Abstract
Metabolic disorders are a major burden for public health systems globally. Regular exercise improves metabolic health. Pharmacological targeting of exercise mediators might facilitate physical activity or amplify the effects of exercise. The peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) largely mediates musculoskeletal adaptations to exercise, including lipid refueling, and thus constitutes such a putative target. Paradoxically, forced expression of PGC-1α in muscle promotes diet-induced insulin resistance in sedentary animals. We show that elevated PGC-1α in combination with exercise preferentially improves glucose homeostasis, increases Krebs cycle activity, and reduces the levels of acylcarnitines and sphingosine. Moreover, patterns of lipid partitioning are altered in favor of enhanced insulin sensitivity in response to combined PGC-1α and exercise. Our findings reveal how physical activity improves glucose homeostasis. Furthermore, our data suggest that the combination of elevated muscle PGC-1α and exercise constitutes a promising approach for the treatment of metabolic disorders.
Metabolic disorders are major threats to public health. Currently, almost two-thirds of adult Americans are overweight (1). It is noteworthy that excessive body weight fosters the development of comorbidities such as hypertension, dyslipidemia, cardiovascular disease, and diabetes (2,3).
Regular exercise improves metabolic parameters (4–6), promotes weight loss (6), and prevents adiposity relapse after successful weight loss (7). People suffering from metabolic disorders often are unable or unwilling to achieve the levels of physical activity that are required to elicit health benefits. The auxiliary use of substances that mimic the plastic adaptations to exercise, so-called exercise mimetics (8), constitutes a seemingly attractive therapeutic approach to ease and support physical activity or amplify the effects of exercise, at least when potential drawbacks and limitations are ignored (9,10). As a key regulator of muscle plasticity (11,12), the peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) constitutes a potential target for such drugs (13).
Elevated expression of PGC-1α in skeletal muscle increases endurance performance (14). PGC-1α acts as a transcriptional coactivator that promotes the expression of several transcription factors, some of which it subsequently coactivates (15). PGC-1α then induces mitochondrial biogenesis (16,17), promotes angiogenesis (18), and increases peak oxygen consumption and fatigue resistance (14,17,19). Moreover, PGC-1α drives slow fiber type–specific calcium handling (17) and switching from fast glycolytic fibers toward slow oxidative fibers (19). PGC-1α also increases metabolic flexibility (20). It is noteworthy that PGC-1α promotes lipid (21) and glucose refueling in skeletal muscle (22) and thereby ensures the provision of substrates during exercise.
Paradoxically, despite the strong promotion of an exercised muscle phenotype, elevated expression of PGC-1α in sedentary mice exacerbates diet-induced insulin resistance as indicated by impairments in glucose disposal rates and muscle glucose uptake under hyperinsulinemic/euglycemic clamp conditions (23). Elevated lipid refueling in muscle-specific PGC-1α transgenic (MPGC-1α TG) animals, in combination with a sedentary lifestyle, likely underlies the pronounced detrimental effects of a high-fat diet (21). We hypothesized that the metabolic impairments in sedentary MPGC-1α TG animals fed a high-fat diet can be improved with exercise. The aim of this study was to longitudinally explore the potential therapeutic use of elevation of PGC-1α in combination with exercise in the treatment of diet-induced metabolic syndrome.
RESEARCH DESIGN AND METHODS
Animals.
Male MPGC-1α TG mice (19) and control littermates were maintained in a fixed 12-h light/dark cycle and received a diet of pellet chow and free access to water. To induce insulin resistance, animals were administered a high-fat diet consisting of 60% fat for 2 weeks. This time span was sufficient to induce impairments in glucose and insulin tolerance, which were more pronounced in MPGC-1α TG animals compared with control littermates (data not shown). Thereafter, all animals continued to consume the high-fat diet for another 3.5 weeks. During these 3.5 weeks, half of the animals remained sedentary (eight wild-type and eight MPGC-1α TG mice), whereas the other half (eight wild-type and eight MPGC-1α TG mice) underwent treadmill training three times per week separated by an intermittent day of recovery. All animals were killed 24 h after the last bout of exercise. Studies were performed according to criteria outlined for the care and use of laboratory animals and with approval of the Swiss authorities.
Body composition.
Body composition was determined by EchoMRI qNMR (Echo Medical Systems).
Locomotor activity, endurance training, and muscle strength.
Locomotor activity was assessed by CLAMS (Columbus Instruments). Endurance training was performed on a motorized treadmill (Columbus Instruments). After acclimatization, maximal endurance capacity was determined. A detailed protocol was followed: 10 m/min for 5 min and then increase by 2 m/min every 5 min up to 26 m/min. The speed of 26 m/min was then kept until exhaustion. Mice subsequently trained 3 days per week for 3.5 weeks. This training period started at 75% of maximal endurance capacity, and exercise levels then were gradually increased to reach 115% by the end of the training period. Maximal force was tested in vivo using a grip strength meter (Chatillon) as previously described (17).
RNA extraction and real-time PCR.
Frozen tissues were homogenized under liquid nitrogen, and total RNA was isolated using TRIzol reagent (Invitrogen). Reverse transcription was carried out using random hexamer primers (Promega). Real-time PCR analysis (Power SYBR Green Master Mix; Applied Biosystems) was performed using the StepONE Detector. Relative expression levels for each gene of interest were calculated with the ΔΔCt method and normalized to the expression of the Tata box-binding protein.
Enzymatic activities.
Citrate synthase (CS) activity was assessed according to the protocol described by Srere (24). Fatty acid synthase (FAS) and glucose-6-phosphate dehydrogenase (G6PDH) activity were measured as previously described (21).
Glucose uptake.
Isolated extensor digitorum longum muscles were blotted on filter paper, weighed, and washed in Krebs-Ringer bicarbonate buffer (KRBB; 117 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 24.6 mmol/L NaHCO3, pH 7.4). 2-Deoxyglucose uptake was assessed in fresh 2 mL KRBB containing 1 mmol/L 2-deoxy-d-[1,2-3H]glucose (1.5 μCi/ml) and 7 mmol/L d-[14C]mannitol (0.45 μCi/ml) (Amersham Biosciences) at 30°C for 10 min (25). To terminate transport, muscles were dipped into ice-cold KRBB buffer containing 80 μmol/L cytochalasin B. Muscles were further processed by incubating in 300 μL 1 N NaOH at 80°C for 10 min, neutralized with 300 μL 1 N HCl, and particles were precipitated by centrifugation at 12,000g for 5 min (26). Radioactivity in the lysates was quantified using a Beckman liquid scintillation counter. All values were corrected for initial tissue weight and expressed as fold change compared with control animals.
Muscle reactive oxygen species.
To monitor intracellular generation of reactive oxygen species (ROS), 2′,7′-dichlorofluorescein diacetate (Sigma) was used as previously described (20).
Blood acylcarnitine and muscle lipid determination.
Acylcarnitine concentrations were measured as previously described (20). In brief, acylcarnitines were extracted from dried blood spots using eight isotopically labeled internal standards that contained methanol (Cambridge Isotopes Laboratories) and analyzed without prior sample derivatization. Precursor ions of m/z 85 in the mass range of m/z 150 to 450 were acquired on a PerkinElmer API 365 LC-ESI-MS/MS instrument.
Analyses of lipids were carried out, also using methods previously described (27,28). Lipid extracts were prepared using a modified Bligh/Dyer extraction procedure, spiked with appropriate internal standards, and analyzed using an Agilent 1200 high-performance liquid chromatography (HPLC) system coupled with an Applied Biosystem triple quadrupole/ion trap mass spectrometer (3200Qtrap). In brief, separation of individual lipid classes of polar lipids by normal phase HPLC was carried out using a Phenomenex Luna 3u silica column (inner diameter [i.d.] 150 × 2.0 mm) with the following conditions: mobile phase A (chloroform-to-methanol-to-ammonium hydroxide ratio of 89.5:10:0.5), phase B (chloroform-to-methanol-to-ammonium hydroxide-to-water ratio of 55:39:0.5:5.5); and a flow rate of 300 µL/min with gradient elution as described previously (28). Individual lipid species were quantified by referencing spiked internal standards. Phosphatidylcholine-14:0/14:0, phosphatidylethanolamine-14:0/14:0, sphingosine-17:0, and C17-Cer were obtained from Avanti Polar Lipids. Triacylglycerol (TAG), diacylglycerol (DAG), and cholesteryl ester were analyzed using a modified version of reverse phase HPLC/electrospray ionization tandem/mass spectrometry described previously (27). In brief, separation of TAG and cholesteryl ester from polar lipids was carried out on a Phenomenex Kinetex C18 column (i.d. 4.6 × 100 mm) using an isocratic mobile phase chloroform-to-methanol-to-0.1 mol/L ammonium acetate (100:100:4) solution at a flow rate of 150 µL/min. TAGs were calculated as contents relative to the spiked d5-TAG 48:0 internal standard (CDN isotopes), whereas cholesterol esters were normalized to corresponding d6-C18 cholesterol ester (CDN isotopes). DAG species were quantified using 4ME 16:0 Diether DG (Avanti Polar Lipids) as an internal standard.
Glucose and insulin tolerance tests.
Animals fasted for 16 h and 4 h before intraperitoneal injection of 2 g/kg glucose and 0.8 units/kg insulin, respectively. Blood was obtained at intervals of 15 min from the tail vein, and glucose levels were determined using a standard glucometer.
Histology.
Muscles were embedded in optimal cutting temperature compound and frozen in cooled isopentane. Succinate dehydrogenase (SDH) stainings were performed on 8-μm cryosections by exposing the sections to succinate (27 mg/mL) and nitroblue tetrazolium (1 mg/mL). Cox staining was similarly performed with cytochrome c (0.1 mg/mL) and diaminobenzidine (0.5 mg/mL). Mitotracker staining was performed as previously described (20).
Cell culture experiments.
Mouse C2C12 myoblasts were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS in a subconfluent culture. C2C12 myoblasts were fused into myotubes by using Dulbecco’s modified Eagle’s medium with 2% horse serum. On the third day of differentiation, myotubes were infected with adenoviral vectors for green fluorescent protein or PGC-1α. Glucose uptake was determined 2 days after adenoviral infection in the presence of vehicle, 50 μmol/L cerulenin, or sphingosine and 100 nmol/L insulin. Both drugs were added 3 min before insulin stimulation. Glucose transport was assessed in 10 µmol/L 2-deoxy-glucose (0.5 µCi/mL 2-deoxy-d-[1,2-3H]glucose) in Hepes buffer (140 mmol/L NaCl, 20 mmol/L Hepes, pH 7.4, 5 mmol/L KCl, 2.5 mmol/L MgSO4, and 1 mmol/L CaCl2). After 10 min, glucose uptake was stopped by the addition of ice-cold PBS, and cells were washed thrice and immediately lysed by the addition of 0.05 mol/L NaOH. Radioactivity in the lysates was quantified using a Beckman liquid scintillation counter. All values were corrected for total protein and expressed as fold change compared with control animals.
Data analysis and statistics.
All data are presented as means ± SE. The data were analyzed by factorial ANOVA for the main effects of genotype (wild-type vs. MPGC-1α TG mice), treatment (sedentary vs. exercised), and group-treatment interactions. Student t tests were applied to assess the effects of treatment (sedentary vs. exercised) and genotype (wild-type vs. MPGC-1α TG mice) between two individual groups. Levels of significance are indicated with one symbol (P < 0.05), two symbols (P < 0.01), or three symbols (P < 0.001).
RESULTS
General parameters.
MPGC-1α TG animals (sedentary and exercised) were significantly heavier than control littermates and tended to display an elevated fat mass but an unaltered lean mass (Table 1). Tibialis anterior and epididymal fat pad mass was significantly increased in MPGC-1α TG animals.
TABLE 1.
General parameters, including body weight, body composition, individual tissue weights, and performance parameters in MPGC-1α TG mice and control littermates
The level of exercise in our study was insufficient to counteract many of the detrimental effects of the high-fat diet, and no general body weight loss, changes in body composition, or reductions in specific tissue weights occurred in response to exercise in MPGC-1α TG or control mice (Table 1). We can therefore exclude that any metabolic changes observed in our study are related to body weight or fat mass loss.
The effect of training is amplified by elevated PGC-1α.
Muscle function was assessed under different experimental settings. No changes in maximal force generation and spontaneous locomotor activity were observed across the four groups (Table 1).
MPGC-1α TG animals display an elevated exercise performance when on a standard diet (14). Interestingly, the initial 2 weeks of high-fat feeding completely abrogated this effect (wild-type and MPGC-1α TG mice ran 445 ± 33 and 449 ± 39 m, respectively). During the subsequent 3.5-week training period, MPGC-1α TG and wild-type animals exercised in parallel at exactly the same relative workload to ascertain maximal comparability between the exercising groups. At the end of the training period, the exercise benefit was more pronounced in MPGC-1α TG animals compared with control littermates and again resembled the improved endurance of PGC-1α TG mice on regular diet (14). In fact, the maximal endurance performance in response to training increased by 43 and 102% in wild-type and MPGC-1α TG animals, respectively (Table 1). The impact of exercise in wild-type controls resembled previously published data in the same mouse strain on the same diet (29).
Endurance exercise preferentially improves glucose homeostasis at elevated PGC-1α levels.
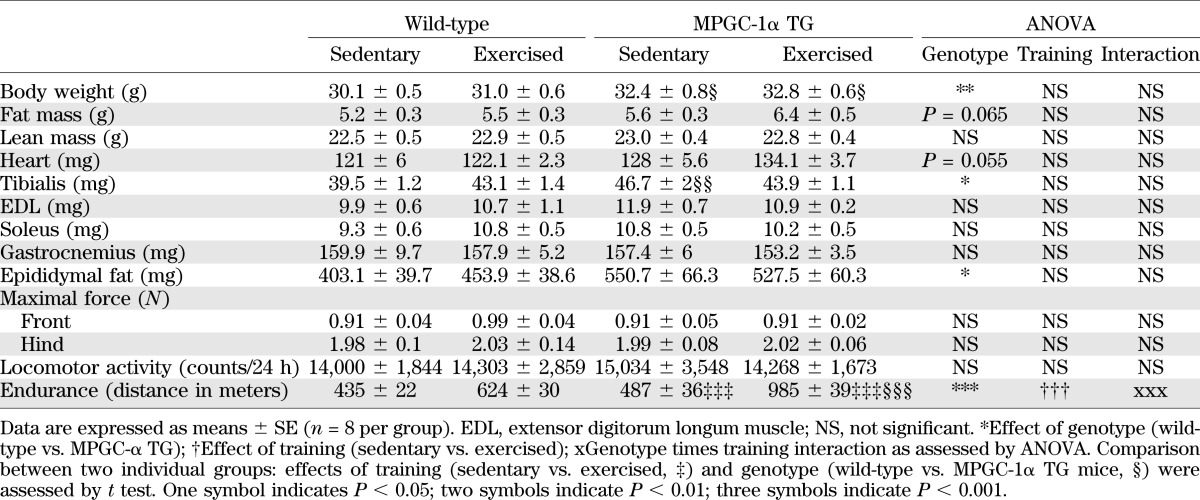
We have reported that overall glucose homeostasis is unaltered in MPGC-1α TG animals under standard laboratory conditions (20). Paradoxically, when challenged by a high-fat diet and under hyperinsulinemic/euglycemic clamp conditions, MPGC-1α TG animals display accelerated development of insulin resistance compared with control littermates (23). Exercise preferentially improved glucose (Fig. 1A and B) and insulin (Fig. 1C and D) tolerance in MPGC-1α TG mice by 28 and 17%, respectively. In comparison, glucose tolerance improved only by 6% (Fig. 1A and B) and insulin tolerance (Fig. 1C and D) was unaltered in response to exercise in wild-type animals. Thus, the beneficial effects of exercise on whole-body glucose homeostasis are amplified by elevated PGC-1α levels.
FIG. 1.
Whole-body and muscle glucose homeostasis. Glucose tolerance test (GTT) excursion curves (A) and corresponding area under the curve (B). Insulin tolerance test (ITT) excursion curves (C) and corresponding area under the curve (D). E: Relative gene expression of mediators of glucose uptake in skeletal muscle assessed by real-time (RT)-PCR. F: Glucose uptake into isolated skeletal muscle measured by the 2-deoxyglucose technique. G: IRS-1-associated muscle PI3K activity. All values are expressed as mean ± SE (n = 8 per group). @Effect of genotype (wild-type [wt] vs. MPGC-1α TG). #Effect of training (sedentary [sed] vs. exercised [ex]). xGenotype times training interaction as assessed by ANOVA. Comparison between two individual groups: *Effects of training (sed vs. ex) and §Genotype (wt vs. MPGC-1α TG mice) were assessed by t test. One symbol indicates P < 0.05, two symbols indicate P < 0.01, and three symbols indicate P < 0.001. HKII, hexokinase II.
Because skeletal muscle is a major contributor to overall glucose homeostasis, we assessed the expression of genes involved in muscle glucose uptake. We primarily focused on gene transcription because exercise alters the transcriptional profile of skeletal muscle and PGC-1α acts as a transcriptional coactivator. Glucose transporter 4 mRNA expression levels were reduced in tibialis anterior of sedentary, high fat diet-fed MPGC-1α TG animals and restored by exercise (Fig. 1E). Moreover, the mRNA expression of hexokinase II was highest in trained MPGC-1α TG animals (Fig. 1E). In line with these findings, muscle glucose uptake was decreased in sedentary animals but was preferentially improved by 93% in trained MPGC-1α TG animals (Fig. 1F). In contrast, exercise increased glucose uptake in wild-type animals by only 22% (Fig. 1F). Intriguingly, muscle phosphoinositide 3-kinase (PI3K) activity, an early marker of insulin sensitivity and a key element in insulin signaling, was higher in MPGC-1α TG animals but unaltered by exercise (Fig. 1G). This absence of an effect of exercise on proximal insulin signaling is further underlined by the fact that the insulin responsiveness within the first 30 min after insulin injection was similar between sedentary and exercised animals (Fig. 1C and D and Supplementary Fig. 1).
Elevated PGC-1α potentiates the effect of exercise on Krebs cycle activity.
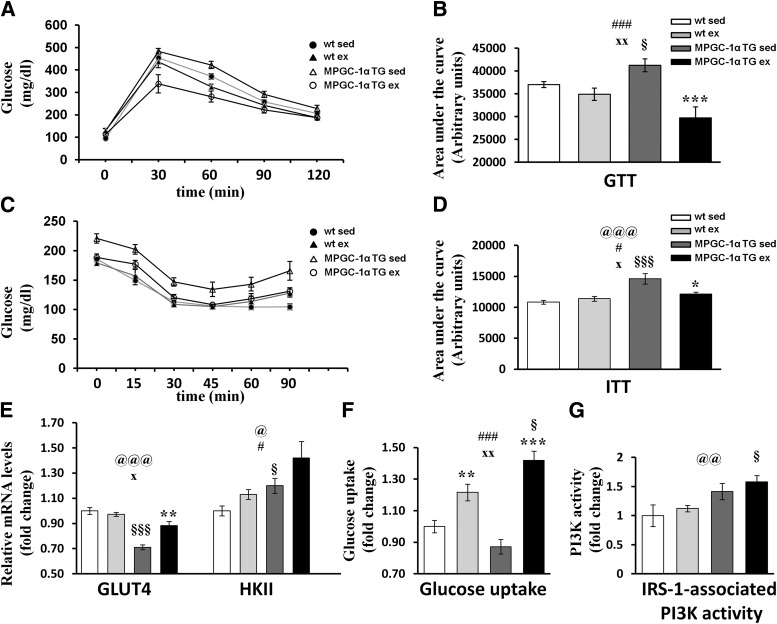
We next assessed the capacity for muscle lipid uptake. MPGC-1α TG mice showed elevated levels of lipoprotein lipase and cluster of differentiation 36, which are involved in lipid uptake (Fig. 2A and B).
FIG. 2.
Lipid uptake. Relative mRNA expression of lipoprotein lipase (LPL) (A) and cluster of differentiation 36 (CD36) (B) in MPGC-1α TG mice and control littermates. All values are expressed as mean ± SE (n = 8 per group). @Effect of genotype (wild-type [wt] vs. MPGC-1α TG). #Effect of training (sedentary [sed] vs. exercised [ex]). xGenotype times training interaction as assessed by ANOVA. Comparison between two individual groups: *Effects of training (sed vs. ex) and §Genotype (wt vs. MPGC-1α TG mice) were assessed by t test. One symbol indicates P < 0.05, two symbols indicate P < 0.01, and three symbols indicate P < 0.001.
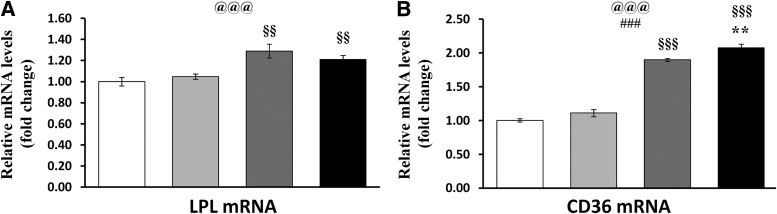
We then examined genes regulating lipid oxidation and Krebs cycle, namely carnitine palmitoyl transferase 1b (CPT-1b), malonyl CoA decarboxylase (MCD), acetyl CoA carboxylase 2 (ACC2), and CS. Although all of the genes were elevated in MPGC-1α TG animals, the majority of them were not significantly altered by exercise (Fig. 3A). In contrast, the enzymatic activity of CS, which is a marker of mitochondrial oxidation and central to both glucose and lipid oxidation, was further boosted by exercise at elevated PGC-1α levels (∼28% compared with sedentary MPGC-1α TG animals) (Fig. 3B). In control littermates, the exercise regimen had no effect on CS activity in accordance with previous studies that used high-fat feed (30,31).
FIG. 3.
β-Oxidation, TCA cycle, and acylcarnitines. A: Relative gene expression of regulators of lipid oxidation and the TCA cycle. B: Citrate synthase activity. C: Levels of saturated acylcarnitines (SFAs). D: Levels of monosaturated acylcarnitines (MUFAs). E: Levels of polysaturated acylcarnitines (PUFAs). F: Total carnitine levels. G: Acetylcarnitine levels. All values are expressed as mean ± SE (n = 8 per group). @Effect of genotype (wild-type [wt] vs. MPGC-1α TG). #Effect of training (sedentary [sed] vs. exercised [ex]). xGenotype times training interaction as assessed by ANOVA. Comparison between two individual groups: *Effects of training (sed vs. ex) and §Genotype (wt vs. MPGC-1α TG mice) were assessed by t test. One symbol indicates P < 0.05, two symbols indicate P < 0.01, and three symbols indicate P < 0.001.
Exercise restores acylcarnitine profiles in MPGC-1α TG animals to wild-type levels.
In sedentary MPGC-1α TG animals fed a high-fat diet, higher amounts of palmitoylcarnitine (C16:0) were released into circulation, whereas exercise diminished the levels of palmitoylcarnitine in MPGC-1α TG animals (Fig. 3C). No major changes were observed across the four groups for the patterns of monounsaturated (Fig. 3D) and polyunsaturated (Fig. 3E) acylcarnitines. Total carnitine and acetylcarnitine (C2) levels were elevated in sedentary MPGC-1α TG animals and returned to wild-type levels in response to exercise (Fig. 3F and G).
Unaltered oxidative phosphorylation activity in response to exercise.
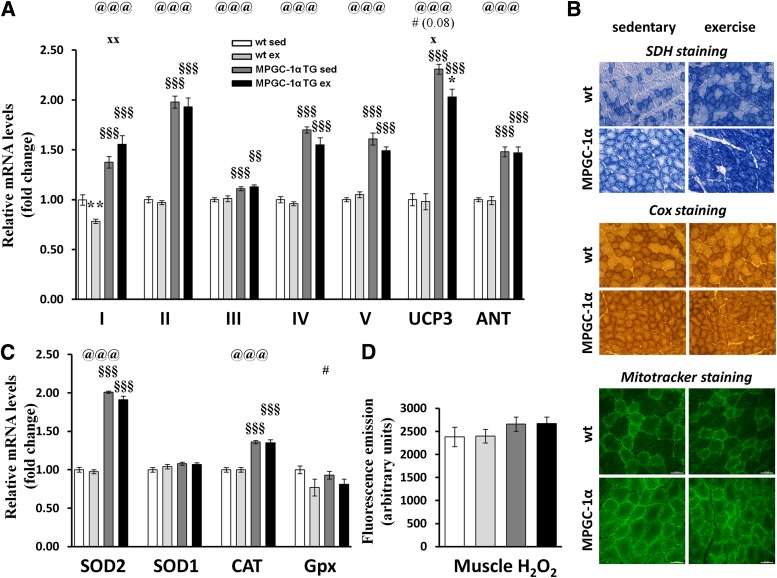
The increased levels of acetylcarnitine in sedentary MPGC-1α TG animals suggest that the amount of lipids taken up and fueled into β-oxidation exceeded the energetic demand of the Krebs cycle and oxidative phosphorylation (OXPHOS). Thus, we tested whether elements of the electron transport chain are altered in response to exercise while eating a high-fat diet. Although all of these genes were elevated in MPGC-1α TG animals, gene expression patterns were not affected by exercise (Fig. 4A). Exercise increased the activity of SDH, an enzyme that contributes to the Krebs cycle and OXPHOS and thereby links these processes (Fig. 4B and Supplementary Fig. 2). In MPGC-1α TG animals, exercise resulted in the highest activity of SDH. In contrast, the activity of cytochrome c oxidase, which constitutes an element of OXPHOS but not of the Krebs cycle, was elevated in MPGC-1α TG animals but no additional effect of exercise could be observed (Fig. 4B and Supplementary Fig. 2). These data suggest that OXPHOS activity is not rate limiting within our experimental context.
FIG. 4.
Oxidative phosphorylation and reactive oxygen species. A: Relative gene expression of elements of oxidative phosphorylation. B: Succinate dehydrogenase (SDH; upper panel), cytochrome c oxidase (Cox; middle panel), and Mitotracker green staining (lower panel). C: Relative gene expression of ROS detoxifying enzymes. D: H2O2 levels in skeletal muscle. All values are expressed as mean ± SE (n = 8 per group). @Effect of genotype (wild-type [wt] vs. MPGC-1α TG). #Effect of training (sedentary [sed] vs. exercised [ex]). xGenotype times training interaction as assessed by ANOVA. Comparison between two individual groups: *Effects of training (sed vs. ex) and §Genotype (wt vs. MPGC-1α TG mice) were assessed by t test. One symbol indicates P < 0.05, two symbols indicate P < 0.01, and three symbols indicate P < 0.001. CAT, catalase; Gpx, glutathione peroxidase; UCP3, uncoupling protein 3; ANT, adenine nucleotide translocator; SOD, superoxide dismutase.
We next determined the relative mRNA expression of genes implicated in the generation of ROS and detoxification because ROS can impair insulin sensitivity (32,33) (Fig. 4C). The mitochondrial superoxide dismutase 2, which transforms superoxides into H2O2, was elevated in MPGC-1α TG animals. Moreover, catalase, which detoxifies H2O2, was increased simultaneously. Therefore, H2O2 was not different overall (Fig. 4D).
Additive increases in de novo lipogenesis by elevated PGC-1α and exercise.
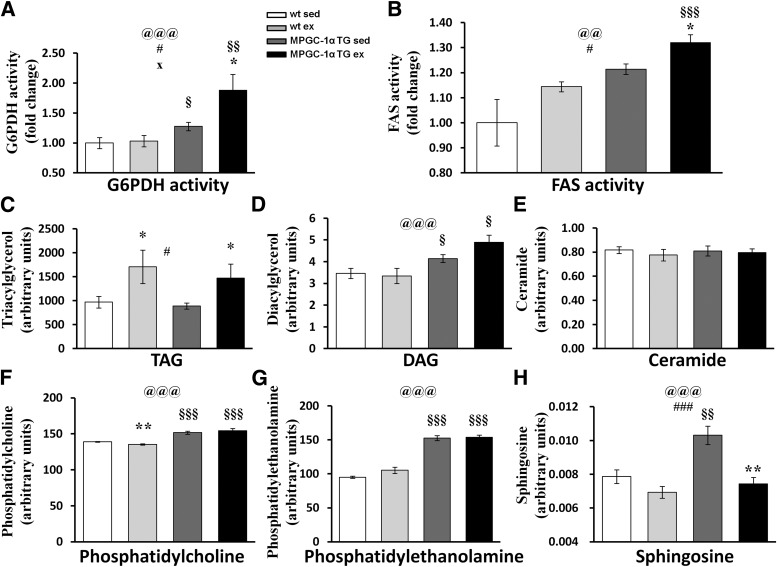
We previously have demonstrated that PGC-1α promotes de novo lipogenesis and pentose phosphate activity (rate limited by G6PDH) and that this partially drives glucose uptake into trained muscle (21). Interestingly, the activities of G6PDH and FAS remained higher in MPGC-1α TG animals on a high-fat diet and increased even further with exercise (Fig. 5A and B).
FIG. 5.
Muscle lipid species. G6PDH activity (A) and FAS activity (B). C: TAGs. D: DAGs. E: Ceramide. F: Phosphatidylcholine. G: Phosphatidylethanolamine. H: Sphingosine. All values are expressed as mean ± SE (n = 8 per group). @Effect of genotype (wild-type [wt] vs. MPGC-1α TG). #Effect of training (sedentary [sed] vs. exercised [ex]). xGenotype times training interaction as assessed by ANOVA. Comparison between two individual groups: *Effects of training (sed vs. ex) and §Genotype (wt vs. MPGC-1α TG mice) were assessed by t test. One symbol indicates P < 0.05, two symbols indicate P < 0.01, and three symbols indicate P < 0.001.
Given the elevated lipogenesis in MPGC-1α TG animals, we next investigated whether lipid partitioning in skeletal muscle is altered. TAG levels were comparable between sedentary wild-type and MPGC-1α TG animals on a high-fat diet and increased to a similar extent in both genotypes after chronic exercise (Fig. 5C).
We found elevated levels of DAGs (Fig. 5D) in the MPGC-1α TG animals, whereas the ceramide content was unaltered (Fig. 5E). Moreover, phosphatidylcholine (Fig. 5F) and phosphatidylethanolamine (Fig. 5G) levels were elevated in MPGC-1α TG animals. Exercise had no additional effect on the levels of these lipid species. In contrast, sphingosine levels were significantly increased in MPGC-1α TG animals and markedly reduced by exercise (Fig. 5H).
Inhibition of FAS or high sphingosine prevent glucose uptake in muscle cells in vitro.
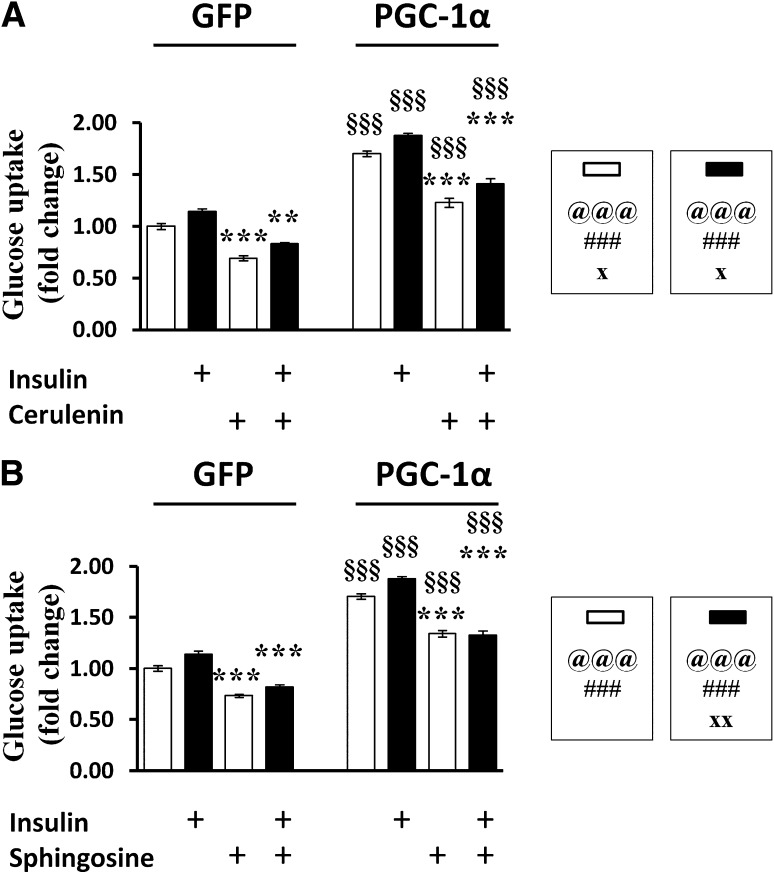
Given the elevated FAS activity in MPGC-1α TG animals that persists even during high-fat feeding, we investigated the general role of FAS in glucose uptake into isolated muscle cells free of interspersed adipose tissue. Pharmacological inhibition of FAS by cerulenin blunted glucose uptake in muscle cells, and this inhibitory effect was more pronounced after overexpression of PGC-1α (Fig. 6A).
FIG. 6.
Metabolic regulation of glucose uptake in skeletal muscle. A: Effect of the FAS inhibitor cerulenin on glucose uptake in the absence (white bars) or presence (black bars) of insulin. B: Effect of the sphingosine on glucose uptake in the absence (white bars) or presence (black bars) of insulin. All values are expressed as mean ± SE (n = 6 per group). @Effect of genotype (wild-type [wt] vs. MPGC-1α TG). #Effect of training (sedentary [sed] vs. exercised [ex]). xGenotype times training interaction as assessed by ANOVA. Comparison between two individual groups: *Effects of training (sed vs. ex) and §Genotype (wt vs. MPGC-1α TG mice) were assessed by t test. One symbol indicates P < 0.05, two symbols indicate P < 0.01, and three symbols indicate P < 0.001. GFP, green fluorescent protein.
The accumulation of sphingosine in the muscle of MPGC-1α TG animals prompted us to investigate the impact of sphingosine on glucose homeostasis. Sphingosine diminished glucose uptake in muscle cells and completely abrogated the stimulating effect of insulin at high levels of PGC-1α (Fig. 6B).
DISCUSSION
The role of skeletal muscle mitochondrial function and, in particular, that of its key regulator PGC-1α in the etiology of type 2 diabetes remains controversial (34,35). Studies in animal models for PGC-1α in skeletal muscle have not conclusively revealed how modulation of this coactivator affects peripheral insulin sensitivity (20,23,36). We now have demonstrated in a model of continuous high-fat feeding that elevated expression of PGC-1α preferentially improves whole-body and muscle glucose homeostasis when combined with exercise. In stark contrast, elevated expression of PGC-1α favors the development of diet-induced insulin resistance in the sedentary state. The beneficial metabolic effects of concerted exercise and PGC-1α expression comprise augmented Krebs cycle activity, reduction in acetylcarnitine levels, elevated glucose uptake for de novo lipogenesis, and altered lipid partitioning in favor of enhanced insulin sensitivity in skeletal muscle (Fig. 7).
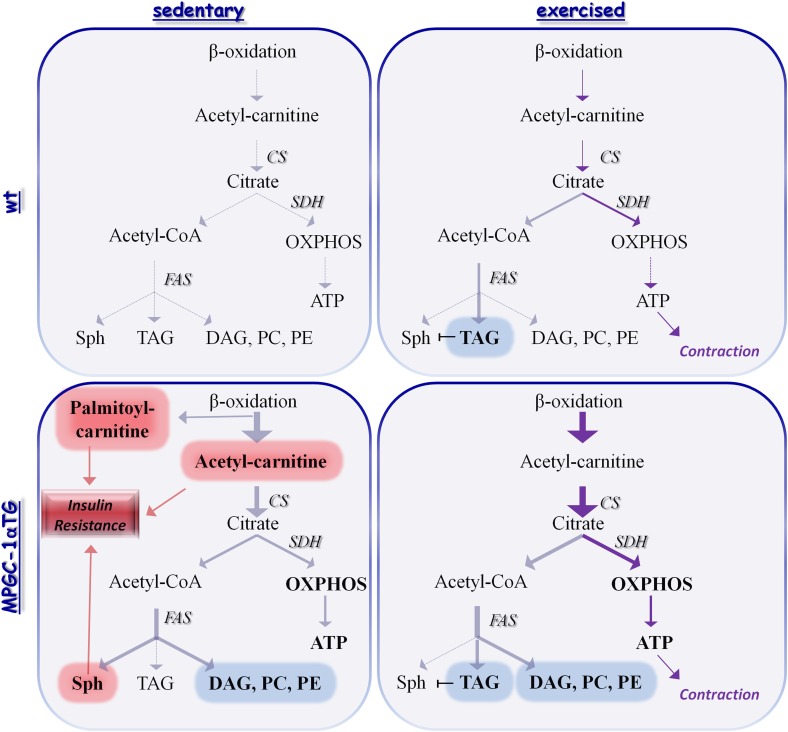
FIG. 7.
Integrative theoretical model. Schematic theoretical interpretation of the findings on metabolic profiles predominating in response to training and PGC-1α levels. Sedentary state: At elevated levels of PGC-1α, the increase in β-oxidation relatively exceeds the increase in citrate synthase activity. Consequently, acetylcarnitine accumulates and impairs insulin sensitivity. Citrate, produced by citrate synthase, is fueled into OXPHOS to generate ATP. In the absence of physical activity, the low ATP turnover ultimately limits OXPHOS activity and directs metabolic fluxes away from catabolism. The elevated citrate synthase activity, without an elevated need to produce ATP for muscle contraction, overloads the Krebs cycle. Citrate is consequently exported into the cytoplasm and promotes de novo lipogenesis through FAS. De novo synthesized fatty acids are subsequently fueled back into lipid oxidation or incorporated into higher lipid species. They then exert distinct effects on glucose homeostasis. DAG, PC, and PE are used to expand mitochondrial mass without impact on glucose homeostasis while sphingosine (Sph) inhibits glucose uptake. Exercised state: At elevated levels of PGC-1α, citrate synthase activity further increases and the levels of acetylcarnitine diminish. Citrate is shunted toward catabolic and anabolic pathways. Acutely, the high turnover of ATP during muscle contraction drives further flux of lipids into the catabolic system. Chronically, endurance training further promotes lipid synthesis and storage as triglycerides. The combination of exercise and elevated muscle PGC-1α consequently results in an elevated activity of lipid synthesis and a concomitant increase in TG, DAG, PC, and PE. Thus, lipid fluxes are diverted away from sphingosine toward triglyceride biosynthesis. Moreover, the elevated levels of TG inhibit the action of sphingosine and relieve any inhibitory effect. The high FAS activity will drive glucose uptake into skeletal muscle and thus removal of glucose from the circulation. Dashed arrows/lines, basal conditions; straight arrows/lines, altered at elevated PGC-1α levels or exercise; light arrows/lines, chronic elevation; dark arrows/lines, additional elevation during acute exercise; red boxes/lines, insulin-desensitizing metabolites; blue boxes/lines, neutral metabolites in respect to insulin sensitivity. PC, phosphatidylcholine; PE, phosphatidylethanolamine.
In sedentary MPGC-1α TG mice fed a high-fat diet, the high lipid provision exceeds the energetic demand of β-oxidation, as indicated by the release of palmitoylcarnitine (C16:0) into the circulation. The parallel increase in CS activity is insufficient to cope with the metabolites deriving from lipid oxidation, and, as a consequence, acetylcarnitine (C2) levels rise, which might impair glucose homeostasis (37,38) (Fig. 7), possibly by activation of insulin-desensitizing nuclear factor (NF) κB. NFκB activation is sufficient to impair glucose homeostasis by interference with insulin receptor substrate and protein kinase B phosphorylation, as well as glucose transporter 4 translocation (39). Acylcarnitine species potently activate NFκB to a degree that is even higher than saturated fatty acids (37), a classic stimulus of NFκB. Whether this effect of acylcarnitines is mechanistically brought about by direct interference with NFκB or activation of upstream signaling events, such as the stimulation of Toll-like receptors, remains unresolved.
In the sedentary state, Krebs cycle activity is less required for ATP regeneration, and citrate is exported into the cytoplasm for de novo lipogenesis. An increase in lipogenesis is indeed corroborated by the augmented phosphate pathway and FAS activity, which persist in sedentary transgenic animals in spite of abundant dietary fat. In MPGC-1α TG mice, the increased mitochondrial biogenesis is associated with elevated levels of phosphatidylcholine and phosphatidylethanolamine, which together account for more than 75% of total membrane phospholipids (40), and of DAG, which constitutes another membrane component. Moreover, sedentary MPGC-1α TG animals display markedly higher levels of sphingosine, which is implicated in PI3K-independent insulin resistance in adipocytes (41–43). We now have analogously demonstrated a direct effect of sphingosine on glucose uptake in skeletal muscle cells in vitro. Consistently, our in vivo data on skeletal muscle show markedly elevated sphingosine levels along with reduced muscle glucose uptake but higher PI3K activity in sedentary MPGC-1α TG animals.
The mechanisms that underlie the detrimental effect of sphingosine remain obscure. In nonmuscle cells, sphingosine exerts pleiotropic effects on insulin signaling. It inhibits protein kinase C isoforms required for proper insulin signaling (42) but activates a truncated form of protein kinase Cδ, which is implicated in the development of insulin resistance (44). Moreover, sphingosine interferes with the mitogen-activated protein kinase pathway and activates protein phosphatases, which target protein kinase B, a downstream target of PI3K (44). Thus, sphingosine seems to target multiple mediators of insulin signaling.
In stark contrast to sedentary mice fed a high-fat diet, regular endurance exercise decreased the levels of C16:0 and C2 in trained MPGC-1α TG animals. Accordingly, exercise increased β-oxidation and Krebs cycle activity in MPGC-1α TG animals, allowing complete oxidation.
Interestingly, an additional elevation in FAS activity occurs in the skeletal muscle of exercised MPGC-1α TG animals. Regular exercise and exercise-independent activation of PGC-1α both promote FAS transcription in skeletal muscle (21). Concomitantly, PGC-1α increases glucose uptake (21,22) but impedes glycolytic fluxes (22), thereby shunting glucose toward glycogen storage (22) and the pentose phosphate pathway for the generation of NADPH (21), a prerequisite for de novo lipogenesis. In adipocytes, metabolic flux through de novo lipogenesis determines insulin sensitivity and glucose uptake to a significant extent (45,46). Using pharmacological intervention, we now have shown that in isolated muscle cells, FAS activity similarly plays a role in regulating glucose uptake. The possibility thus arises that the elevated pentose phosphate pathways activity and de novo lipogenesis in exercised animals eating a high-fat diet might similarly be implicated in driving glucose uptake in skeletal muscle.
Trained TG animals subsequently display a different pattern of lipid partitioning. In addition to the elevated levels of membrane components (DAG, phosphatidylcholine, and phosphatidylethanolamine), triglycerides are increased in response to exercise. According to the “athlete’s paradox,” elevated lipogenesis and high levels of intramyocellular triglycerides and even diacylglycerides coexist with improved insulin sensitivity and thus do not seem to constitute predictors of muscle insulin sensitivity per se (47,48).
It is noteworthy that exercise reduced the levels of sphingosine in MPGC-1α TG mice and thus relieved its potential inhibitory effect on glucose uptake. The negative effect of sphingosine on glucose uptake is further counteracted in exercised MPGC-1α TG mice by elevated levels of triglycerides, which have been shown to inhibit sphingosine activity in a dose-dependent manner (49). Thus, exercise at elevated muscle levels of PGC-1α decreased palmitoylcarnitine and acetylcarnitine levels and diverted de novo synthesized lipids away from sphingosine biosynthesis toward triglyceride storage. Therefore, combined elevated expression of PGC-1α and exercise promote lipid partitioning in favor of enhanced insulin sensitivity (Fig. 7).
In our study, exercise exerts only moderate effects on glucose homeostasis in the context of continuous high-fat feeding similar to those of other studies in which daily high-fat feeding largely antagonized the beneficial effects of exercise (50–52). We specifically chose a mild exercise regimen to avoid potentially confounding effects of reduced adiposity. Nevertheless, during the development of insulin resistance (2 weeks of high-fat feeding in the sedentary state), the transgenic elevation of PGC-1α levels led to a more severe starting (pre-exercise) insulin resistance in MPGC-1α TG animals. This was unavoidable in our model but partially compromises the interpretation of our data. Nonetheless, the strong interactive effect of exercise and PGC-1α clearly demonstrates that exercise exerts its beneficial effects preferentially at elevated levels of PGC-1α in muscle and that the significant differences in exercise responses are unlikely related to differences in the absolute amount of muscle work because of the slightly higher body weight in MPGC-1α TG animals. Timely coordination of the expression of PGC-1α and dietary intervention would, therefore, presumably culminate in an exclusively positive outcome on metabolic health.
In conclusion, we demonstrate that exercise preferentially improves exercise capacity and metabolic parameters at high levels of muscle PGC-1α in the context of energy-dense nutrition. Moreover, we provide novel insights into metabolic alterations that affect glucose homeostasis in the insulin-resistant and trained muscle, respectively. Although our data suggest that elevation of PGC-1α, as a monotherapy, is detrimental in sedentary patients who consume a Western diet, targeting PGC-1α to further amplify the effects of exercise regimens might represent a novel avenue to improve skeletal muscle function and to achieve metabolic benefits. In light of the growing prevalence of metabolic disorders, which are favored by a sedentary lifestyle, the use of adjuvants to exercise to ease physical activity and enhance exercise effects in people with a low drive to move might gain profound medical and economical interest in the coming years.
ACKNOWLEDGMENTS
This project was funded by the Swiss National Science Foundation (SNF PP00A-110746), the Muscular Dystrophy Association USA (MDA), the SwissLife “Jubiläumsstiftung für Volksgesundheit und medizinische Forschung,” the Swiss Society for Research on Muscle Diseases (SSEM), the Swiss Diabetes Association, the Roche Research Foundation, the United Mitochondrial Disease Foundation (UMDF), the Association Française contre les Myopathies (AFM), and the University of Basel. M.R.W. has received grants from the Singapore National Research Foundation (CRP Award No. 2007-04) and the SystemsX.ch RTD project LipidX.
No potential conflicts of interest relevant to this article were reported.
S.S., G.Sh., D.M., and G.Sa. performed experiments and analyzed data. S.S. and C.H. wrote the manuscript. S.S., G.Sh., D.M., M.R.W., and C.H. contributed to discussion and reviewed and edited the manuscript. S.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0291/-/DC1.
See accompanying commentary, p. 34.
REFERENCES
- 1.Bogers RP, Barte JC, Schipper CM, et al. Relationship between costs of lifestyle interventions and weight loss in overweight adults. Obes Rev 2010;11:51–61 [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–643 [DOI] [PubMed] [Google Scholar]
- 3.Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E, Obesity Canada Clinical Practice Guidelines Expert Panel 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ 2007;176:S1–S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 5.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 2009;9:407–416 [DOI] [PubMed] [Google Scholar]
- 6.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011;378:129–139 [DOI] [PubMed] [Google Scholar]
- 7.Jakicic JM. The effect of physical activity on body weight. Obesity (Silver Spring) 2009;17(Suppl 3):S34–S38 [DOI] [PubMed] [Google Scholar]
- 8.Matsakas A, Narkar VA. Endurance exercise mimetics in skeletal muscle. Curr Sports Med Rep 2010;9:227–232 [DOI] [PubMed] [Google Scholar]
- 9.Summermatter S, Handschin C. PGC-1alpha and exercise in the control of body weight. Int J Obes (Lond). 31 January 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise’s complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol 2009;587:5527–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728–735 [DOI] [PubMed] [Google Scholar]
- 12.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008;454:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handschin C. The biology of PGC-1α and its therapeutic potential. Trends Pharmacol Sci 2009;30:322–329 [DOI] [PubMed] [Google Scholar]
- 14.Calvo JA, Daniels TG, Wang X, et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol 2008;104:1304–1312 [DOI] [PubMed] [Google Scholar]
- 15.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 2006;116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98:115–124 [DOI] [PubMed] [Google Scholar]
- 17.Summermatter S, Thurnheer R, Santos G, et al. Remodeling of calcium handling in skeletal muscle through PGC-1α: impact on force, fatigability, and fiber type. Am J Physiol Cell Physiol 2012;302:C88–C99 [DOI] [PubMed] [Google Scholar]
- 18.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008;451:1008–1012 [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002;418:797–801 [DOI] [PubMed] [Google Scholar]
- 20.Summermatter S, Troxler H, Santos G, Handschin C. Coordinated balancing of muscle oxidative metabolism through PGC-1α increases metabolic flexibility and preserves insulin sensitivity. Biochem Biophys Res Commun 2011;408:180–185 [DOI] [PubMed] [Google Scholar]
- 21.Summermatter S, Baum O, Santos G, Hoppeler H, Handschin C. Peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J Biol Chem 2010;285:32793–32800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wende AR, Schaeffer PJ, Parker GJ, et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem 2007;282:36642–36651 [DOI] [PubMed] [Google Scholar]
- 23.Choi CS, Befroy DE, Codella R, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 2008;105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srere PA. [1] Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol 1969;13:3–11 [Google Scholar]
- 25.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 2001;50:241–247 [DOI] [PubMed] [Google Scholar]
- 26.Brüning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 1998;2:559–569 [DOI] [PubMed] [Google Scholar]
- 27.Shui G, Guan XL, Low CP, et al. Toward one step analysis of cellular lipidomes using liquid chromatography coupled with mass spectrometry: application to Saccharomyces cerevisiae and Schizosaccharomyces pombe lipidomics. Mol Biosyst 2010;6:1008–1017 [DOI] [PubMed] [Google Scholar]
- 28.Chan RB, Oliveira TG, Cortes EP, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem 2012;287:2678–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol 2005;288:R1006–R1013 [DOI] [PubMed] [Google Scholar]
- 30.Cheng B, Karamizrak O, Noakes TD, Dennis SC, Lambert EV. Time course of the effects of a high-fat diet and voluntary exercise on muscle enzyme activity in Long-Evans rats. Physiol Behav 1997;61:701–705 [DOI] [PubMed] [Google Scholar]
- 31.Lee JS, Bruce CR, Spriet LL, Hawley JA. Interaction of diet and training on endurance performance in rats. Exp Physiol 2001;86:499–508 [DOI] [PubMed] [Google Scholar]
- 32.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948 [DOI] [PubMed] [Google Scholar]
- 33.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003;100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 36.Handschin C, Choi CS, Chin S, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 2007;117:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009;139:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Wu W, Li D, Guo Y, Ding H. Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine 2010;37:157–166 [DOI] [PubMed] [Google Scholar]
- 40.Takagi A. Lipid composition of sarcoplasmic reticulum of human skeletal muscle. Biochim Biophys Acta 1971;248:12–20 [PubMed] [Google Scholar]
- 41.Merrill AH, Jr, Stevens VL. Modulation of protein kinase C and diverse cell functions by sphingosine—a pharmacologically interesting compound linking sphingolipids and signal transduction. Biochim Biophys Acta 1989;1010:131–139 [DOI] [PubMed] [Google Scholar]
- 42.Smal J, De Meyts P. Sphingosine, an inhibitor of protein kinase C, suppresses the insulin-like effects of growth hormone in rat adipocytes. Proc Natl Acad Sci U S A 1989;86:4705–4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 1998;18:5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol 2008;9:162–176 [DOI] [PubMed] [Google Scholar]
- 45.Fried SK, Lavau M, Pi-Sunyer FX. Role of fatty acid synthesis in the control of insulin-stimulated glucose utilization by rat adipocytes. J Lipid Res 1981;22:753–762 [PubMed] [Google Scholar]
- 46.Foley JE, Laursen AL, Sonne O, Gliemann J. Insulin binding and hexose transport in rat adipocytes. Relation to cell size. Diabetologia 1980;19:234–241 [DOI] [PubMed] [Google Scholar]
- 47.Amati F, Dubé JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 2011;60:2588–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 49.Robertson DG, DiGirolamo M, Merrill AH, Jr, Lambeth JD. Insulin-stimulated hexose transport and glucose oxidation in rat adipocytes is inhibited by sphingosine at a step after insulin binding. J Biol Chem 1989;264:6773–6779 [PubMed] [Google Scholar]
- 50.Duggan GE, Hittel DS, Sensen CW, Weljie AM, Vogel HJ, Shearer J. Metabolomic response to exercise training in lean and diet-induced obese mice. J Appl Physiol 2011;110:1311–1318 [DOI] [PubMed] [Google Scholar]
- 51.Grimditch GK, Barnard RJ, Hendricks L, Weitzman D. Peripheral insulin sensitivity as modified by diet and exercise training. Am J Clin Nutr 1988;48:38–43 [DOI] [PubMed] [Google Scholar]
- 52.Richard D, Labrie A, Lupien D, Tremblay A, LeBlanc J. Role of exercise-training in the prevention of hyperinsulinemia caused by high energy diet. J Nutr 1982;112:1756–1762 [DOI] [PubMed] [Google Scholar]