble DPP4 and assessment of ADA content on cell surface. The flow cytometric contour plots depict inhibition of ADA binding to cells by exogenously added soluble DPP4 (sDPP4). C: Representative mean fluorescent intensity of ADA on DPP4+ cells are shown. *P < 0.05 when compared with control; #P < 0.05 when compared with ADA-treated cells. (A high-quality digital representation of this figure is available in the online issue.)
DPP4 on antigen-presenting cells promotes T-cell proliferation.
To investigate the underlying mechanisms by which DPP4 expression on DCs/macrophages may promote adipose inflammation, we investigated both innate and adaptive immune response. We first detected the secretion of proinflammatory cytokines, an important innate function of DC, after knockdown of DPP4 by siRNA. As shown in Fig. 6A, transfection efficiency of MDDCs was as high as 67%. As detected by flow cytometry, DPP4 expression on MDDC was successfully knocked-down by siRNA (decreased to one-third; Fig. 6B). Surprisingly, the secretion of proinflammatory cytokines (TNF-α and IL-6) was not affected by the downregulation of DPP4 (Fig. 6C and D).
FIG. 6.
DPP4 on human DCs promotes T-cell proliferation. A: Human MDDCs were transfected with unlabeled siRNA (control) or FITC-labeled siRNA (siRNA) and subjected to flow cytometric analysis after 24 h. MDDCs were gated based on the expression of CD11c and HLA-DR (left). Transfection efficiency (right) showed >67% of MDDCs were successfully transfected with siRNA. B: Human MDDCs were transfected with DPP4 siRNA (siDPP4) or control siRNA (siCtrl) and subjected to flow cytometric analysis after 24 h. Expression of DPP4 was reduced from 31.1% to 13.2% after transfection with DPP4-specific siRNA. C and D: At 24 h after siRNA transfection, MDDCs were stimulated with 1μg/mL LPS. Supernatant was collected for ELISA detection of TNF-α (C) and IL-6 (D). E: T cells isolated from human peripheral blood were labeled with CFSE. High concentration (Hi; 5 × 106/mL) or low concentration (Lo; 5 × 105/mL) CFSE-labeled T cells were stimulated with untreated or ADA-pretreated MDDCs (10 ng/mL of ADA). Proliferation of T cells was detected by analyzing CFSE intensity on flow cytometer after 5 days of coculture. *P < 0.05 when compared with untreated group. F: CFSE-labeled T cells were stimulated by MDDCs pretreated with indicated concentrations of ADA in the presence of adenosine (0–2 mmol/L). Proliferation of T cells was detected by analyzing CFSE intensity on flow cytometer after 3 days of coculture. G: In the presence of 1 mmol/L adenosine (Ado), CFSE-labeled T cells were cocultured with MDDCs that were pretreated with indicated concentrations of ADA. Unstimulated T cells were used as a negative control (− Ctrl), and T cells stimulated with anti-CD3 Ab in the absence of Ado were used as a positive control (+ Ctrl). Proliferation of T cells was detected using flow cytometry after 3 days. *P < 0.05 when compared with 0 nmol/L ADA group. H: MDDCs were transfected with DPP4-specific or control siRNA and pretreated with 10 ng/mL ADA. The cells were then cocultured with CFSE-labeled T cells (5 × 105/mL) with or without the presence of 1 mmol/L adenosine. Proliferation of T cells was detected using flow cytometry after 3 days. *P < 0.05 when compared with control siRNA-transfected group. (A high-quality color representation of this figure is available in the online issue.)
DPP4 on T cells has been shown to provide costimulatory signaling via its binding to extracellular ADA (2). However, the function of DPP4 on antigen-presenting cells (APCs) in the context of T-cell proliferation is not fully understood. In mixed lymphocyte reaction (MLR) assays using T-cell/DC cocultures (4:1 ratio), preincubation with ADA promoted T-cell proliferation at high cell concentrations but not low cell concentrations (Fig. 6E). In contrast, inhibition of DPP4 catalytic function with a specific inhibitor, sitagliptin, did not improve T-cell proliferation at high cell numbers. Consistent with this, addition of soluble DPP4 with enzymatic function showed a similar level of proliferation (Supplementary Fig. 8). Considering the fact that ADA can degrade adenosine, a cellular metabolic product able to suppress T-cell proliferation (19), our results suggest that the relative abundance of ADA--DPP4 complex or the concentrations of adenosine in the MLR may determine T-cell proliferation. MLR was then performed to detect T-cell proliferation stimulated by ADA-pulsed DCs with different concentrations of adenosine. As shown in Fig. 6F and G, adenosine dose-dependently suppressed T-cell proliferation, whereas preincubation of DCs with ADA relieved the inhibitory effect of adenosine in a dose-dependent manner. The siRNA knockdown of DPP4 on DC suppressed proliferation of both CD4+ and CD8+ T cells in a MLR culture system with the presence of adenosine (Fig. 6H and Supplementary Fig. 9). These results are consistent with the in vivo findings in obese subjects (Fig. 3). Consistent with this, T-cell receptor signaling andSTAT3, an important signaling molecule that regulates T-cell proliferation and activation (20,21), were regulated by both adenosine and DPP4-expressing DC (Supplementary Fig. 10). The activation of STAT3 increased on T-cell activation, which is suppressed by adenosine. Coculture with DC relieved the suppressive effect of adenosine, and knockdown of DPP4 by siRNA attenuated the effect of DC. Similar results were observed on TCR signaling. Lck is a Src family tyrosine kinase that mediates TCR signaling during T-cell activation. After TCR engagement, Lck is activated and phosphorylates ZAP-70 to mediate downstream signaling events. Phospho-Lck (Tyr505), the inactive form of Lck (22), increased in T cells cocultured with DPP4 siRNA-transfected DC (DC-siDPP4) compared with that of control siRNA-tranfected DC (DC-siCtrl), suggesting a lower activation of TCR signaling. These data support a role for DC-expressing DPP4 as an important regulator of T-cell proliferation, predominantly via the regulation of ADA function.
DISCUSSION
In this article we demonstrate a number of findings: 1) DPP4 expression in VAT macrophages/DCs is significantly higher than that of corresponding peripheral cells; 2) VAT DCs/macrophages in human and experimental obesity (diet-induced and ob/ob) express higher levels of DPP4 compared with lean controls; 3) DPP4 expression in DCs/macrophages is directly related to the degree of insulin resistance and T-cell activation in adipose tissue; 4) DPP4 expression increases with functional maturation of DCs/macrophages; and 5) DC/macrophage-expressing DPP4 binds ADA and contributes to T-cell proliferation via modulation of adenosine concentrations.
DPP4 is widely known for its regulatory role in glycemia control through enzymatic degradation of incretin peptides (23,24). Its role in inflammation has been traditionally postulated as occurring via its enzymatic function, whereby DPP4, through its N-terminal dipeptidase activity, cleaves X-Pro or X-Ala dipeptides from numerous chemokines. This cleavage can result in both inactive and active fragments of the targeted chemokines, resulting in unique effects that may depend on local milieu and disease context. The nonenzymatic function (especially the immune regulatory aspects) of DPP4 on immune cells and their putative role in modulating inflammation in type 2 diabetes remain poorly characterized. Although a role of DPP4 in inflammation has been postulated by many previous investigations primarily based on its expression on T cells, considerably less attention has been given to its role in macrophages and DCs because of its relatively low expression on such cells in the blood and lymphoid tissue (6–8). In our study, we noticed much higher expression of DPP4 on APCs (macrophages and DCs) in adipose tissue compared with that of peripheral blood (Fig. 1A), suggesting that DPP4 might have regional functions in VAT depots. To further explore its role in DC function, we quantified DPP4 expression during differentiation and activation of APCs. During the in vitro differentiation of DCs and macrophages from monocyte precursors, the expression of DPP4 was significantly increased (Fig. 1B and Supplementary Fig. 3). Our subsequent findings demonstrated that DPP4 expression increased with functional maturation of DCs and macrophages (Fig. 4B–D), which further supports the hypothesis that DPP4 may regulate immune responses. Consistent with our findings, Ohnuma et al. (25) previously reported a link between DPP4 expression and maturation of APCs. DPP4 upregulates CD86 via IRAK-1 and nuclear factor-κB pathways (26). To investigate the role of DPP4 in DC-mediated innate immune response, we tested the proinflammatory cytokines secretion on DPP4-siRNA–transfected DCs. Surprisingly, no difference in TNF-α and IL-6 secretion was noted between control and DPP4-siRNA–transfected DCs (Fig. 6C and D). These results do not rule out the possibility that DPP4 may regulate the secretion of other proinflammatory cytokines (27,28). The maturation of APC might influence another major role of these cells, initiating the adaptive immune response. We therefore investigated T-cell proliferation in VAT of obese humans and mice. Higher percentages of CD4 and CD8 T-cells and higher levels of T-cell activation in SVF from VAT of ob/ob mice were observed (Fig. 3). Moreover, the cell number of CD4+ T cells in the SVF of human VAT was strongly correlated with the expression of DPP4 on adipose macrophages (Fig. 3E), suggesting a potential role for DPP4 in the activation of adipose T cells. Further studies in obese humans demonstrated a connection between adipose DPP4 expression and insulin resistance (Fig. 2B). To investigate whether the catalytic function of DPP4 is involved in this effect, we added either soluble DPP4 (has preserved catalytic function) or specific inhibitor for DPP4 enzymatic activity into a DC--lymphocyte coculture system. Neither soluble DPP4 nor DPP4 enzymatic inhibition significantly changed DC-mediated T-cell proliferation (Supplementary Fig. 8), suggesting that the catalytic function may not be involved in this process. The nonenzymatic function of DPP4 (interacting with ADA) is probably responsible for the enhanced T-cell proliferation. However, preincubation of ADA with DCs had no discernible effect on T-cell proliferation at lower cell concentrations (5 × 105/mL), suggesting the costimulation might not be the major contributor for the proinflammatory effect of APC-expressing DPP4. The failure of ADA pretreatment in our study to stimulate T-cell proliferation under low cell concentrations may be related to the relatively low expression of DPP4 on APCs. Furthermore, commercially available ADAs are exclusively ADA1, the major isoform of ADA, which is known to have weak costimulatory effects even after 7 days of coculture with T cells (29,30). In contrast, incubation of T cells with ADA-pulsed DC at high concentrations (5 × 106/mL), albeit at the same ratio of T/DC, had a discernible impact on promoting T-cell proliferation cells (Fig. 6E).
As a metabolite of ATP, adenosine is released from cells in a variety of inflammatory states, ischemia, and infection, and has marked suppressive effects on immune cells, including T and innate immune cells (19,31). ADA functions as a pivotal enzyme in modulating adenosine concentrations by converting adenosine into inosine, a nontoxic degradation product (32). In tissue stress, higher levels of adenosine signal through adenosine receptors to exert strong anti-inflammatory effects. Elevated adenosine potently downregulates the activation of lymphocytes during inflammation. The regulation of adenosine levels in VAT may be of importance in DC responses to pathogen-associated molecular patterns and their activation of T cells. In our experiments, a high cell concentration coculture system was designed to mimic the in vivo context of inflammation (high cellularity) and elevated levels of adenosine. Our findings confirmed that ADA dose-dependently relieved the inhibitory effect of adenosine on T-cell proliferation (Fig. 6F and G). Furthermore, knockdown of DPP4 by siRNA suppressed the stimulatory function of DC in the presence of adenosine (Fig. 6H), suggesting DPP4 on DCs is responsible for the binding of ADA and for creating a microenvironment suitable for T-cell proliferation. Considering the fact that adipose inflammation is an important contributor for type 2 diabetes (33,34), our results are strongly suggestive of an effect of DPP4 in modulating ADA activity, thereby regulating adenosine concentrations and adipose inflammation in obesity and type 2 diabetes.
Our findings highlight the complex regulatory role of DPP4 in obesity. We demonstrate in the current study that in addition to its well-known function in regulation of glycemic control through its enzymatic function, DPP4 expression in inflammatory cells such as macrophages and DCs may play an important role in regulating the adipose inflammatory environment in obesity through its nonenzymatic function. During the development of obesity-induced insulin resistance, the expression of DPP4 may increase in adipose mononuclear immune cells. This increased expression may facilitate rapid DPP4-mediated degradation of incretin peptides via its enzymatic function, thereby contributing to hyperglycemic effects. The nonenzymatic function of DPP4, however, may help augment DC/macrophage maturation, resulting in DPP4-mediated promotion of T-cell activation and proliferation via its interaction with ADA. T-cell proliferation occurs early in the course of the development of obesity and contributes to insulin resistance and metabolic complications (35–37). Both CD8+ T cells and various subsets of CD4+ T cells have been demonstrated to play a critical role in the regulation of body weight, adipocyte hypertrophy, insulin resistance, and glucose tolerance, implicating these cells as fundamental regulators of disease progression (35–37). DPP4 expression on DCs/macrophages may facilitate T-cell proliferation and may constitute a maladaptive feed-forward loop that may contribute to adipose inflammation.
Understanding nonenzymatic functions of DPP4 in the development of obesity-induced adipose inflammation/insulin resistance might be of importance in the treatment of type 2 diabetes and may provide additional targets for intervention. Because inflammation is a powerful regulator of metabolic dysfunction in type 2 diabetes, and because metabolic dysregulation plays an obvious role in facilitating inflammatory cascades, one may hypothesize that DPP4 plays a pernicious role in both processes. To what extent the indirect effects of enzymatic DPP4 inhibition on inflammation versus direct nonenzymatic regulation of innate and adaptive immunity will require further investigation.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant (R01 ES017290, R01 ES015146, and R21 DK088522; to S.R.). J.Z. was supported by the National Natural Science Foundation of China (81101553/H1604), and J.D. was supported by a National Research Service Award (F32DK083903).
No potential conflicts of interest relevant to this article were reported.
J.Z. and X.R. researched data and wrote the manuscript. J.D., Z.B., and A.R.S. helped with data analysis. V.N., J.H., D.M., and B.N. contributed to the human adipose sample collection. S.R. designed the experiments and edited the manuscript. S.R. is the guarantor of this work and, as such, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Rebecca Dettorre, CCRC, Clinical Research Coordinator, Center for Minimally Invasive Surgery, The Ohio State University Medical Center, Columbus, Ohio, for her invaluable help with the project.
REFERENCES
- 1.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009;30:600–607 [DOI] [PubMed] [Google Scholar]
- 2.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol 2008;29:295–301 [DOI] [PubMed] [Google Scholar]
- 3.Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy 2010;30:463–484 [DOI] [PubMed] [Google Scholar]
- 4.Dong RP, Kameoka J, Hegen M, et al. Characterization of adenosine deaminase binding to human CD26 on T cells and its biologic role in immune response. J Immunol 1996;156:1349–1355 [PubMed] [Google Scholar]
- 5.Gorrell MD, Wickson J, McCaughan GW. Expression of the rat CD26 antigen (dipeptidyl peptidase IV) on subpopulations of rat lymphocytes. Cell Immunol 1991;134:205–215 [DOI] [PubMed] [Google Scholar]
- 6.Herrera C, Morimoto C, Blanco J, et al. Comodulation of CXCR4 and CD26 in human lymphocytes. J Biol Chem 2001;276:19532–19539 [DOI] [PubMed] [Google Scholar]
- 7.Martín M, Huguet J, Centelles JJ, Franco R. Expression of ecto-adenosine deaminase and CD26 in human T cells triggered by the TCR-CD3 complex. Possible role of adenosine deaminase as costimulatory molecule. J Immunol 1995;155:4630–4643 [PubMed] [Google Scholar]
- 8.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 1993;261:466–469 [DOI] [PubMed] [Google Scholar]
- 9.Zhong J, Yang P, Muta K, et al. Loss of Jak2 selectively suppresses DC-mediated innate immune response and protects mice from lethal dose of LPS-induced septic shock. PLoS ONE 2010;5:e9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah Z, Kampfrath T, Deiuliis JA, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011;124:2338–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao X, Zhong J, Zhang S, et al. Loss of methyl-CpG-binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind-limb ischemic injury. Circulation 2011;123:2964–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deiuliis J, Shah Z, Shah N, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS ONE 2011;6:e16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace PK, Muirhead KA. Cell tracking 2007: a proliferation of probes and applications. Immunol Invest 2007;36:527–561 [DOI] [PubMed] [Google Scholar]
- 14.Su AI, Wiltshire T, Batalov S, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 2004;101:6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto C, Torimoto Y, Levinson G, et al. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol 1989;143:3430–3439 [PubMed] [Google Scholar]
- 16.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–2180 [DOI] [PubMed] [Google Scholar]
- 17.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preller V, Gerber A, Togni M, et al. CD26/DP IV in T cell activation and autoimmunity. Adv Exp Med Biol 2006;575:187–193 [DOI] [PubMed] [Google Scholar]
- 19.Dong RP, Tachibana K, Hegen M, et al. Determination of adenosine deaminase binding domain on CD26 and its immunoregulatory effect on T cell activation. J Immunol 1997;159:6070–6076 [PubMed] [Google Scholar]
- 20.Oh HM, Yu CR, Golestaneh N, et al. STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J Biol Chem 2011;286:30888–30897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston JA, Bacon CM, Finbloom DS, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci USA 1995;92:8705–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi H, Hendrickson WA. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature 1996;384:484–489 [DOI] [PubMed] [Google Scholar]
- 23.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 2007;30:1335–1343 [DOI] [PubMed] [Google Scholar]
- 24.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011;124(Suppl):S3–S18 [DOI] [PubMed] [Google Scholar]
- 25.Ohnuma K, Munakata Y, Ishii T, et al. Soluble CD26/dipeptidyl peptidase IV induces T cell proliferation through CD86 up-regulation on APCs. J Immunol 2001;167:6745–6755 [DOI] [PubMed] [Google Scholar]
- 26.Ohnuma K, Yamochi T, Uchiyama M, et al. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci USA 2004;101:14186–14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii T, Ohnuma K, Murakami A, et al. CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci USA 2001;98:12138–12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ta NN, Li Y, Schuyler CA, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits TLR4-mediated ERK activation and ERK-dependent MMP-1 expression by U937 histiocytes. Atherosclerosis 2010;213:429–435 [DOI] [PubMed] [Google Scholar]
- 29.Zavialov AV, Gracia E, Glaichenhaus N, Franco R, Zavialov AV, Lauvau G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol 2010;88:279–290 [DOI] [PubMed] [Google Scholar]
- 30.Gakis C. Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2: diagnostic and biological role. Eur Respir J 1996;9:632–633 [DOI] [PubMed] [Google Scholar]
- 31.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 2004;25:33–39 [DOI] [PubMed] [Google Scholar]
- 32.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 1998;161:95–109 [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 34.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 35.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1304–1310 [DOI] [PubMed] [Google Scholar]
- 37.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]