Among the end-organ complications that ravage patients afflicted with diabetes, kidney disease is associated with the highest morbidity and mortality (1,2). Diabetic kidney disease is the most common cause of chronic kidney disease (CKD) and end-stage renal disease in the U.S. and the world (3). While tighter glycemic control and the use of inhibitors of the renin-angiotensin-aldosterone system (in types 1 and 2 diabetes) have helped to slow the progression of diabetic kidney disease, the beneficial effects are modest (4). Moreover, an increase in the prevalence of type 2 diabetes (4) and the high cardiovascular risk associated with CKD and end-stage renal disease (5–7) suggest that diabetic kidney disease will absorb a disproportionate fraction of scarce health care resources in the coming decades.
Basic research to understand the pathogenesis of this disease and to develop novel therapies has been slowed by the lack of reliable mouse models that fully recapitulate the severity of the human condition. In recent years, the Animal Models of Diabetic Complications Consortium sponsored by the National Institutes of Health put forth two position papers outlining the pros and cons of various rodent models of diabetic kidney disease based on histological and clinical criteria (8,9). However, in terms of gene expression, it is unknown to what degree these data are applicable to humans. Perhaps particular strains of diabetic mice may accurately reflect specific elements of human diabetic nephropathy (e.g., aberrant transforming growth factor-β or vascular endothelial growth factor signaling) (10), and mice strains, which are seemingly protected from more advanced kidney disease, may express protective gene transcripts that have not yet been identified.
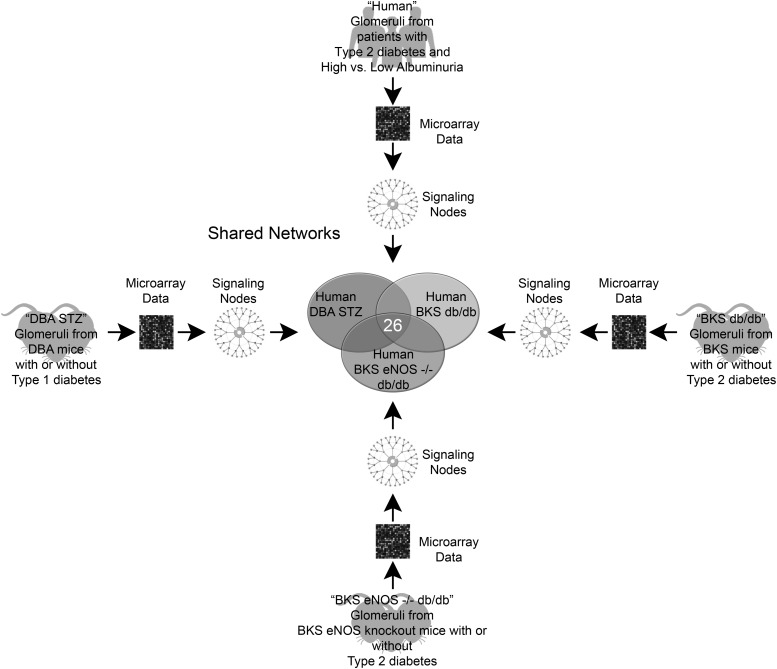
In this issue of Diabetes, Hodgin et al. (11) use an unbiased approach to compare and contrast glomerular gene expression profiles in human and murine diabetic nephropathy. The authors map the glomerular transcripts from humans with biopsy-proven disease and compare these patterns with data from three diabetic murine models that develop glomerular pathology similar to the early stages of human diabetic nephropathy (Fig. 1). Using microarray experiments and bioinformatic tools to organize and synthesize the data, the authors create transcriptional networks from the individual diabetes-specific transcripts (i.e., differentially expressed genes between diabetic and nondiabetic control subjects) within each of four groups (one human; three mouse) and then compare these four networks. They then validate a small subset of gene transcripts by qPCR across these groups. The authors ultimately identify shared and unique pathways from the “glomerular transcriptomes” of human and murine diabetic kidneys. This study supports the role of the JAK/STAT signaling pathway in human disease as previously described (12) and validates these three diabetic mouse strains as models of this pathway. Importantly, the authors also identify novel genes common to all four groups and identify specific pathways for which a subset of the mouse models might be most appropriate for preclinical studies.
FIG. 1.
The investigators used microarrays to analyze differential gene expression from glomeruli in four different comparisons and then generated signaling networks to represent each comparison. Nodes from each of these networks were then compared to examine the overlap between pathways that are altered in glomeruli from patients with clinical disease and three separate mouse models of diabetic nephropathy. In total, 26 shared nodes were present in all four models of the disease. BKS db/db, C57BLKS db/db mice; DBA STZ, streptozotocin-treated DBA/2 mice; eNOS, endothelial nitric oxide synthase.
The data generated by Hodgin et al. will be exceptionally valuable to researchers in the field. Nevertheless, we should refrain from considering these findings as a blueprint for biomarkers or drug discovery, else we fall prey to two logical fallacies. While a technical tour de force, the identified genes associated with disease are not yet proven to be mediators or markers of disease (cum hoc ergo propter hoc). The analyses are also cross-sectional and do not allow for the possibility that certain gene transcripts proceed and others may follow the development of histologically or clinically apparent disease (post hoc ergo propter hoc). Moreover, the human biopsies are from a Pima Indian cohort that is well-characterized clinically; however, Pima Indians are known to experience rapid progression of kidney disease, and data from the Pima may not optimally represent disease characteristics in other racial or ethnic groups. In addition, the authors compare gene transcripts from patients with early diabetic nephropathy with normal urine albumin excretion to patients with elevated urine albumin excretion, and they speculate on the observed trends. However, the transcriptional networks are from patients who have not yet reached a point of diminished kidney function (i.e., reduced glomerular filtration rate). While these glomerular transcripts certainly correlate with histologic disease and with albuminuria, they may not represent genes that determine the clinically important problem of progression. Less well-studied and absent in this manuscript are a cohort of patients with reduced glomerular filtration rate and normal urine albumin excretion, observed in at least 30–50% of patients with types 1 and 2 diabetes (13,14). These patients have diabetic kidney disease, but slower rates of progression compared with patients with classic Kimmelstiel-Wilson lesions and micro- or macroalbuminuria (i.e., diabetic nephropathy), although the risks of cardiovascular disease and other sequelae of CKD are still high in this population (5,15). Finally, as with any well-conducted venture in bioinformatics, the authors define cut-offs for what was considered a significant difference in expression among diabetic and nondiabetic samples, and they use matching algorithms, either or both of which may contribute to misclassification in the final analysis.
In summary, Hodgin et al. provide exciting, novel data that will allow investigators to compare gene expression profiles across different study cohorts and across species. By focusing on glomerular gene expression, the authors have enriched these profiles for specific gene products, which may be missed in transcriptome studies from the whole kidney as glomerular RNA comprises less than 5% of the total transcripts within the kidney (16), yet histological changes in glomeruli are the first visible signs of this disease (17). Investigators interested in studying a pathway relevant to human disease (e.g., epidermal growth factor signaling) can now choose from a menu of mouse models. Finally, patients with diabetes and kidney disease are rarely biopsied and thus, while cancers and other diseases have long been categorized by their molecular phenotype (18), kidney disease in patients with diabetes is crudely defined. This work may help to redefine the taxonomy of diabetic kidney disease based on glomerular gene expression rather than on nonspecific markers such as albuminuria and serum creatinine.
The authors have painstakingly derived, organized, and now shared a wealth of transcriptional data. Future experiments should build upon this impressive foundation and usher in an era of unprecedented progress in the treatment (and prevention) of diabetic kidney disease.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 299.
REFERENCES
- 1.Parving HH, Hommel E, Mathiesen E, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed) 1988;296:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borch-Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1985;28:590–596 [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012;59(Suppl. 1):e1–e420 [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 6.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 1996;313:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005;16:489–495 [DOI] [PubMed] [Google Scholar]
- 8.Breyer MD, Böttinger E, Brosius FC, 3rd, et al. AMDCC Mouse models of diabetic nephropathy. J Am Soc Nephrol 2005;16:27–45 [DOI] [PubMed] [Google Scholar]
- 9.Brosius FC, 3rd, Alpers CE, Bottinger EP, et al. Animal Models of Diabetic Complications Consortium Mouse models of diabetic nephropathy. J Am Soc Nephrol 2009;20:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract 2008;82Suppl. 1S38–S41 [DOI] [PubMed] [Google Scholar]
- 11.Hodgin JB, Nair V, Zhang H, et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 2013;62:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 2009;58:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, UKPDS Study Group Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006;55:1832–1839 [DOI] [PubMed] [Google Scholar]
- 14.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011;20:246–257 [DOI] [PubMed] [Google Scholar]
- 16.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 2002;161:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond K, Mauer M, International Diabetic Nephropathy Study Group The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 2002;51:1580–1587 [DOI] [PubMed] [Google Scholar]
- 18.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–511 [DOI] [PubMed] [Google Scholar]