Abstract
OBJECTIVES
Examine the relationship of influenza and activities of daily living (ADL) decline and other clinical indicators among nursing home (NH) residents.
DESIGN
retrospective, NH aggregated longitudinal study.
SETTING
2,351 NHs in 122 US cities during years 1999–2005.
PARTICIPANTS
Long-stay (>90 days) NH residents.
MEASUREMENTS
Quarterly, city-level influenza mortality rate, state-level influenza severity. Quarterly incidence of MDS-derived ADL decline (≥ 4 points), weight loss, new or worsening pressure ulcers, and infections. Outcome variables chosen as clinical controls: antipsychotic use, restraint use, persistent pain.
RESULTS
City-level influenza mortality and state-level influenza severity were both associated with higher rates of large (4 points or more) ADL decline (mortality β=0.20, p<.001; severity β=0.18, p<.001), weight loss (β=0.19, p<.001; β=0.24, p<.001), worsening pressure ulcers (β=0.04, p=0.08; β=0.12, p<.001), and infections (β=0.41, p<.001; β=0.47, p<.001), but not with restraints use, antipsychotic use, or persistent pain. NH influenza vaccination rates were very weakly associated with our outcomes (e.g. β= −0.009, p=0.03 for ADL decline, β= 0.008, p=0.07 for infections). Compared to the summer quarter of lowest influenza activity, our results for the other quarters translate to an additional 12,284 NH residents experiencing large ADL decline annually, 15,168 experiencing significant weight loss, 6,284 new or worsening pressure ulcers, and 29,753 experiencing infections due to influenza.
CONCLUSION
Our results suggest a substantial and potentially costly impact of influenza on NH residents. The effect of influenza vaccination on preventing further ADL decline and other clinical outcomes in NH residents should be studied further.
Keywords: influenza, nursing home, functional decline, ADL, MDS
Introduction
Influenza is an important cause of excess morbidity and mortality which disproportionately affects elderly people, exacerbates underlying cardiovascular and cerebrovascular conditions, and can lead to hospitalization and death. Older individuals incur more than 90% of the estimated 36,000 influenza-related deaths each year,1 and the majority of influenza-associated hospitalizations due to bacterial infections, pulmonary, cardio- and cerebrovascular complications. 2–4
Nursing home (NH) residents represent a subset of elderly people particularly vulnerable to the effects of influenza. Their immune and physiologic senescence, multi-morbidity, and increased exposure risk through close living quarters and shared caregivers contribute to their increased susceptibility to disease. 5–9
Studies delineating influenza-related outcomes among NH residents have traditionally focused on rates of clinician visits, hospitalizations, and deaths from influenza-associated illness. 10, 11 The latter are usually categorized by the International Classification of Diseases, Ninth Revision (ICD-9) codes. Although specific, these codes fail to adequately describe the significant heterogeneity that exists in the clinical presentation and outcomes of geriatric patients with influenza-related complications. As such, they are insufficiently sensitive to measure the morbidity and functional loss experienced by different NH residents diagnosed with the same medical condition.12 An assessment of functional status and other clinical outcomes, on the other hand, provides additional insight into how residents’ health status is compromised by influenza, and is a meaningful quality of life indicator for the NH elderly.
The importance of functional status as both a risk-factor, as well as an outcome of infections in older adults is well-recognized. 13–15 Instruments measuring specific aspects of functional status have already been developed 15. In NH residents, functional status is often described by the ability to independently perform the basic activities of daily living (ADLs), such as dressing, eating, toileting, personal hygiene, and ambulation.16 .Other clinical findings, such as unintentional weight loss, infections, and pressure ulcers have also been associated with functional decline.17–21
The impact of influenza on ADL decline and other functional status measures in the NH elderly has not been investigated, however. The objective of this study was to characterize the relationship between seasonal influenza, measured by city-level influenza mortality rate and state-level influenza severity, and various markers of functional status in nursing homes, with particular attention to ADL decline.
METHODS
Study Design and Data Sources
We performed a retrospective, NH facility-aggregated longitudinal study over 6 influenza seasons, from October 1, 1999 through September 30, 2005, in order to examine the relationship between the regional prevalence and severity of influenza and the prevalence of functional decline and other selected measures of clinical outcomes among long-stay NH residents, defined as those with a NH stay exceeding 90 days. We focused on long-stay NH residents to insure that we are studying influenza acquired while being a NH resident, and to exclude short stay admissions for whom functional change outcomes are not measured and whose other acute conditions leading to their short stay admission could confound an outcome that affects functional status.
Two main factors determined our cohort and time frame used in the study. The Centers for Disease Control and Prevention (CDC) collects and publishes online an ongoing weekly series of data on the total reported number of deaths attributable to influenza in 122 major U.S. cities. For this reason, we restricted our study to NH residents in NHs located in the 122 U.S. cities monitored by the CDC. The Minimum Data Set (MDS) provides the source for our outcome measures. Because the number of resident assessments in a NH during a given week can be small, we decided to conduct the study at the quarterly level, creating quarterly NH-level outcome measures based on resident-level MDS outcomes reported during a given quarter, and using quarterly averages of the weekly influenza mortality rate and of the weekly influenza severity. Quarters were defined as follows: Q1 = (calendar) weeks 1–13; Q2 = weeks 14–26, Q2 = weeks 27–39, Q4 = weeks 40–52. For inclusion in our analysis, we required that the NH was a freestanding (not hospital based) facility, and that it was observed for at least 12 of the 24 quarters in our six influenza seasons (102 facilities were excluded for having less than 12 quarters). The final analysis was carried out using NH quarter level resident outcomes among 2,351 freestanding NHs in the 122 U.S. cities monitored by the CDC.
Independent Variables
Our main independent variables were influenza mortality, influenza severity, and NH influenza vaccination rates. We calculated city-level influenza-related mortality rates using the CDC weekly reports of pneumonia and influenza (P&I) related deaths of all ages in 122 major US cities (http://cdc.gov/mmwr). These cities are spread across 39 states and the District of Columbia (Washington DC). Weekly mortality rates were calculated at the city-level as the number of influenza deaths per 100,000 population (based on the US Census Bureau data (http://www.census.gov) on annual population for each city).
The CDC also publishes a weekly series of influenza severity data at the state level, available for all 50 states from calendar week 40 through calendar week 20, the period with significant seasonal influenza activity (http://www.cdc.gov/flu/weekly/fluactivitysurv.htm). The report provides state-level influenza activity on a scale of 1 (no activity) through 5 (widespread). Therefore, influenza severity in our study is interpreted as a marker of influenza prevalence and activity, not as a marker of clinical disease severity. Prior studies have shown that influenza mortality correlates closely with influenza activity and severity. 22, 23 We examined the degree to which this measure of influenza severity adds information content to the influenza mortality in our NH population.
Finally, the rate of influenza vaccination for each NH facility was obtained from the On-line Survey & Certification Automated Record (OSCAR), an annual survey that, starting in 1999, reports the number of residents known to have received the influenza immunization within the prior 12 months (per HCFA-672 Form instructions). Unfortunately, because vaccination in NHs is typically done once a year during late fall/early winter, and because of continued admissions and discharges in the facility throughout the year, the month in which the annual OSCAR survey is done influences the number of residents known to be vaccinated and reported in the OSCAR survey. For example, the rates in surveys done in the December through May six month period in any given city are on average 12% higher than in surveys done June through November in the same facility or city, making it hard to impute vaccination rates for the facility during the months when they have no OSCAR survey. For this reason, and taking advantage of the fact that the month of OSCAR survey reporting is independent of facility characteristics, rather than using facility-level vaccination rates, we used city-level vaccination rates for a flu season. We calculated these city-level rates as the average vaccination rate among all NHs in that city that have an OSCAR survey during the December through May months, a period when the OSCAR-reported rates are close measures of the actual vaccination rates. As a consequence, the analyses using this city-level NH vaccination measure were restricted to cities where we had at least five NHs reporting vaccination rates in the December through May months for all six flu seasons, to ensure that our vaccination measure is reliable. While an imperfect measure, it allowed us to evaluate whether city-level NH vaccination rates confounds the relationship between influenza and our functional status outcomes.
Outcome Variables
Our NH resident outcome variables were constructed from the Minimum Data Set (MDS), a federally-mandated comprehensive resident assessment which includes measures of resident functioning, diagnoses, and demographics (MDS 2.0, http://www.cms.hhs.gov/nursinghomequalityinits/20_NHQIMDS20.asp). 16, 24, 25 The MDS is completed by facility nurses upon admission and at least quarterly thereafter, and it has been shown to have high inter-observer reliability. 26 Computerization of the MDS data is mandated, and all data are compiled in a national repository. The repository was made available to us through a Data Use Agreement with CMS (DUA #15293).
We chose the following indicators of impairment and dependence to assess functional status in the NH elderly, based on prior literature: 17, 18, 20, 21 1) physical functional decline, defined by an increase of at least 4 points in the additive ADL scale over a 90-day period. The MDS-derived ADL scale (MDS 2.0, section G.1) assigns a score on a scale from 0 (independent) to 4 (total dependence) to each of the following categories: bed mobility, transfer, walk in the room and corridor, locomotion on and off unit, dressing, eating, toileting, and personal hygiene, for a maximum total score of 28. 27, 28 Higher scores reflect higher dependence on the NH staff for performing each task, and indicate a poorer ADL status. The scale demonstrates high levels of internal consistency, with an alpha reliability exceeding 0.85.29 This measure was restricted to residents who were at-risk of declining at least this much by excluding those already at the highest 3 levels of impairment (a score 26–28) in the 0–28 scale. We required a minimum of a 4 point worsening in the ADL performance because less than 10% of long stay residents who have this much functional loss revert to their baseline state in the following assessment, suggesting that the decline is likely permanent, and not merely a measurement artifact;{Mor, 2011 #26} 2) weight loss of 5% or more in the last 30 days, or 10% or more in the last 180 days (MDS 2.0, section K.3.1); 3) Pressure ulcer (PU) incidence, defined as reporting a PU (MDS 2.0, section V.16) for a resident who did not have one reported at baseline, or reporting a more advanced stage PU than reported previously; 4) infections prevalence, defined as residents with any of the following infections: pneumonia, respiratory infection, septicemia, urinary tract infection, wound infection (MDS 2.0, section I.2), fever (J1h), or recurrent lung aspiration (J1k).
We also chose the following three NH quality indicators not expected to vary with seasonal influenza as clinical controls: 1) use of physical restraints (MDS 2.0, section P.4) scoring 0, 1 or 2; 2) antipsychotic use (MDS 2.0, section O.4.a) scoring 0–7; and 3) persistent pain (MDS 2.0, section J.2.a), defined as daily pain, at any level, reported on two consecutive assessments (90 days +/−30). 28
Construction of the longitudinal facility quality measures recording change or persistence (i.e. decline of 4 points in ADL, new or worsening pressure ulcer, and persistent moderate to severe pain over two successive assessments) required that residents were present in the facility at two successive MDS assessments, and we required at least 20 observations in the facility risk group (denominator) during that quarter, or the measures were set to missing for that quarter. Following recommendations from recent research, the measures of ADL decline, weight loss, PU incidence and pain persistence were risk adjusted at the patient level before aggregation. 21, 27, 30, 31 For our analysis, we followed the methodology described in detail in Mor et al. 21
The requirement, for accuracy purposes, that there are at least 20 residents in the facility risk group (denominator) during that quarter for the outcomes measures to be calculated and not set to missing for that quarter, has the implication that small NHs can have a large number of quarters with missing outcomes. For any given outcome, we restricted analyses to NHs that had at most 21% of their quarter outcome measures missing (2 if N=12 quarters, 5 if N=24 quarters). In addition, because our analyses were carried out weighting NHs by their corresponding NH population size for a given outcome (the risk group used to calculate that outcome rate), NHs with missing data were naturally weighted less, thus minimizing the impact of the few missing outcomes still present.
Additional Variables
NH facility characteristics were obtained from the On-line Survey & Certification Automated Record (OSCAR), an annual survey that reports organizational, staffing and residents characteristics. The OSCAR data can be linked to the MDS data through the facility provider number available on the MDS with match rates that now approach 100%.
Statistical analysis
For each outcome measure, a facility fixed effects multivariate regression model with an autoregressive error term of order one (AR(1)) was used to analyze the relationship between influenza mortality and influenza severity and our chosen measures of NH resident outcomes. This model compares outcomes from a facility in a given quarter to outcomes from the same facility in other quarters; thus, it controls for unobserved time-invariant NH factors that may otherwise bias our estimates if they correlate with the NH resident outcomes. The AR(1) error term controls for correlation across successive observations of the NH induced by unobserved time-varying factors.
All models were corrected for secular trends by including dummy variables for each influenza season. This allowed us to examine the seasonal association between our outcomes and the influenza measures—our main interest—without influence from associations between secular time trends in our variables that are more likely due to non-influenza related factors. The regression models also used weights to account for the different number of observations (NH residents) used to derive the NH outcome quarterly averages. This provides estimates that are more representative of the overall NH population in these cities. All statistical analysis was performed in Stata (version 11, College Station, TX).
RESULTS
Using OSCAR, we identified a total of 2,351 freestanding NHs, whose city address corresponded to one of the 122 cities in which CDC documents weekly influenza mortality data. The majority (2,179; 92.7%) had observations in all 24 quarters of our six influenza seasons. Table 1 summarizes some of the structural, organizational, staffing and resident characteristics of the NHs in our sample. The average bed size was 128, almost 70% were for-profit, and the average occupancy rate was around 85%. During this six year study period there was an increase in Medicare skilled care short stay (SNF) residents from 9% to 13% and a corresponding drop in the proportion of Medicaid residents. This change in resident insurance mix is reflected in slight increases in total direct care staff hours per resident day (3.3 to 3.4), average nursing case-mix acuity index (0.94 to 0.99), and number of admissions per bed (1.06 to 1.2).
Table 1.
Characteristics of Free-Standing Nursing Homes in the 122 CDC Monitored Cities
| Influenza Season |
||
|---|---|---|
| Characteristic | 1999 Q4-2000 Q3 (N=2,326) |
2004 Q4-2005 Q3 (N=2,284) |
| Number of Beds, mean ± SD (IQR) | 128.2±76.7 (79) | 127.6±71.7 (74) |
| Occupancy Rate, mean ± SD (IQR) | 84.9±14.8 (15.7) | 86.4±12.7 (13.5) |
| For-profit, % | 69.8 | 69.6 |
| Part of a Chain, % | 56.9 | 52.3 |
| Total Direct-Care Staffing hours per resident day, mean ± SD (IQR) | 3.3±2.0 (0.88) | 3.4±1.3 (0.9) |
| Percent Medicaid, mean ± SD (IQR) | 68.0±25.9 (30.9) | 65.3±24.3 (28.2) |
| Percent Medicare, mean ± SD (IQR) | 9.2±14.8 (8.1) | 12.8±14.0 (10.3) |
| Percent Private Pay, mean ± SD (IQR) | 22.8±22.3 (26.1) | 21.8±19.6 (22.4) |
| Nursing Case-Mix Index (Admission), mean ± SD (IQR) | 0.94±0.12 (0.14) | 0.99±0.12 (0.13) |
| No. of Admissions per Bed, mean ± SD (IQR) | 1.06±1.5 (0.69) | 1.2±1.1 (0.86) |
SD = Standard Deviation; IQR = Inter-Quartile Range.
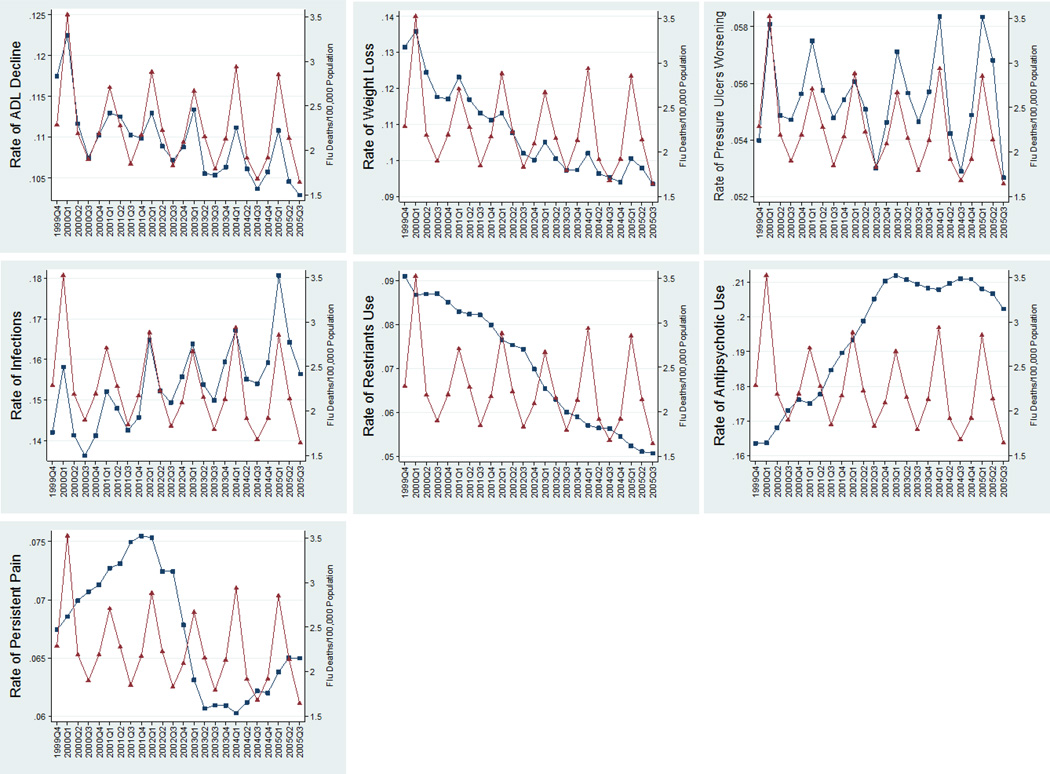
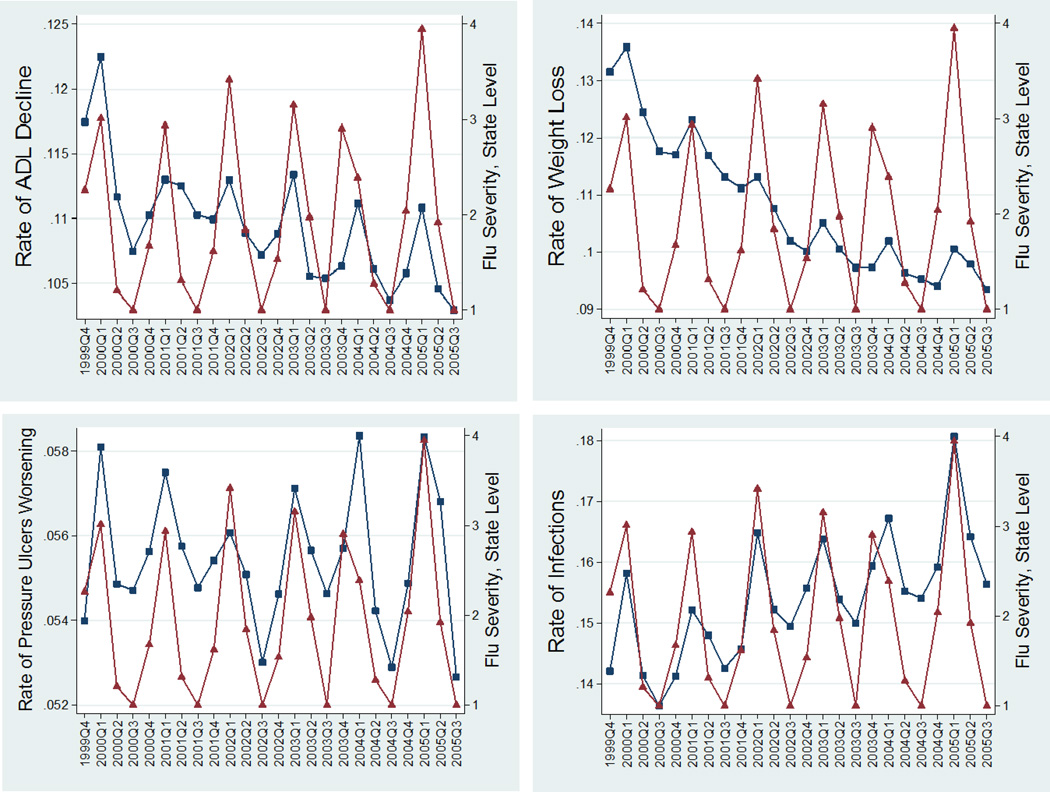
Influenza related mortality is well known to exhibit seasonal variation, with peaks during winter weeks and troughs during the summer weeks of each year. Figures 1A–1G and 2A–2D, based on averages for all NHs, show that our physical function outcome measures—ADL decline, weight loss, pressure ulcer worsening, and infections prevalence—also exhibit a similar (procyclical) and strong seasonal trend corresponding to influenza mortality rates (per 100,000 population) and to influenza severity, respectively. In these figures, the NH outcomes were weighted by the number of residents at risk in the NH, thus representing a population-based quarterly aggregate comparable to the CDC city influenza mortality rates which are standardized per 100,000 population in each city. Our “control” quality indicators—physical restraints, antipsychotic use, and persistent pain—exhibit a very weak (countercyclical) seasonal pattern. The intensity and timing of the seasonal patterns vary from city to city, and from year to year, but their patterns are roughly those observed in the figures.
Figure 1.
1A–1G. Quarterly Patterns of Functional Outcomes ( ) versus Influenza City-Level Mortality (
) versus Influenza City-Level Mortality ( ) (functional outcomes rates (range 0–1) on the left vertical axis, influenza mortality (Deaths/100,000 population) on the right vertical axis) for long-stay (>90 days) nursing home residents in 122 CDC-monitored cities in the USA. The outcomes (from left to right and top to bottom) are prevalence rates of 1. Large ADL decline (>3 points in the 0–28 ADL scale); 2. Weight Loss; 3. Worsening Pressure Ulcers; 4. Infections; 5. Use of Restraints; 6. Use of Antipsychotics; 7. Persistent Pain.
) (functional outcomes rates (range 0–1) on the left vertical axis, influenza mortality (Deaths/100,000 population) on the right vertical axis) for long-stay (>90 days) nursing home residents in 122 CDC-monitored cities in the USA. The outcomes (from left to right and top to bottom) are prevalence rates of 1. Large ADL decline (>3 points in the 0–28 ADL scale); 2. Weight Loss; 3. Worsening Pressure Ulcers; 4. Infections; 5. Use of Restraints; 6. Use of Antipsychotics; 7. Persistent Pain.
Figure 2.
2A–2D. Quarterly Patterns of Functional Outcomes ( ) versus Influenza State-Level Severity (
) versus Influenza State-Level Severity ( ) (functional outcomes rates (range 0–1) on the left vertical axis, influenza severity scales (range 1–5) on the right vertical axis). for long-stay (>90 days) nursing home residents in 122 CDC-monitored cities in the USA. The outcomes (from left to right and top to bottom) are prevalence rates of 1. Large ADL decline (>3 points in the 0–28 ADL scale); 2. Weight Loss; 3. Worsening Pressure Ulcers; 4. Infections.
) (functional outcomes rates (range 0–1) on the left vertical axis, influenza severity scales (range 1–5) on the right vertical axis). for long-stay (>90 days) nursing home residents in 122 CDC-monitored cities in the USA. The outcomes (from left to right and top to bottom) are prevalence rates of 1. Large ADL decline (>3 points in the 0–28 ADL scale); 2. Weight Loss; 3. Worsening Pressure Ulcers; 4. Infections.
The results of our multivariate analyses using influenza mortality (Table 2) show that influenza mortality was strongly associated with high (≥4 points) ADL decline (β = 0.32, p < 0.001), weight loss (β = 0.36, p < 0.001), and infections (β = 0.73, p < 0.001), less so with new or worsening pressure ulcers (β = 0.11, p < 0.001), and anti-psychotics use (β = − 0.08, p = 0.02), and not significantly related to restraint use (β = −0.02, p = 0.13), or persistent pain (β = = − 0.01, p = 0.55). Of note, both the percent of variance explained by the facility-specific component of the error term and the size of the auto-regressive coefficient are particularly high for restraint use, antipsychotic use, persistent pain, and infections, indicating that there are strong and persistent time-invariant and time-varying unobserved NH factors affecting those outcomes that go beyond the effects of the seasonal influenza mortality factor. Adding influenza severity to the model has the overall effect of diminishing the impact of influenza mortality, particularly so for the rate of infections in the NH, to the point of influenza severity having as strong or stronger effect on our four main outcomes as influenza mortality (Table 3). The influenza severity estimates are all significantly different from zero, and in absolute value, influenza severity has a larger impact than influenza mortality on infections (β = 0.47, p < 0.001), weight loss (β = 0.24, p < 0.001), and pressure ulcers (β = 0.12, p < 0.001). As expected, restraints use, antipsychotic use, and persistent pain prevalence were not significantly associated with influenza mortality and severity.
Table 2.
Regression Results Using Influenza Death Rates, AR(1) Fixed Effects Model
| ADL ≥ 4 pt Decline |
Weight Loss |
Pressure Ulcer Worse |
Infections | Restraints | Anti Psychotics |
Persistent Pain |
|
|---|---|---|---|---|---|---|---|
| Influenza Death Rate | 0.32 † | 0.36 † | 0.11 † | 0.73 † | −0.02 | −0.08 * | −0.01 |
| % Variance explained by Facility | 34 | 32 | 27 | 53 | 87 | 59 | 60 |
| AR coefficient (−1,1) | 0.19 | 0.30 | 0.10 | 0.36 | 0.78 | 0.58 | 0.67 |
Note: Outcomes are percentages (0–100 range). The model also adjusted for secular trend by including indicator variables for each influenza season.
coefficient estimates are significant at the 5% level;
coefficient estimates are significant at the .01% level.
Table 3.
Regression Results Using Influenza Death and Severity, AR(1) Fixed Effects Model
| ADL ≥ 4 pt Decline |
Weight Loss |
Pressure Ulcer Worse |
Infection | Restraints | Anti Psychotics |
Persistent Pain |
|
|---|---|---|---|---|---|---|---|
| Influenza Death Rate | 0.20 † | 0.19 † | 0.04 | 0.41 † | −0.03 | −0.05 | −0.01 |
| Influenza Severity | 0.18 † | 0.24 † | 0.12 † | 0.47 † | 0.01 | −0.04 | −0.00 |
| % Variance explained by Facility | 34 | 31 | 27 | 53 | 87 | 60 | 61 |
| AR coefficient (−1,1) | 0.19 | 0.29 | 0.10 | 0.36 | 0.78 | 0.58 | 0.67 |
Note: Outcomes are percentages (0–100 range). The model also adjusted for secular trend by including indicator variables for each influenza season.
coefficient estimates are significant at the 5% level;
coefficient estimates are significant at the .01% level.
Adding city-level NH vaccination rate to our model had very little impact on the coefficients of influenza mortality and severity, and the magnitude of the impact of vaccination rates was not statistically significantly different from zero, or marginally significantly different from zero, and much smaller than the impact of influenza mortality and influenza severity (results not shown). As an example, the biggest impact was in the ADL decline model, where the coefficient for vaccination was β = −0.009 (p = 0.03), almost negligible.
DISCUSSION
Our study found that the NH quality as measured by ADL decline, weight loss, pressure ulcers, and infections are significantly and negatively impacted by influenza. This finding was reproduced consistently in each of our analyses. As expected, our control measures of nursing home quality, including persistent pain and restraint use, were largely unaffected by influenza severity, nor did they correlate with influenza mortality. In a typical year, with approximately 1.23 million long-stay NH residents, using the average increases in influenza severity and mortality during the influenza season (quarters 1, 2, and 4) relative to the summer months (quarter 3) of lowest flu activity, our model estimated that an additional 12,284 long-stay NH residents experience an ADL decline of four points or larger; an additional 15,168 NH residents experience significant weight loss; an additional 6,284 NH residents experience new or worsening pressure ulcers; and an additional 29,753 NH residents experience an infection.
Taken together, these findings suggest that seasonal influenza has a major negative impact on the quality of life for the surviving NH elderly population. The ADL decline, weight loss, worsening pressure ulcers—presumed secondary to immobility, and increased rates of infections reflect an advanced level of disability and functional dependence. This likely results in increased personnel and health-care expenditures for the NH facilities.32
The association between influenza and ADL decline has been reported previously in a case-control study among frail NH residents from a limited geographical location, over 2 consecutive influenza seasons.33 Our study confirms these findings at the NH facility level, using a 6-year longitudinal analysis of a large-sample size, over the entire country. The scope of the effects we find on ADL decline and the other outcomes of interest provide insight into the strength of the forces at work over which even the best nursing facilities have little control. Influenza season, particularly in a city hard hit by influenza, will increase the work load on staff as they struggle to keep residents mobile, their skin from breaking down as they are sick in bed, and their food intake at reasonable levels. However, in the case of restraints, persistent pain, antipsychotic use, and infection rates, there appear to be NH specific unobserved factors that explain a large portion the variance (above 50%) of these outcomes beyond the explanation provided by seasonal effects of influenza. This indicates that these outcomes are potentially more amenable to reductions through increased staffing or other quality improvement measures than outcomes like large ADL decline, weight loss, or pressure ulcers, whose variance appears better explained by seasonal influenza. This suggests that research is needed on how high vs. less high quality homes cope with exposure to influenza.
Our findings have policy implications for the Center for Medicare/Medicaid Services current public reporting model, which posts facilities’ quality measures using a 5-star scale based in part on ADL decline and pressure ulcer prevalence, since only the facility’s most recent measure is posted. Those facilities in cities or states in which influenza was particularly severe will necessarily have worse performance on these measures. 34, 35 This indicates the importance and need for seasonal adjustment for these quality measures. For persons considering NHs in different cities or states, proper seasonal adjustment would provide a better comparative measure of NH quality. More indirectly, given that our functional decline outcomes are risk factors for hospitalizations of NH residents, and gven the introduction by CMS payment penalties for hospitals which have high risk-adjusted re-hospitalization rates, hospitals in cities/regions that have more severe influenza seasons could be unfairly penalized relative to those in regions with more benign influenza seasons. Careful examination of the effect of seasonal variation on re-hospitalizations from nursing homes (and from the community) is certainly warranted.36
Our study has several limitations that deserve mention. First, influenza mortality is measured indirectly, as P& I deaths, as opposed to laboratory-confirmed influenza, and as such, limits sensitivity and specificity. Other viruses that circulate in the winter, notably respiratory syncytial virus, can cause clinical symptoms indistinguishable from influenza, and their mortality can be mistakenly attributed to influenza, 1 reducing the specificity of our influenza mortality variable. On the other hand, influenza-related deaths from causes other than pulmonary, such as cardiovascular events, for example, are not included in our variable, limiting its sensitivity. Reassuringly, the strong relationship we observed with influenza mortality at the city level on the local functional status outcomes persisted.
Since influenza severity is the result of local and regional epidemiological surveillance based on laboratory-confirmation and clinical symptoms, its increased specificity may explain the stronger associations with the decline in functional status outcomes we found, validating our results.
A second limitation is that our analysis includes data derived from the MDS, a large, administrative, non-audited dataset that may be subject to reporting errors. However, the MDS has been shown to have high levels of inter-rater reliability particularly on the measures of ADL performance. 37 Finally, because our data on clinical outcomes have been aggregated at the NH-facility level, some of the differences present at the individual-level may have been lost in the analysis. Further studies examining the impact of influenza on functional status in NH residents using non-aggregated, large cohort individual-level data are important to confirm our findings.
Our longitudinal facility fixed effects models control for any unobserved facility level characteristics that are time invariant, or quasi time-invariant, and the use of autoregressive errors corrects standard errors for correlation over time. But there is a possibility that some time-varying confounders have not been accounted for.
A final important limitation was the imprecision of influenza vaccination as reported in the OSCAR NH surveys, which prevented us from having reliable vaccination measures for all NHs, plus lack of information on NH staff vaccination. As such, the lack of significance of influenza vaccination on functional decline outcomes is an open question that requires additional research.
CONCLUSION
Our study suggests that influenza significantly negatively affects functional status in NH residents. This finding has important implications both for the individual resident’s quality of life, as well as for the facility’s ability to effectively cope with this decline. Influenza vaccination remains one of the mainstays of prevention for this disease, even as its effectiveness in reducing mortality and cardiopulmonary complications in older individuals has been called into question. 38, 39 Our analysis was not structured to estimate the effect of vaccine effectiveness due to lack of precise vaccination data. It would be of utmost interest, therefore, to evaluate the vaccine’s effectiveness among the NH population in preventing functional decline. We need to better understand whether some facilities are better able to ameliorate the effects of influenza either by initially protecting their residents, or by identifying flu cases early and working to minimize their impact. Similarly, it is important to understand the cumulative effect of exposure to influenza on patients’ functioning, particularly whether recovery from influenza is a sign of residual resilience or of superior facility performance.
ACKNOWLEDGMENTS
This research was funded by the National Institute on Aging Research Grants (R01AG020557 and P01AG027296), and by the Agency for Healthcare and Quality Research Grant (R01HS018462).
Funding Sources: This research was funded by National Institute of Aging Research Grants (R01AG020557 and P01AG027296), and by the Agency for Healthcare and Quality Research Grant (R01HS018462).
Sponsor’s Role: The funding sources had no role in the design of the study; data collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
| Elements of Financial/Personal Conflicts |
*Author 1 Pedro L Gozalo |
Author 2 Aurora Pop- Vicas |
Author 3 Zhanlian Feng |
Author 4 Stefan Gravenstein |
Author 5 Vincent Mor |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | |||||
| Grants/Funds | X | X | X | X | X | |||||
| Honoraria | X | X | X | X | X | |||||
| Speaker Forum | X | X | X | X | X | |||||
| Consultant | X | X | X | X | X | |||||
| Stocks | X | X | X | X | X | |||||
| Royalties | X | X | X | X | X | |||||
| Expert Testimony | X | X | X | X | X | |||||
| Board Member | X | X | X | X | X | |||||
| Patents | X | X | X | X | X | |||||
| Personal Relationship | X | X | X | X | X | |||||
For “yes”, provide a brief explanation: Vincent Mor, Ph.D. is a founder and on the board of directors of PointRight, Inc. an information services company serving nursing homes on quality measurement and improvement. Dr. Mor receives no research funding, data or consultation on his research from PointRight.
Author Contributions: All authors contributed in the study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med. 1998;158:1769–1776. doi: 10.1001/archinte.158.16.1769. [DOI] [PubMed] [Google Scholar]
- 4.Bradley SF. Prevention of influenza in long-term-care facilities. Long-Term-Care Committee of the Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 1999;20:629–637. doi: 10.1086/501687. [DOI] [PubMed] [Google Scholar]
- 5.Menec VH, Black C, MacWilliam L, Aoki FY. The impact of influenza-associated respiratory illnesses on hospitalizations, physician visits, emergency room visits, and mortality. Can J Public Health. 2003;94:59–63. doi: 10.1007/BF03405054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns EA, Goodwin JS. Immunodeficiency of aging. Drugs Aging. 1997;11:374–397. doi: 10.2165/00002512-199711050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kingston BJ, Wright CV., Jr. Influenza in the nursing home. Am Fam Physician. 2002;65:75–78. 72. [PubMed] [Google Scholar]
- 8.Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36:870–876. doi: 10.1086/368197. [DOI] [PubMed] [Google Scholar]
- 9.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 10.Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ. 2006;333:1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deguchi Y, Nishimura K. Efficacy of Influenza Vaccine in Elderly Persons in Welfare Nursing Homes: Reduction in Risks of Mortality and Morbidity During an Influenza A (H3N2) Epidemic. J Gerontol A Biol Sci Med Sci. 2001;56:M391–M394. doi: 10.1093/gerona/56.6.m391. [DOI] [PubMed] [Google Scholar]
- 12.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35:345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 13.Bula CJ, Ghilardi G, Wietlisbach V, Petignat C, Francioli P. Infections and functional impairment in nursing home residents: a reciprocal relationship. J Am Geriatr Soc. 2004;52:700–706. doi: 10.1111/j.1532-5415.2004.52205.x. [DOI] [PubMed] [Google Scholar]
- 14.High KP. The importance of geriatric-specific instruments and functional status assessment in infectious diseases research: time to start preaching to the congregation instead of the choir. J Am Geriatr Soc. 2004;52:1768–1770. doi: 10.1111/j.1532-5415.2004.52491.x. [DOI] [PubMed] [Google Scholar]
- 15.High KP, Bradley S, Loeb M, Palmer R, Quagliarello V, Yoshikawa T. A new paradigm for clinical investigation of infectious syndromes in older adults: assessment of functional status as a risk factor and outcome measure. Clin Infect Dis. 2005;40:114–122. doi: 10.1086/426082. [DOI] [PubMed] [Google Scholar]
- 16.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 17.Chang JI, Katz PR, Ambrose P. Weight loss in nursing home patients: prognostic implications. J Fam Pract. 1990;30:671–674. [PubMed] [Google Scholar]
- 18.Min LC, Wenger NS, Reuben DB, Saliba D. A short functional survey is responsive to changes in functional status in vulnerable older people. J Am Geriatr Soc. 2008;56:1932–1936. doi: 10.1111/j.1532-5415.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore SA, Robinson G, Posthauer ME, Raymond J. Clinical indicators associated with unintentional weight loss and pressure ulcers in elderly residents of nursing facilities. J Am Diet Assoc. 1995;95:984–992. doi: 10.1016/S0002-8223(95)00271-5. [DOI] [PubMed] [Google Scholar]
- 20.Ferrell BA, Josephson K, Norvid P, Alcorn H. Pressure ulcers among patients admitted to home care. J Am Geriatr Soc. 2000;48:1042–1047. doi: 10.1111/j.1532-5415.2000.tb04778.x. [DOI] [PubMed] [Google Scholar]
- 21.Mor V, Gruneir A, Feng Z, Grabowski DC, Intrator O, Zinn J. The effect of state policies on nursing home resident outcomes. J Am Geriatr Soc. 2011;59:3–9. doi: 10.1111/j.1532-5415.2010.03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viboud C, Boelle PY, Pakdaman K, Carrat F, Valleron AJ, Flahault A. Influenza epidemics in the United States, France, and Australia, 1972–1997. Emerg Infect Dis. 2004;10:32–39. doi: 10.3201/eid1001.020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowell G, Miller MA, Viboud C. Seasonal influenza in the United States, France, and Australia: transmission and prospects for control. Epidemiol Infect. 2008;136:852–864. doi: 10.1017/S0950268807009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips CD, Morris JN, Hawes C, et al. Association of the Resident Assessment Instrument (RAI) with changes in function, cognition, and psychosocial status. J Am Geriatr Soc. 1997;45:986–993. doi: 10.1111/j.1532-5415.1997.tb02971.x. [DOI] [PubMed] [Google Scholar]
- 25.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42:III50–III59. doi: 10.1097/01.mlr.0000120104.01232.5e. [DOI] [PubMed] [Google Scholar]
- 26.Mor V, Angelelli J, Jones R, Roy J, Moore T, Morris J. Inter-rater reliability of nursing home quality indicators in the U.S. BMC Health Serv Res. 2003;3:20. doi: 10.1186/1472-6963-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fries BE, Schneider DP, Foley WJ, Gavazzi M, Burke R, Cornelius E. Refining a case-mix measure for nursing homes: Resource Utilization Groups (RUG-III) Med Care. 1994;32:668–685. doi: 10.1097/00005650-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Wu N, Miller SC, Lapane K, Roy J, Mor V. The quality of the quality indicator of pain derived from the minimum data set. Health Serv Res. 2005;40:1197–1216. doi: 10.1111/j.1475-6773.2005.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mor VI O, Unruh M, Cai S. Temporal and Geographic variation in the validity and internal consistency of the Nursing Home Resident Assessment Minimum Data Set 2.0. BMC-Health Services Research. 2011;11:1–14. doi: 10.1186/1472-6963-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukamel DB, Glance LG, Li Y, et al. Does risk adjustment of the CMS quality measures for nursing homes matter? Med Care. 2008;46:532–541. doi: 10.1097/MLR.0b013e31816099c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Z, Grabowski DC, Intrator O, Mor V. The effect of state medicaid case-mix payment on nursing home resident acuity. Health Serv Res. 2006;41:1317–1336. doi: 10.1111/j.1475-6773.2006.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161:2602–2607. doi: 10.1001/archinte.161.21.2602. [DOI] [PubMed] [Google Scholar]
- 33.Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med. 1998;158:645–650. doi: 10.1001/archinte.158.6.645. [DOI] [PubMed] [Google Scholar]
- 34.Werner RM, Konetzka RT. AcademyHealth. Chicago, Ill: 2009. What drives nursing home quality improvement under public reporting? An examination of post-acute care. [Google Scholar]
- 35.Werner RM, Konetzka RT, Kruse GB. Impact of public reporting on unreported quality of care. Health Serv Res. 2009;44:379–398. doi: 10.1111/j.1475-6773.2008.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood) 2010;29:57–64. doi: 10.1377/hlthaff.2009.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snowden M, McCormick W, Russo J, et al. Validity and responsiveness of the Minimum Data Set. J Am Geriatr Soc. 1999;47:1000–1004. doi: 10.1111/j.1532-5415.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 38.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35:337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 39.Simonsen L, Viboud C, Taylor R. Influenza vaccination in elderly people. Lancet. 2005;366:2086. doi: 10.1016/S0140-6736(05)67884-1. [DOI] [PubMed] [Google Scholar]