Abstract
Two methods of assay, measuring (i) stimulation of host cell DNA synthesis by [3H]thymidine incorporation and (ii) morphological transformation in microtiter plates, were employed to determine what effect treatment during infection with adenine arabinoside or 5-iodo-5′-amino-2′,5′-dideoxyuridine has on Epstein-Barr virus (EBV) (B95-8)-induced transformation of human umbilical cord leukocytes. It was found that adenine arabinoside inhibited EBV-induced transformation in a dose-dependent manner in both assays, beginning at drug concentrations (<2 μg/ml) which had little effect on either spontaneous or phytohemagglutinin-induced host cell DNA synthesis. Adenine arabinoside was more effective in inhibiting morphological transformation than EBV-induced host DNA synthesis. Adenine arabinoside treatment was also effective in reducing both EB viral capsid antigen expression and production of biologically active extracellular transforming virus in EBV-transformed cells. In contrast, 5-iodo-5′-amino-2′,5′-dideoxyuridine, which inhibited herpes simplex virus replication, had little effect on EBV-induced transformation as measured by either method of assay. However, 5-iodo-5′-amino-2′,5′-dideoxyuridine was found to be effective in inhibiting viral capsid antigen expression and production of extracellular transforming virus in EBV-transformed cells.
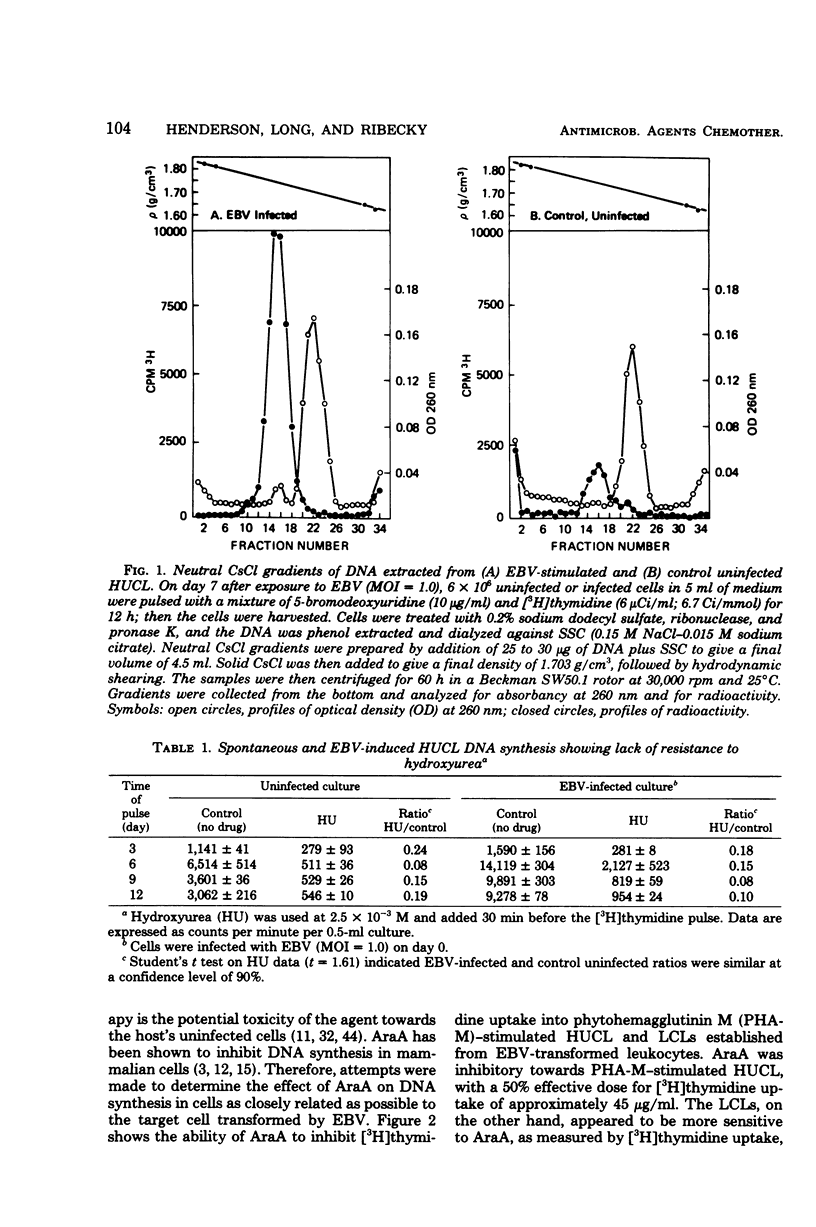
Full text
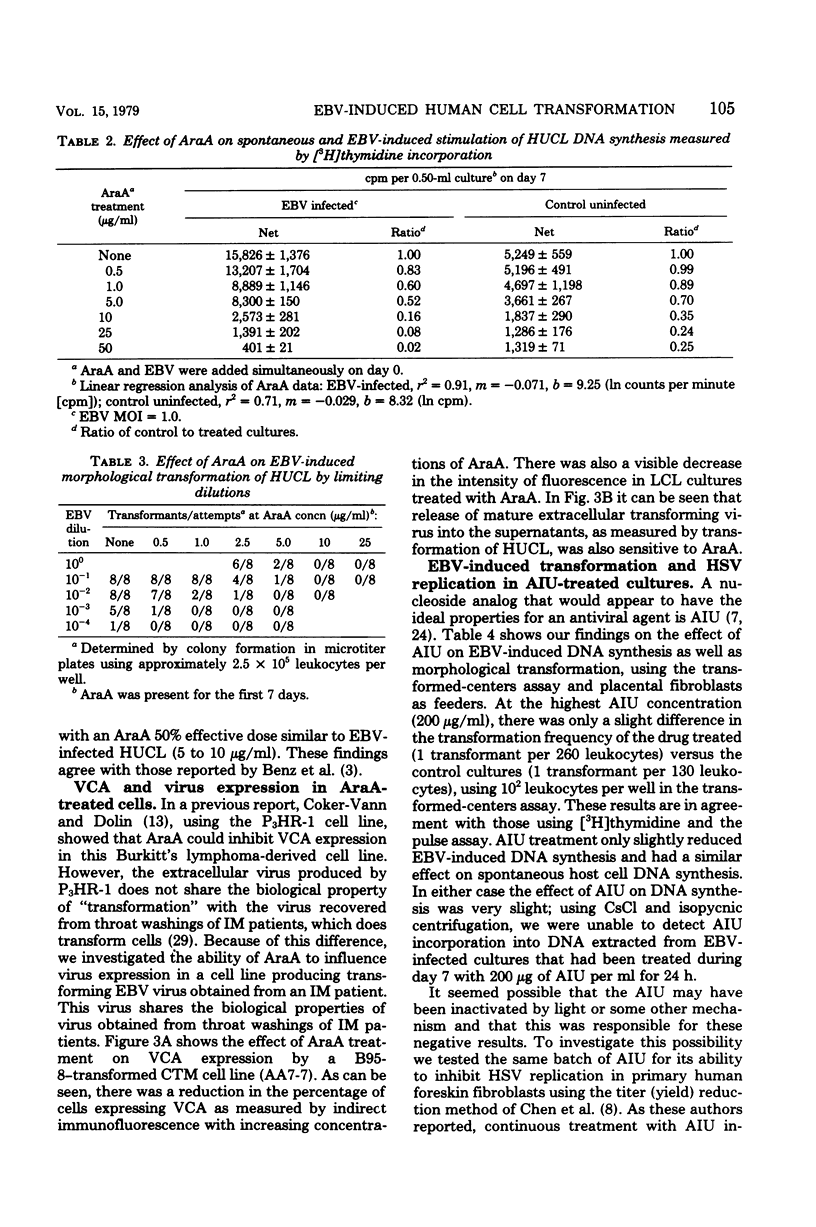
PDF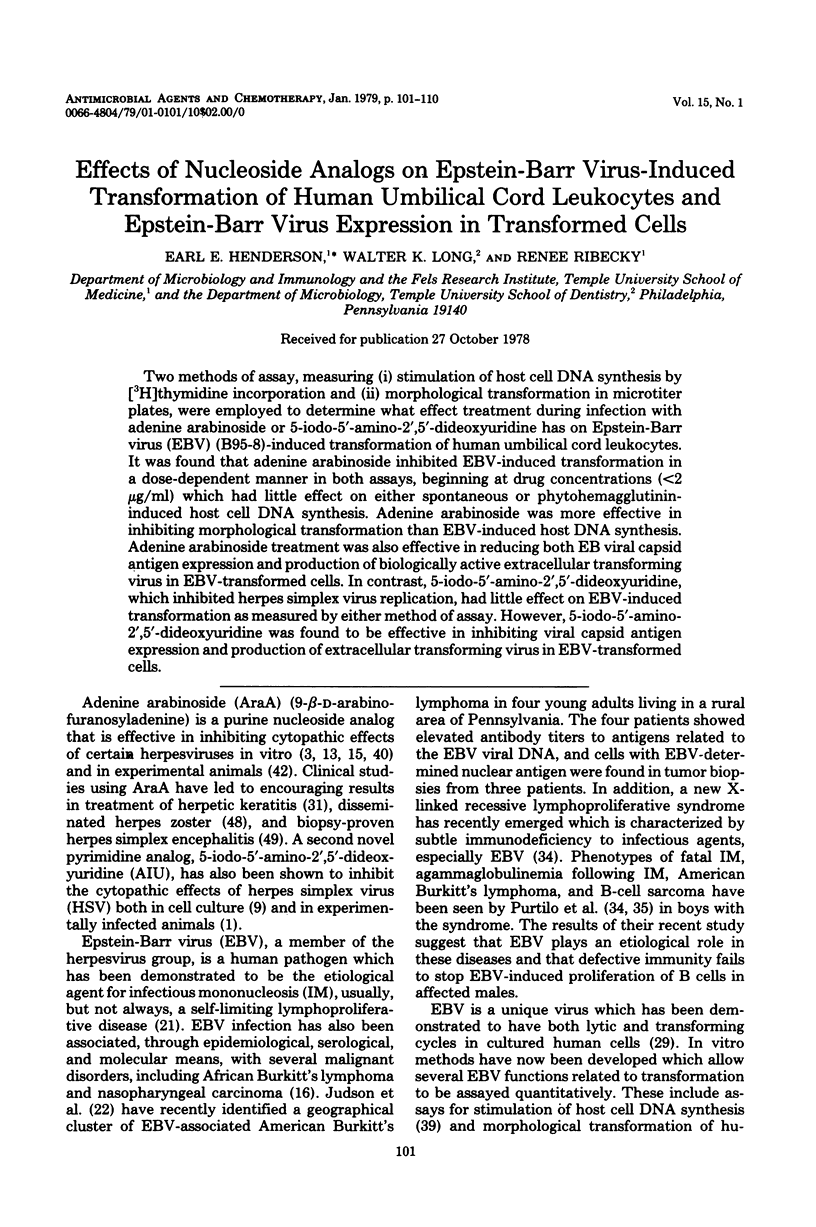


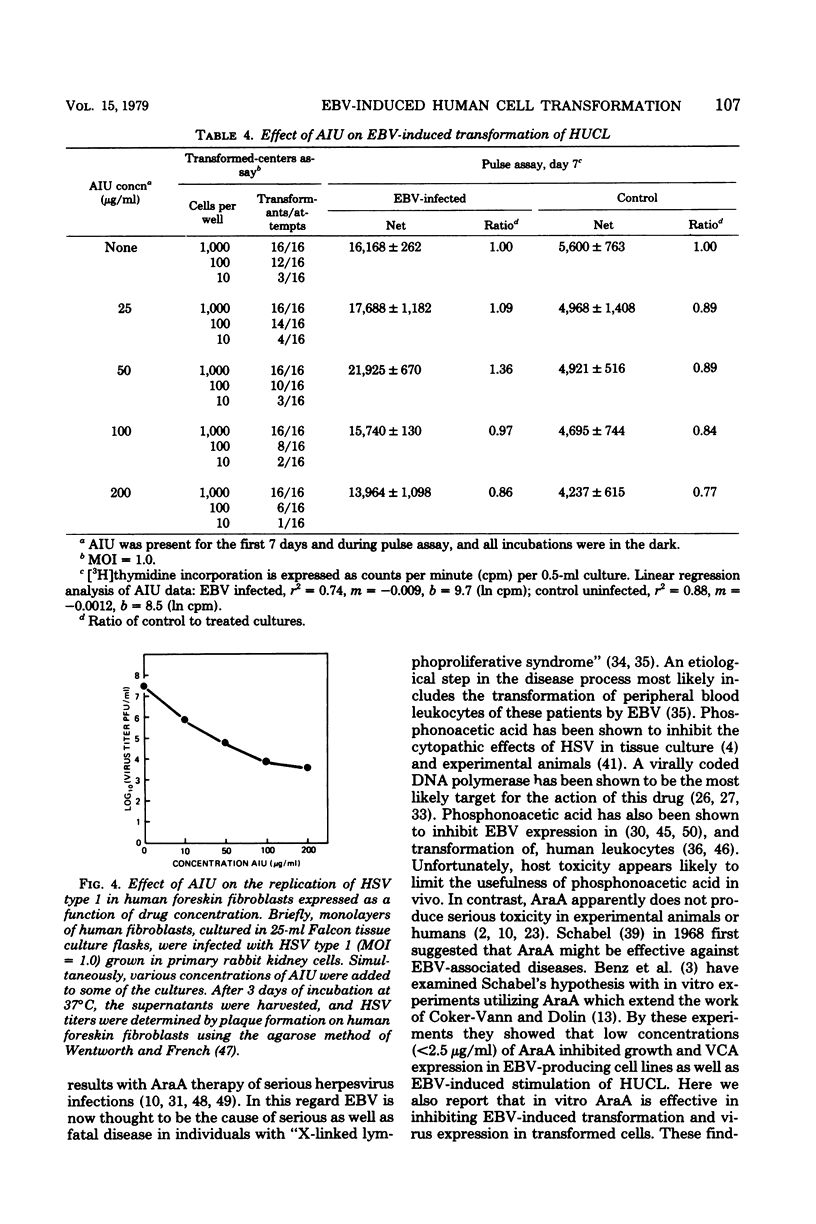
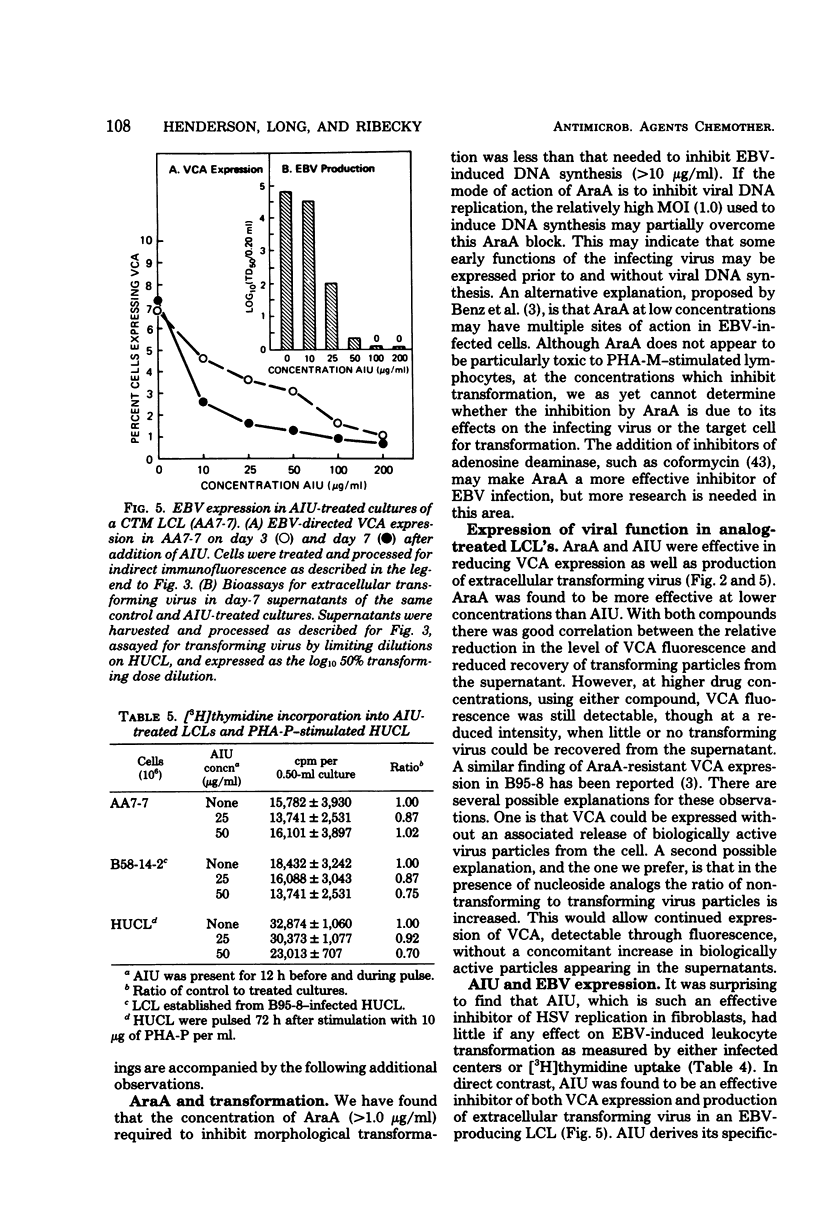


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. M., Lahav M., Bhatt P. N., Reid T. W., Ward R. E., Cykiert R. C., Lin T. S., Ward D. C., Prusoff W. H. Successful therapy of herpes hominis keratitis in rabbits by 5-iodo-5'-amino-2'5'-dideoxyuridine (AIU): a novel analog of thymidine. Invest Ophthalmol. 1976 Jun;15(6):470–478. [PubMed] [Google Scholar]
- Aronson M. D., Phillips C. F., Gump D. W., Albertini R. J., Phillips C. A. Vidarabine therapy for severe herpesvirus infections. An unusual syndrome of chronic varicella and transient immunologic deficiency. JAMA. 1976 Mar 29;235(13):1339–1342. doi: 10.1001/jama.235.13.1339. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Siegel P. J., Baer J. Effects of adenine arabinoside on lymphocytes infected with Epstein-Barr virus. J Virol. 1978 Sep;27(3):475–482. doi: 10.1128/jvi.27.3.475-482.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W. N., Flamm W. G., Bernheim N. J. The value of hydroxyurea in assessing repair synthesis of DNA in HeLa cells. Chem Biol Interact. 1972 Oct;5(5):327–339. doi: 10.1016/0009-2797(72)90072-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ch'ien L. T., Whitley R. J., Nahmias A. J., Lewin E. B., Linnemann C. C., Jr, Frenkel L. D., Bellanti J. A., Buchanan R. A., Alford D. A., Jr Antiviral chemotherapy and neonatal herpes simplex virus infecition: a pilot study--experience with adenine arabinoside (ARA-A). Pediatrics. 1975 May;55(5):678–685. [PubMed] [Google Scholar]
- Chen M. S., Ward D. C., Prusoff W. H. 5-Iodo-5'-amino-2',5'-dideoxyuridine-5'-N'-triphosphate. Synthesis, chemical properties, and effect on Escherichia coli thymidine kinase activity. J Biol Chem. 1976 Aug 25;251(16):4839–4842. [PubMed] [Google Scholar]
- Chen M. S., Ward D. C., Prusoff W. H. Specific herpes simplex virus-induced incorporation of 5-iodo-5'-amino-2',5'-dideoxyuridine into deoxyribonucleic acid. J Biol Chem. 1976 Aug 25;251(16):4833–4838. [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Neenan J. P., Ward D. C., Prusoff W. H. Selective inhibition of herpes simplex virus by 5-amino-2,5-dideoxy-5-iodouridine. J Virol. 1975 May;15(5):1284–1285. doi: 10.1128/jvi.15.5.1284-1285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. A strategy for the chemotherapy of infectious disease. Science. 1977 Jul 29;197(4302):431–432. doi: 10.1126/science.195340. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. The mechanisms of lethal action of arabinosyl cytosine (araC) and arabinosyl adenine (araA). Cancer. 1977 Jul;40(1 Suppl):509–518. doi: 10.1002/1097-0142(197707)40:1+<509::aid-cncr2820400717>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Coker-Vann M., Dolin R. Effect of adenine arabinoside on Epstein-Barr virus in vitro. J Infect Dis. 1977 Mar;135(3):447–453. doi: 10.1093/infdis/135.3.447. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Dolin R., Smith H. A. Antiviral activity of adenine arabinoside and iododeoxyuridine in human fetal intestinal and tracheal organ cultures. J Infect Dis. 1975 Sep;132(3):287–295. doi: 10.1093/infdis/132.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G. The EB virus. Annu Rev Microbiol. 1973;27:413–436. doi: 10.1146/annurev.mi.27.100173.002213. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Miller G., Robinson J., Heston L. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology. 1977 Jan;76(1):152–163. doi: 10.1016/0042-6822(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Henderson E., Robinson J., Frank A., Miller G. Epstein-Barr virus: transformation of lymphocytes separated by size or exposed to bromodeoxyuridine and light. Virology. 1977 Oct 1;82(1):196–205. doi: 10.1016/0042-6822(77)90042-3. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson S. C., Henle W., Henle G. A cluster of Epstein-Barr-virus-associated American Burkitt's lymphoma. N Engl J Med. 1977 Sep 1;297(9):464–468. doi: 10.1056/NEJM197709012970902. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Neenan J. P., Cheng Y. C., Prusoff W. H. Synthesis and antiviral activity of 5- and 5'-substituted thymidine analogs. J Med Chem. 1976 Apr;19(4):495–498. doi: 10.1021/jm00226a009. [DOI] [PubMed] [Google Scholar]
- Lorentz A. K., Munk K., Darai G. DNA repair replication in human embryonic lung cells infected with herpes simplex virus. Virology. 1977 Oct 15;82(2):401–408. doi: 10.1016/0042-6822(77)90015-0. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele J., Glaser R., Nonoyama M., Zimmerman J., Rapp F. Observations on the resistance of Fpstein-Barr virus DNA synthesis to hydroxyurea. Virology. 1974 Nov;62(1):102–111. doi: 10.1016/0042-6822(74)90306-7. [DOI] [PubMed] [Google Scholar]
- Nyormoi O., Thorley-Lawson D. A., Elkington J., Strominger J. L. Differential effect of phosphonoacetic acid on the expression of Epstein-Barr viral antigens and virus production. Proc Natl Acad Sci U S A. 1976 May;73(5):1745–1748. doi: 10.1073/pnas.73.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusoff W. H., Ward D. C. Nucleoside analogs with antiviral activity. Biochem Pharmacol. 1976 Jun 1;25(11):1233–1239. doi: 10.1016/0006-2952(76)90083-6. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. Herpes simplex virus DNA polymerase as the site of phosphonoacetate sensitivity: temperature-sensitive mutants. J Virol. 1977 Nov;24(2):470–477. doi: 10.1128/jvi.24.2.470-477.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo D. T., DeFlorio D., Jr, Hutt L. M., Bhawan J., Yang J. P., Otto R., Edwards W. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N Engl J Med. 1977 Nov 17;297(20):1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Hutt L., Bhawan J., Yang J. P., Cassel C., Allegra S., Rosen F. S. Immunodeficiency to the Epstein-Barr virus in the X-linked recessive lymphoproliferative syndrome. Clin Immunol Immunopathol. 1978 Feb;9(2):147–156. doi: 10.1016/0090-1229(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Finerty S., Epstein M. A. Mechanism of the establishment of Epstein-Barr virus genome-containing lymphoid cell lines from infectious mononucleosis patients: studies with phosphonoacetate. Int J Cancer. 1977 Dec 15;20(6):861–868. doi: 10.1002/ijc.2910200607. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Andiman W. A., Henderson E., Miller G. Host-determined differences in expression of surface marker characteristics on human and simian lymphoblastoid cell lines transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):749–753. doi: 10.1073/pnas.74.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabel F. M., Jr The antiviral activity of 9-beta-D-arabinofuranosyladenine (ARA-A). Chemotherapy. 1968;13(6):321–338. doi: 10.1159/000220567. [DOI] [PubMed] [Google Scholar]
- Shipkowitz N. L., Bower R. R., Appell R. N., Nordeen C. W., Overby L. R., Roderick W. R., Schleicher J. B., Von Esch A. M. Suppression of herpes simplex virus infection by phosphonoacetic acid. Appl Microbiol. 1973 Sep;26(3):264–267. doi: 10.1128/am.26.3.264-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Shipman C., Jr, Drach J. C. Deoxyadenosine antagonism of the antiviral activity of 9-beta-D-arabinofuranosyladenine and 9-beta-D-arabinofuranosylhypoxanthine. Cancer Res. 1978 Jul;38(7):1916–1921. [PubMed] [Google Scholar]
- Stalder H. Antiviral therapy. Yale J Biol Med. 1977 Sep-Oct;50(5):507–532. [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D., Strominger J. L. Transformation of human lymphocytes by Epstein-Barr virus is inhibited by phosphonoacetic acid. Nature. 1976 Sep 23;263(5575):332–334. doi: 10.1038/263332a0. [DOI] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of Herpesvirus hominis on human embryonic fibroblasts. Proc Soc Exp Biol Med. 1969 Jun;131(2):588–592. doi: 10.3181/00379727-131-33932. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Ch'ien L. T., Dolin R., Galasso G. J., Alford C. A., Jr Adenine arabinoside therapy of herpes zoster in the immunosuppressed. NIAID collaborative antiviral study. N Engl J Med. 1976 May 27;294(22):1193–1199. doi: 10.1056/NEJM197605272942201. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Soong S. J., Dolin R., Galasso G. J., Ch'ien L. T., Alford C. A. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med. 1977 Aug 11;297(6):289–294. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]



