Apoptosis is now recognized as a predominant mechanism by which β cells are destroyed in both type 1 and type 2 diabetes (1). Pro- and antiapoptotic members of the Bcl-2 family are central regulators involved in β-cell fate decision between life and death in response to physiological insults. The intricate interplay and balance between members of this family regulate apoptosis by controlling mitochondrial cell death signaling, the so-called intrinsic pathway (2). The Bcl-2 family is divided into three subgroups as follows: 1) the antiapoptotic Bcl-2–like proteins (Bcl-2, Bcl-xL, Bcl-W, Mcl-1, and A1/Bfl1) that possess four independent and highly conserved Bcl-2 homology (BH) domains (BH-1 to -4); 2) the proapoptotic Bax-like proteins (Bax, Bak, and Bok/Mtd), which also harbor the four BH domains; and 3) proapoptotic members typified by a single BH-3 domain (Bid, Bim/Bod, Bad, Bmf, Bik/Nbk, Blk, NoxA, Puma/Bbc3, and Hrk/DP5). Members of this last subgroup initiate the apoptotic-signaling cascade by interacting with antiapoptotic members (3). In addition, intracellular calcium activity ([Ca2+]i) is an important second messenger that initiates the apoptotic program (4) as well as being essential in metabolism secretion coupling that drives the Ca2+ sensor of the exocytotic machinery (5). Given the importance of Bcl-2 and Bcl-xL as prosurvival sentinels of the mitochondria, combined with the indispensable function of this organelle in β-cell metabolism secretion coupling, these proteins have been the targets of numerous studies in which expression levels were manipulated with the goal of improving islet viability. The study by Zhou et al. (6) in 2000 was the first to provide proof of concept that overexpression of Bcl-xL in β cells blunted stressed-induced apoptosis. Unexpectedly, one of the transgenic lines expressing high levels of Bcl-xL also displayed severe hyperglycemia. Glucose intolerance in these animals was linked to impaired nutrient-induced insulin secretion caused by alterations in mitochondrial ATP generation and intracellular Ca2+ handling (6). Substantiating this study, Pax4-induced Bcl-xL expression in rat islets also resulted in curtailed β-cell metabolism-secretion coupling through blunted mitochondrial Ca2+ concentrations and decreased ATP production (7). These early studies cautioned that Bcl-2 protein family members might not only be involved in regulating apoptosis but also be implicated in a day-to-day control of cellular function. Consistent with this premise was the elegant finding by Danial et al. (8) that Bad forms a complex with glucokinase in order to regulate glucose-driven mitochondrial metabolism and insulin secretion in islets. Nonetheless, other studies have tended to argue against the role of these proteins in glucose metabolism. Indeed, transgenic mice either overexpressing Bcl-2 or bearing a deletion in the BCLXL gene specifically in β cells were not reported to display any aberrant alterations in glucose metabolism (9,10). Thus, the relative contributions of antiapoptotic versus alternative functions of Bcl-2 and Bcl-xL for overall β-cell survival and performance remain controversial.
In this issue of Diabetes, Luciani et al. (11) address this controversy and convincingly demonstrate that Bcl-2 or Bcl-xL dampens glucose-induced insulin secretion and highlight the role of these prosurvival proteins as critical physiological integrators balancing life and death with metabolism secretion coupling in the β cell. In a first approach to authenticate this dual functionality, the authors used the small-molecule antagonist compound 6 (C6) and YC137 to pharmacologically hinder Bcl-2 and Bcl-xL. These antagonists bind to and displace proapoptotic members such as Bad from Bcl-2 and Bcl-xL, ultimately inducing apoptosis. In these experiments, C6 caused a rapid disruption of the Bcl-xL/Bad complex as well as a redistribution of Bax from the cytosol to mitochondria resulting in the release of cytochrome c, activation of caspase-3, and β-cell death. As antagonist-induced apoptosis was usually detected 2 h posttreatment, the authors argued that cellular events occurring within this time frame were likely independent of the central apoptotic events. In this context, the most impressive physiological event occurring subsequent to antagonistic treatment was the rapid triggering of [Ca2+]i in cells that mimicked the effect of glucose signaling. Yet, cells were cultured in the presence of low glucose, suggesting increased performance of mitochondrial metabolism leading to Ca2+ influx and potentially insulin secretion. Luciani et al. (11) methodically dissect the pathway leading to glucose-induced insulin secretion using various inhibitors and demonstrate that antagonizing Bcl-2/Bcl-xL in islets recapitulates cellular events associated with metabolism secretion coupling in β-cells: increased ATP production causing closure of the ATP-sensitive K+ channel with the subsequent depolarization of the plasma membrane and opening of the L-type Ca2+ channel resulting in submembranous increase in [Ca2+]i and ultimately insulin exocytosis. Low glucose levels as well as a sustained mitochondrial proton gradient were necessary to convey the effect of C6 and YC137. These results indicate that antagonist-mediated disruption of Bcl-2/Bcl-xL increases basal glucose-driven mitochondrial metabolism. A genetic loss-of-function approach was then used to substantiate the nonapoptotic role of Bcl-2/Bcl-xL in metabolism secretion coupling. Islets derived from transgenic animals bearing either a global knockout of BCL2 or a β-cell–specific deletion of BCLXL (BclxβKO) displayed significant increases in [Ca2+]i in response to low glucose. Nonetheless, only Bcl-2–ablated islets exhibited precocious insulin secretion in response to low glucose. However, glucose tolerance was moderately improved in BclxβKO mice. Using double transgenic animals in which both BAX and BAK were deleted, the authors ruled out the contribution of these two proapoptotic proteins in mediating the effect of Bcl-2 and Bcl-xL in mitochondrial metabolism (11).
Taken together, these data are noteworthy, as they provide the first convincing evidence that Bcl-2 and Bcl-xL take on dual functions in β cells: on the one hand, they are the gatekeepers of life and death, and on the other they are the “thermostat” of energy production in mitochondria. In fact, we would like to propose the term “energystat” to describe this new regulatory function of Bcl-2 and Bcl-xL. This is particularly relevant in a cell that lacks the Pasteur effect, a condition for being a nutrient sensor (12). In fact, these two roles are likely not mutually exclusive, as they converge on mitochondrial processes that will ultimately preserve β cells from deleterious stress. Indeed, as proposed by Luciani et al., restricting glucose metabolism may be a means by which the nonapoptotic function of Bcl-2 and Bcl-xL protects β cells against reactive oxygen species generated through oxidative phosphorylation while the antiapoptotic function preserves mitochondrial integrity under metabolic stress conditions such as hyperglycemia. Interestingly, Bax, another member of the Bcl-2 family, was recently linked to mitochondrial energy production. Indeed, BAX-deficient HCT-116 colorectal cancer cells were shown to have blunted ATP biosynthesis, a metabolic alteration associated with reduced citrate synthase activity. In contrast, overexpression of Bcl-2 in wild-type HCT-116 cells caused a drastic decrease in ATP production. The authors of this study concluded that Bcl-2 impedes Bax action on mitochondrial bioenergetics by potentially blunting its interaction with other mitochondrial proteins (13). However, it is now clear from the study of Luciani et al. (11) that Bcl-2 suppresses glucose-driven mitochondrial ATP biosynthesis independently of Bax or Bak. Thus, Bcl-2 and Bcl-xL join the armada of apoptotic factors along with Bad and Bax that possesses dual functionality. Despite these findings, one outstanding question remains to be clearly resolved from the study of Luciani et al.: what are the downstream mitochondrial targets mediating the nonapoptotic effects of Bcl-2 and Bcl-xL on β-cell bioenergetics? Experimental data would tend to suggest that the answer to this question lies within the tricarboxylic acid (TCA) cycle and/or the oxidative phosphorylation metabolic pathways. Indeed, 1) levels of acetyl-CoA as well as those of citrate, α-ketoglutarate, and succinate, three key metabolites of the TCA cycle, were reduced in Bcl-xL-overexpressing Jurkat cells (14); 2) pyruvate, the main substrate for the generation of mitochondrial acetyl-CoA that fuels the TCA cycle, was unable to rescue impaired insulin secretion in Bcl-xL–overexpressing islets (6); and 3) Bcl-xL was shown to interact with the ATP synthase β-subunit and to regulate mitochondrial energetics by stabilizing the inner membrane potential in neuronal cells (15). In addition, Bax was found to interact with citrate synthase, which is involved in the production of citrate from acetyl-CoA and oxaloacetate (16). Thus, it is tempting to speculate that Bcl-2 family members interact with and regulate the activity of key TCA cycle enzymes and proteins of the respiratory chain to energystat levels of ATP biosynthesis (Fig. 1). Two potential candidate targets could be the pyruvate dehydrogenase complex or the pyruvate carboxylase. These control the entry of pyruvate in the form of acetyl-CoA and oxaloacetate, respectively, into the TCA cycle. Alternatively, Bcl-2 or Bcl-xL could alter levels of acetyl-CoA by interacting with the citrate carrier, which is responsible for the efflux of this metabolite from the mitochondria to the cytosol in the form of citrate. Luciani et al. (11) also propose that the voltage-dependent anion channel and the mitochondrial adenine nucleotide translocator, which are the main venues by which ATP and ADP are exchanged between the mitochondrial matrix and the cytosol, could also be targets of Bcl-2 and Bcl-xL.
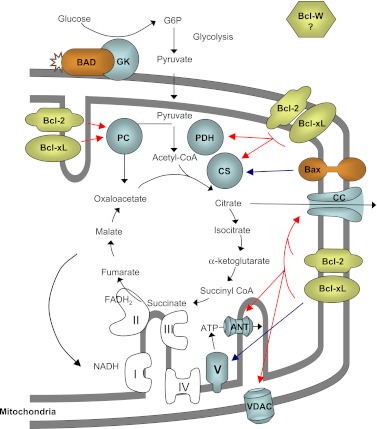
FIG. 1.
Nonapoptotic function of Bcl-2 family members as energystat of mitochondrial metabolism. Through multiple interactions with mitochondrial proteins (cyan), antiapoptotic (yellow) as well as proapoptotic (orange) Bcl-2 family members regulate glucose-driven mitochondrial ATP synthesis, thereby acting as energystat. Dark blue arrows depict interactions reported in the literature, while red arrows portray putative interactions that remain to be confirmed. Oxidative phosphorylation complexes are depicted as I, II, III, IV and V. ANT, adenine nucleotide transporter; CC, citrate carrier; CS, citrate synthase; GK, glucokinase; G6P, glucose 6-phosphate; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; VDAC, voltage-dependent anion channel.
While these various targets are plausible Bcl-2 and Bcl-xL regulatory checkpoints, several issues remain to be addressed from the study of Luciani et al. in order to firmly validate the bona fide implication of these survival factors in the management of mitochondrial metabolism. Indeed, the antagonists C6 and YC137 were shown to preferentially bind to and inhibit Bcl-2 while displaying low affinity for Bcl-xL. Consistent with the latter, islets derived from Bcl-2–ablated transgenic mice displayed a stronger precocious insulin secretion response to lower glucose concentrations compared with BclxβKO islets (11). Thus, although rapid triggering of [Ca2+]i was also observed in BclxβKO islets, the direct impact of Bcl-xL on insulin secretion needs to be further investigated. Furthermore, as YC137 also inhibits Bcl-W, which is strongly expressed in islets, the role of this prosurvival factor in regulating mitochondrial bioenergetics needs to be addressed (17,18). This is of particular interest, as human islets express low levels of Bcl-2 relative to other family members such as Bcl-W (19).
In summary, the study by Luciani et al. raises serious concerns about the feasibility of using prosurvival members of the Bcl-2 family as therapeutic targets for the treatment of diabetes. Indeed, human islets cultured in high glucose were found to have reduced levels of Bcl-xL (20). This most likely alleviates constraints on glucose-driven mitochondrial ATP production, thereby allowing increased insulin secretion to restore normal circulating glucose levels. Nonetheless, decreased Bcl-xL levels also tilt the balance between pro- and antiapoptotic factors favoring apoptosis. Paradoxically, cell death may be instrumental in this context to induce β-cell renewal in order to maintain a healthy and functional β-cell mass. Indeed, a recent study demonstrated that insulinoma INS-1 cells undergoing caspase-dependent apoptosis promoted the regenerative capacity of neighboring cells by shedding microparticles harboring the pancreatic stone protein/regenerating protein (21). Thus, Bcl-2 protein family members clearly act as double-edged swords in the context of diabetes. Extreme care should be taken to establish whether increased or decreased expression of these proteins would provide the most favorable outcome for the treatment of diabetes.
ACKNOWLEDGMENTS
B.S. and B.R.G. are supported by grants from the Consejeria de Salud; Fundacion Publica Andaluza Progreso y Salud; Junta de Andalucia (PI-0727-2010 to B.R.G.), Instituto de Salud Carlos III, cofunded by Fondo Europeo de Desarrollo Regional (PI10/00871 to B.R.G. and Red TERCEL, RD06/0010/0025, and PI10/00964 to B.S.); and Consejeria de Economia, Innovacion y Ciencia (P10.CTS.6505 to B.S. and P10.CTS.6359).
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 170.
REFERENCES
- 1.Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. Beta cell apoptosis in diabetes. Apoptosis 2009;14:1389–1404 [DOI] [PubMed] [Google Scholar]
- 2.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria—specificity in membrane targeting for death. Biochim Biophys Acta 2011;1813:532–539 [DOI] [PubMed] [Google Scholar]
- 3.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 2011;21:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol 2008;70:73–91 [DOI] [PubMed] [Google Scholar]
- 5.Nadal A, Valdeolmillos M, Soria B. Metabolic regulation of intracellular calcium concentration in mouse pancreatic islets of Langerhans. Am J Physiol 1994;267:E769–E774 [DOI] [PubMed] [Google Scholar]
- 6.Zhou YP, Pena JC, Roe MW, et al. Overexpression of Bcl-x(L) in beta-cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am J Physiol Endocrinol Metab 2000;278:E340–E351 [DOI] [PubMed] [Google Scholar]
- 7.Brun T, Franklin I, St-Onge L, et al. The diabetes-linked transcription factor PAX4 promotes beta-cell proliferation and survival in rat and human islets. J Cell Biol 2004;167:1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danial NN, Walensky LD, Zhang CY, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 2008;14:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison J, Thomas H, Beck D, et al. Transgenic overexpression of human Bcl-2 in islet beta cells inhibits apoptosis but does not prevent autoimmune destruction. Int Immunol 2000;12:9–17 [DOI] [PubMed] [Google Scholar]
- 10.Carrington EM, McKenzie MD, Jansen E, et al. Islet beta-cells deficient in Bcl-xL develop but are abnormally sensitive to apoptotic stimuli. Diabetes 2009;58:2316–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luciani DS, White SA, Widenmaier SB, et al. Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells. Diabetes 2013;62:170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellman B, Idahl LA, Sehlin J, Täljedal IB. Influence of anoxia on glucose metabolism in pancreatic islets: lack of correlation between fructose-1,6-diphosphate and apparent glycolytic flux. Diabetologia 1975;11:495–500 [DOI] [PubMed] [Google Scholar]
- 13.Boohaker RJ, Zhang G, Carlson AL, Nemec KN, Khaled AR. BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells. Am J Physiol Cell Physiol 2011;300:C1466–C1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi CH, Pan H, Seebacher J, et al. Metabolic regulation of protein N-alpha-acetylation by Bcl-xL promotes cell survival. Cell 2011;146:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alavian KN, Li H, Collis L, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol 2011;13:1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Lo A, Young JB, et al. Targeted quantitative mass spectrometric identification of differentially expressed proteins between Bax-expressing and deficient colorectal carcinoma cells. J Proteome Res 2009;8:3403–3414 [DOI] [PubMed] [Google Scholar]
- 17.Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS ONE 2010;5:e8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Reilly LA, Print C, Hausmann G, et al. Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ 2001;8:486–494 [DOI] [PubMed] [Google Scholar]
- 19.Campbell PD, Weinberg A, Chee J, et al. Expression of pro- and antiapoptotic molecules of the Bcl-2 family in human islets postisolation. Cell Transplant 2012;21:49–60 [DOI] [PubMed] [Google Scholar]
- 20.Federici M, Hribal M, Perego L, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 2001;50:1290–1301 [DOI] [PubMed] [Google Scholar]
- 21.Bonner C, Bacon S, Concannon CG, et al. INS-1 cells undergoing caspase-dependent apoptosis enhance the regenerative capacity of neighboring cells. Diabetes 2010;59:2799–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]