Abstract
As Rocky Mountain Spotted Fever is the most common tick-borne disease in South America, the presence of Rickettsia sp. in Amblyomma ticks is a possible indication of its endemicity in certain geographic regions. In the present work, bacterial DNA sequences related to Rickettsia amblyommii genes in A. dubitatum ticks, collected in the Brazilian state of Mato Grosso, were discovered. Simultaneously, Paracoccus sp. was detected in aproximately 77% of A. cajennense specimens collected in Rio de Janeiro, Brazil. This is the first report of Paracoccus sp. infection in a specific tick population, and raises the possibility of these bacteria being maintained and/or transmitted by ticks. Whether Paracoccus sp. represents another group of pathogenic Rhodobacteraceae or simply plays a role in A. cajennense physiology, is unknown. The data also demonstrate that the rickettsial 16S rRNA specific primers used forRickettsia spp. screening can also detect Paracoccus alpha-proteobacteria infection in biological samples. Hence, a PCR-RFLP strategy is presented to distinguish between these two groups of bacteria.
Keywords: Paracoccus, Rickettsia amblyommii, Amblyomma, ixodidae ticks
Ticks are blood-feeding arthropods harboring several associated bacteria, viruses, fungi and protozoans. Although some of the bacteria are classified as either symbionts, or human pathogens, the biological functions of most of these, only recently described prokaryotes, are still unknown (Niebylski et al., 1997; Noda et al., 1997; Marin and Schmidtman, 1998; Marquez et al., 1998; Yparraguirre et al., 2007; Stromdhal et al., 2008; Machado-Ferreira et al., 2009, 2011). In South America, Amblyomma cajennense is considered the most important tick species, representing the main vector of the Rhodobacteraceae Rickettsia rickettsii, the etiological agent of Rocky Mountain Spotted Fever (Sexton et al., 1993; Lemos 2000; Galvão et al., 2003; Guedes et al., 2005). A. cajennense may also harbor several other bacteria, such as Coxiella, Francisella and other Rickettsia species, which may represent new agents of human diseases or symbionts (Sexton et al., 1993; Parola et al., 1998; Machado-Ferreira et al., 2009, 2011).
Emerging infectious disease (EID) agents are comprised of pathogens that recently entered human populations, most of these with still unknown reservoirs or associated arthropod vectors (Salyers and Whitt, 2002). In this scenario, the CDC Group EO-2 Paracoccus yeeii, recently isolated from patients in the US, Canada and Europe, could be classified as another Rhodobacteraceae EID agent, with a currently unidentified source or transmission route (Daneshvar et al., 2003; Funke et al., 2004).
In the present study, an attempt was made to detect Rickettsia spp. in Amblyomma ticks, collected in the Brazilian states of Rio de Janeiro, Minas Gerais and Mato Grosso. We showed that the use of 16S rRNA specific primers for screening Rickettsia DNA in biological samples, also proved to be functional in detecting tick-associated Paracoccus sp.. This species, by being highly prevalent in A. cajennense ticks collected in a sampling site in Rio de Janeiro, possibly represents another group of tick-borne alpha-proteobacteria.
A total of 213 actively feeding adult A. cajennense and 20 A. dubitatum ticks were collected from horses and a domestic dog in the cities of Sambaetiba, Rio do Ouro and Três Rios (Rio de Janeiro), Pouso Alto (Minas Gerais) and São José do Xingu (Amazonia region of Mato Grosso) between May and November, 2006 (Table 1). After washing engorged females four times in 70% (v/v) ethanol, individuals and pools of ten ticks were snap-frozen in liquid nitrogen. After homogenization in 1 mL of a lysis solution (sucrose 5%, SDS 1%, 100 mM Tris pH 7.5, 600 mM NaCl and 100 mM EDTA), samples were incubated at 56 °C for 3 h with 0.4 mg/mL of proteinase K and 0.1 mg/mL of ribonuclease A. The resultant DNA was purified by phenol-chloroform extraction and re-suspended in 200 μL of 1 mM Tris pH 7.4 and 0.1 mM EDTA. DNA integrity was analyzed by agarose gel electrophoresis. Tick morphological classification was confirmed by PCR amplification of mitochondrial 16S rRNA genes, using the primer set 16S+1/16S-1 (Black and Piesman, 1994) followed by sequencing and BLAST analyses.
Table 1.
Total number of ticks analyzed for Rickettsia sp. infection.
| Sampling area1 | Host | Tick species | No. ticks analyzed2 | Minimum infection rate3 |
|---|---|---|---|---|
| Sambaetiba (RJ) | Horse | A. cajennense | 67 | 0 |
| Rio do Ouro (RJ) | Horse | A. cajennense | 12 | 0 |
| Três Rios (RJ) | Horse | A. cajennense | 49 | 38/494 |
| Pouso Alto (MG) | Horse | A. cajennense | 85 | 0 |
| São Jose do Xingu (MT) | Dog | A. dubitatum | 20 (2 X 10) | 1/205 |
RJ, state of Rio de Janeiro; MG, state of Minas Gerais; MT, state of Mato Grosso.
Most of the ticks were individually analyzed. Samples analyzed by pools are indicated in the parentheses (No. of pools X pool size).
The minimum infection rate was obtained by assuming that only one tick was infected.
Minimum number of Paracoccus sp. 4or Rickettsia sp. 5positive ticks / total number of ticks analyzed.
The Rickettsia genus-specific primer set fD1-Rc16S.452n (Marquez et al., 1998; Simser et al., 2002) was used to amplify the 16S rRNA gene (16S rDNA). Presumptive Rickettsia positive samples were then characterized by PCR amplification by using the Spotted Fever group primer sets, Rr190.70p/Rr190.602n for ompA (Regnery et al., 1991; Simser et al., 2002), the Rickettsia-specific primer set named Rr120 for ompB (Gage et al., 1992; Noda et al., 1997), Rr17.61p/Rr17.491n for htrA (Williams et al., 1992; Simser et al., 2002), and RpCS.877p/RpCS.1258n for gltA (Regnery et al., 1991; Simser et al., 2002). PCR temperature/time cycling conditions were 35 cycles of 94 °C for 1 min, 55 °C for 30 s and 72 °C for 1 min. PCR products from four independent reactions were then merged and directly purified with a GFX PCR DNA and Gel band purification kit (GE Healthcare, Buckinghamshire, UK), according to manufacturer’s instructions. In the case of suspect spurious PCR products, bands of expected size were cut from agarose gels and used as PCR templates with the same primer set. Purified PCR products were sequenced using the BigDye Terminator DNA sequencing kit (Applied Bio-systems, Foster City, Calif., U.S.A.), and analyzed in a Megabace 1000 DNA sequencer (Amersham Biosciences). Sequences were edited using the SeqMan program (DNASTARinc package for the Windows platform, 1989–1999), and gene identities obtained by BLAST analyses (blastn).
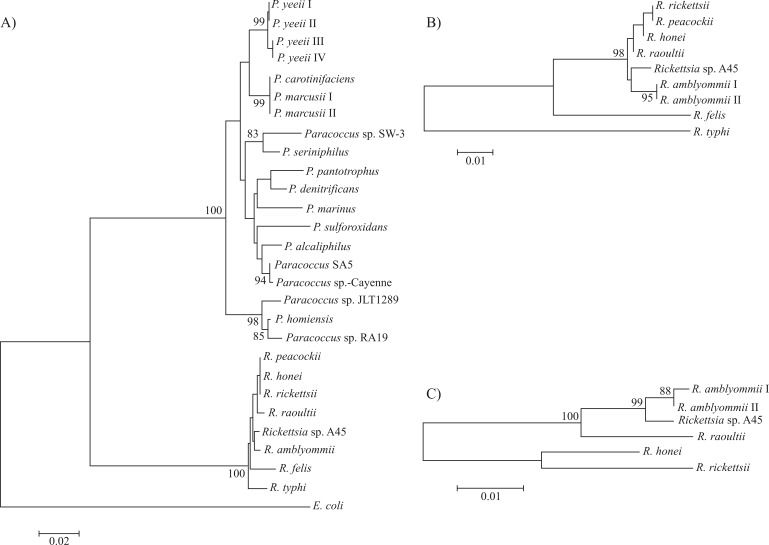
From the 233 ticks analyzed from distinct geographic locations, 94 DNA samples presented the expected amplicon size of ∼430 bp for Rickettsia spp. 16S rDNA (Named “Major-94” sample group) (data not shown). However, only one DNA sample (A45), from a pool of 10 A. dubitatum ticks collected in São José do Xingu was also PCR positive for the Rickettsia genes ompA (∼530 bp), ompB (∼500 bp), gltA (∼380 bp) and htrA (∼430 bp) (data not shown). Sequence analyses confirmed that sample A45 contained gene sequences corresponding to 16S rDNA (GenBank EF611737), htrA (GenBank EF611738) and ompA (GenBank Bankit_1476251), these, respectively, being 99%, 98% and 100% identical to the related genes of Rickettsia amblyommii (GenBank U11021; DQ517291 and AY062007). Phylogenetic analysis with 16S rDNA gene and htrA sequences clearly included the sample A45, denoted “Rickettsia sp. A45,” within the Rickettsia Spotted-Fever group (Figure 1A,B). The ompA sequences gave an even better resolution with the Rickettsia Spotted-Fever group. In this tree the close relationship between Rickettsia sp. A45 and R. amblyommii was evident, with a 99% bootstrap support (Figure 1C).
Figure 1.
Phylogenetic inferences by Neighbour-Joining (NJ) with Kimura 2-parameter. GenBank accession numbers are presented in parenthesis. A) 16S rDNA gene sequence alignment of Paracoccus sp-Cayenne and Rickettsia sp. A45, P. yeeii I (AY014178), P. yeeii II (DQ857285), P. yeeii III (AY014179), P. yeeii IV (AY014179), P. carotinifaciens (AB006899), P. marcusii I (DQ298023), P. marcusii II (DQ298022), Paracoccus sp. SW-3 (FJ593906), P. seriniphilus (AJ428275), P. pantotrophus (AB098590), P. denitrificans (CP000490), P. marinus (AB681212), P. sulforoxidans (JQ291588), P. alcaliphilus (AJ294415), Paracoccus SA5 (AY864654), Paracoccus sp. JLT1289 (EU650196), P. homiensis (NR_043733), Paracoccus sp. RA19 (AJ507806), R. peacockii (DQ062433), R. honei (U17645), R. rickettsii (U11021), R. raoultii (DQ365809), R. amblyommii (U11012), R. felis (CP000053), R. typhi (AE017197) and E. coli (AY84014). B) htrA gene sequence alignment of Rickettsia sp. A45, R. rickettsii (DQ176856), R. peacockii (AF260571), R. honei (AF060704), R. raoultii (EF392727), R. amblyommii I (DQ517291), R. amblyommii II (AY375162), R. felis (CP000053) and R. typhi (AE017197). C) ompA sequence aligment of Rickettsia sp. A45, R. amblyommii I (AY062007), R. amblyommii II (EF194096), R. raoultii (DQ365799), R. honei (AF018075) and R. rickettsii (DQ002504). Sequences were aligned using the CLUSTAL W 2.0 program, and phylogenetic inferences obtained using MEGA 5 software. Internal node supports were calculated using bootstrap with 1000 replicas. Bootstrap values below 80% are not presented.
In the Major-94 group, DNA samples from the 55 A. cajennense ticks collected in Sambaetiba (RJ) yielded numerous spurious PCR bands. According to initial-replicon analysis, re-amplification and sequencing, they were not identical to either Rickettsia sp., or to any other bacterial DNA. From these apparently positive samples, 17 tick-bulk DNA samples were also PCR analyzed, in an attempt to detect ompA, ompB, htrA and gltA genes. No amplification was obtained, confirming that they were in fact free of Rick-ettsia spp. (data not shown). Four randomly selected DNA samples of A. cajennense collected at Três Rios (RJ), and pertaining to the same group, were also sequenced and analyzed. Although these nucleotide sequences (GenBank EF531228) were all identical, there was no similarity to any Rickettsia spp. sequence. Even so, there was a close relationship to soil and invertebrate-associated alpha-proteobacteria Paracoccus spp., the latter mostly of the Paracoccus sp. SA5 strain (GenBank AY864654) and the soil P. alcaliphilus KTC002 (GenBank AJ294415), with 99% and 98% identity, respectively (Figure 1A). Thus, there is every indication that an A. cajennense population at Três Rios is infected with a specific Paracoccus sp. population, denoted here as “Paracoccus sp-Cayenne”. This bacterial population shows 96% 16S rRNA gene identity to the clinical P. yeeii strain G3060 (GenBank AY014179). PCR products from a total of 38 Três Rios tick samples, positive for the ∼430 bp 16S rDNA amplicon were subjected to additional endonuclease digestion. A RFLP strategy was designed to specifically assign these amplicons to either the Rickettsia or the Paracoccus 16S rDNA group. After analyzing the 16S rRNA gene sequences presented in Figure 1A, we observed that every Rickettsia 16S rRNA gene sequence included in the primer set fD1-Rc16S.452n amplicon generated the same HinfI digestion profile, with the presence of 117 bp, 124 bp and 195 bp bands, whereas all the Paracoccus sequences generated a profile with bands of 25 bp, 100 bp and 297 bp, this including P. sp-Cayenne. All 38 samples from Três Rios showed the typical restriction enzyme digestion profile expected for the Paracoccus group (Figure 2). The 14 Três Rios Cayenne-tick DNA samples were all negative for additional PCR reactions when using primer sets for ompA, ompB, gltA and htrA genes (data not shown), thereby confirming the absence of Rickettsia sp.
Figure 2.
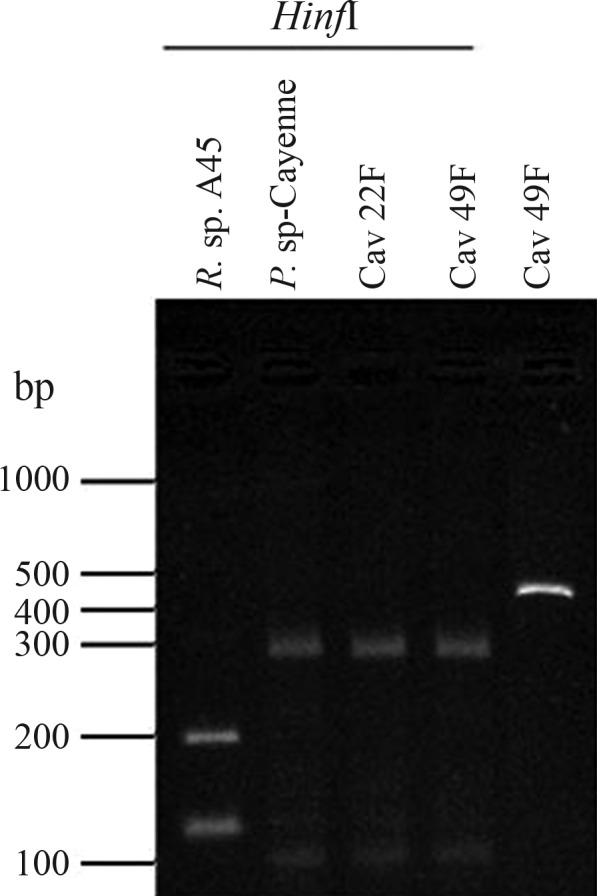
Agarose gel of 16S rDNA PCR products digested with HinfI. PCR reactions were achieved using the Rickettsia spp. specific primer set, as described in the text. Lanes include amplicon digestions for Rickettsia sp. A45 (Lane 1) and Paracoccus sp-Cayenne (Lane 2), as well as the tick-total DNA samples Cav22F and Cav49F (Lanes 3–4). The non-digested PCR product for Cav49F was run in parallel (Lane 5). The molecular marker is presented in base pairs (bp). The expected bands for the Rickettsia sample include the 195 bp, 117 bp and 124 bp bands, the last two appearing as a single band in the gels. The Paracoccus sp-Cayenne expected profile includes the bands of 297 bp and 100 bp, as well as the faint band of 25 bp, not observed in the gels.
The genus Paracoccus comprises metabolically versatile bacteria, which are either aquatic or terrestrial free-living microbes or are associated with other organisms, such as plants, humans and invertebrates, including insects (Baker et al., 1998; Baj, 2000; Daneshvar et al., 2003; Funke et al., 2004; Amarasekare et al., 2008; Huang et al., 2012; Kämpfer et al., 2012; Singh et al., 2012). Interestingly, even though Paracoccus spp. have never been identified in ticks, the application of primers designed for Rickettsia 16S rDNA, followed by sequence analysis and RFLP, revealed the presence of P. sp-Cayenne in ∼77% of the cayenne ticks collected at Três Rios (Table 1). Phylogenetic inference, using partial 16S rDNA sequences, places P. sp-Cayenne into a soil-associated Paracoccus group, closely related to the Paracoccus sp. SA5 strain (Figure 1A). Due to the proximity of P. sp-Cayenne and free-living soil isolated Paracoccus, the possibility of an accidental contamination was assessed, in an attempt to detect Paracoccus sp-Cayenne in soil samples from the same area at Três Rios. Soil samples were collected from four distinct areas used for horse pasture. A mixture of 7 g of each soil sample with 6 mL of TE was shaken, and after particle decantation, DNA was purified by phenol-chloroform or DNeasy Kit (Qiagen, Valencia, CA) extraction. After PCR reactions using Eubacteria universal primers 27f/1492R (Lane, 1991) and a Rickettsia sp. 16S rDNA specific primer set, all samples proved to be positive for Eubacteria, although only one presented positive amplification using the Rickettsia primer set. Subsequent sequence analysis of this DNA sample indicated sequence relationship to a soil Acidobacteria (data not shown). Nevertheless, this geographically localized analysis does not rule out the soil origin of this tick-associated Paracoccus sp.
Over the last two decades, several new members of the Rickettsia genus were identified in association with ticks. Most were classified as tick symbionts, while others were considered EID agents (Parola et al., 1998; Fournier et al., 2000; Beninati et al., 2002; Nilsson et al., 2002). Many of the symbionts were further reclassified as pathogenic agents (Fournier et al., 2000; Nilsson et al., 2002). Within this scenario, R. amblyommii, which was identified in Amblyomma ticks from Brazil and the United States, could possibly represent an unkown ricketsiosis agent (Stothard and Fuerst, 1995; Labruna et al., 2004; Stromdahl et al., 2008), due to a suggestive association of R. amblyommii infections with several cases of a Rocky Mountain spotted-fever-like disease, ascertained by serological assays (Apperson et al., 2008), and the fact that R. amblyommii antigens cross-react with antibodies against R. rickettsii (Walker et al., 2008). In the present study, the presence of Rickettsia sp. A45 DNA in A. dubitatum collected in the Amazon region of São Jose do Xingu (MS), was confirmed. Sequence analysis showed that Rickettsia sp. A45 is closely related to R. amblyommii (Figure 1), which was also recently described in Amblyomma ticks collected in the Amazon (Labruna et al., 2004). Although the pathogenic potential of Rickettsia sp. A45 was not assessed, ompA phylogenetic analysis (Figure 1C) indicated certain similarities to R. amblyommii, through the OmpA protein being directly associated with host-cell adherence and rickettsial pathogenicity (Blanc et al., 2005).
Primer sets for the Rickettsia genes ompA, ompB, htrA, gltA and 16S rDNA have been collectively and successfully applied to diagnose rickettsial infection (Regnery et al., 1991; Fournier et al., 2000; Simser et al., 2002; Pacheco et al., 2007; Phan et al., 2011; Radulovic et al., 2011; Zou et al., 2011). However, our results suggest a limitation in the use of the 16S rDNA primer set fD1-Rc16S, since PCR reactions using these primers also revealed the presence of P. sp-Cayenne in approximately 77% of A. cajennense ticks collected at Três Rios (Figures 1A and 2). This suggests certain limitations when rickettsial 16S rRNA specific primers are solely used to assess Rickettsia infection in a biological sample.
Phylogenetic inference clustered P. sp-Cayenne 16S rDNA sequences with soil Paracoccus sp. Interestingly, the phylogenetic reconstruction did not keep soil, invertebrate associated, and clinical Paracoccus clusters robustly apart (bootstrap values < 80%) (Figure 1A), thereby reflecting an intimate phylogenetic relatedness grouping all these organisms (Baker et al., 1998). A robust Paracoccus cluster was observed, keeping the marine group with the isolates P. sp. JLT1284, P. homiensis and P. sp. RA19 apart. It is important to note that the human-infectious P. yeeii cluster is not only closely related to the insect-associated Paracoccus marcusii cluster, but is often detected in patients with leg or foot wounds (Daneshvar et al., 2003; Funke et al., 2004), thus, a possible indication of vector bites. Recently a P. yeeii infection, associated with a peritonitis in a habitual horse rider (Wallet et al., 2010), could be correlated with the presence of P. sp-Cayenne encountered in horse-fed ticks at Três Rios. Also recently, several uncultured bacteria phylotypes with 16S rRNA gene sequences 99% identical to P. sp-Cayenne, were identified as composing the skin microbiota associated with human dermatitis (Grice et al., 2009; Kong et al., 2012) and infecting gastrointestinal tissues (Frank et al., 2007) (data not shown). It is not clear whether P. sp-Cayenne represents a potential pathogen, or is capable of competing with, or even exacerbating, other human tick-borne infectious agents. Even though possibly tick symbionts, other recognized symbiotic bacteria in Ixodidae are also potentially pathogenic (Burgdorfer et al., 1973). Although Paracoccus sp. was not specifically isolated in this study, this is the first report of Paracoccus sp. detection in ticks, thereby indicating that this bacterial group is harbored in Brazilian tick populations. Future studies should focus on isolating this Paracoccus sp. and on infected tick metagenomics, to clearly define both its pathogenic potential and/or its role in promoting aspects of A. cajennense biophysiology.
Acknowledgments
We thank Dr. Hector Seuanez and Dr. Miguel A.M. Moreira (Instituto Nacional do Câncer/Disvisão de Genética – Rio de Janeiro, Brazil) for sequencing support, Manoel Itamar do Nascimento for providing ticks, and Maria de Fátima S. Cardoso, Luiz F.P. Frade and Sílvio P. Nascimento for technical assistance. This research was financially supported in part by Brazilian federal agencies CNPq and CAPES.
Footnotes
Associate Editor: Ana Tereza Vasconcelos
References
- Amarasekare KG, Mannion CM, Osborne LS, Epsky ND. Life history of Paracoccus marginatus (Hemiptera, Pseudococcidae) on four host plant species under laboratory conditions. Environ Entomol. 2008;37:630–635. doi: 10.1603/0046-225x(2008)37[630:lhopmh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Apperson CB, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick borne disease in North Carolina: Is Rickettsia amblyommii a possible cause of rickettsiosis reported as Rocky Mountain Spotted Fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- Baj J. Taxonomy of the genus Paracoccus. Acta Microbiol Pol. 2000;49:185–200. [PubMed] [Google Scholar]
- Baker SC, Ferguson ST, Ludwig B, Page MD, Richter OMH, van Spanning RJM. Molecular genetics of the genus Paracoccus: Metabolic versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev. 1998;62:1046–1078. doi: 10.1128/mmbr.62.4.1046-1078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninati T, Lo N, Noda H, Esposito F, Rizzoli A, Favia G, Genchi C. First detection of spotted fever group rickettsiae in Ixodes ricinus in Italy. Emerg Infect Dis. 2002;8:983–986. doi: 10.3201/eid0809.020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W, IV, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari, Ixodidae) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc G, Ngwamidiba M, Ogata H, Fournier PE, Claverie JM, Raoult D. Molecular evolution of Rickettsia surface antigens: Evidence of positive selection. Mol Biol Evol. 2005;22:2073–2083. doi: 10.1093/molbev/msi199. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Brinton LP, Hughes LE. Isolation and characterization of symbionts from the rocky mountain wood tick, Dermacentor andersoni. J Invertebr Pathol. 1973;22:424–434. doi: 10.1016/0022-2011(73)90173-0. [DOI] [PubMed] [Google Scholar]
- Daneshvar MI, Hollis DG, Weyant RS, Steigerwalt SG, Whitney AM, Douglas MP, Macgregor JP, Jordan JG, Mayer LW, Rassouli SM, et al. Paracoccus yeeii sp. nov. (Formerly CDC Group EO-2), a novel bacterial species associated with human infection. J Clin Microbiol. 2003;41:1289–1294. doi: 10.1128/JCM.41.3.1289-1294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Grunnenberger F, Jaulhac B, Gastinger G, Raoult D. Evidence of Rickettsia helvetica infection in humans, eastern France. Emerg Infect Dis. 2000;6:389–392. doi: 10.3201/eid0604.000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke G, Frodl R, Sommer H. First comprehensively documented case of Paracoccus yeei infection in a human. J Clin Microbiol. 2004;42:3366–3368. doi: 10.1128/JCM.42.7.3366-3368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage KL, Gilmore RD, Karstens RH, Schwam TG. Detection of Rickettsia rickettsii in saliva, hemolymph and triturated tissues of infected Dermacentor andersonii ticks by polymerase chain reaction. Mol Cell Probes. 1992;6:333–341. doi: 10.1016/0890-8508(92)90010-u. [DOI] [PubMed] [Google Scholar]
- Galvão MA, Mafra CL, Moron C, Anaya E, Walker DH. Rickettsiosis of the genus Rickettsia in South America. Ann NY Acad Sci. 2003;990:57–61. doi: 10.1111/j.1749-6632.2003.tb07337.x. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes E, Leite RC, Prata MC, Pacheco RC, Walker DH, Labruna MB. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever-endemic area in the state of Minas Gerais. Mem Inst Oswaldo Cruz. 2005;100:841–845. doi: 10.1590/s0074-02762005000800004. [DOI] [PubMed] [Google Scholar]
- Huang S, Sheng P, Zhang H. Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (Coleoptera, Scarabaeidae) Int J Mol Sci. 2012;13:2563–2577. doi: 10.3390/ijms13032563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpfer P, Lai WA, Arun AB, Young CC, Rekha PD, Martin K, Busse HJ, Chen WM. Paracoccus rhizosphaerae sp. nov., a novel species isolated from the rhizosphere of a plant Crossostephium chinense (L.) Makino (Seremban) Int J Syst Evol Microbiol. 2012. (Ahead of Print). [DOI] [PubMed]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, NISC Comparative Sequence Program et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LMA, Camargo EP, Popov V, Walker DH. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon, Brazil. J Med Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rDNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematic. Wiley and Sons; Chichester: 1991. pp. 115–175. [Google Scholar]
- Lemos ERS. Rickettsial diseases in Brazil. Virus Rev Res. 2000;7:7–16. [Google Scholar]
- Machado-Ferreira E, Piesman J, Zeidner NS, Soares CAG. Francisella-like endosymbiont DNA and Francisella tularensis virulence-related genes in Brazilian ticks (Acari, Ixodidae) J Med Entomol. 2009;46:369–374. doi: 10.1603/033.046.0224. [DOI] [PubMed] [Google Scholar]
- Machado-Ferreira E, Dietrich G, Hojgaard A, Levin M, Piesman J, Zeidner NS, Soares CAG. Coxiella symbionts in the Cayenne tick Amblyomma cajennense. Microb Ecol. 2011;62:134–142. doi: 10.1007/s00248-011-9868-x. [DOI] [PubMed] [Google Scholar]
- Marin PA, Schmidtman ET. Isolation of aerobic microbes from Ixodes scapularis (Acari, Ixodidae), the vector of Lyme disease in the eastern United States. J Econ Entomol. 1998;91:864–868. doi: 10.1093/jee/91.4.864. [DOI] [PubMed] [Google Scholar]
- Márquez FJ, Muniain MA, Soriguer RC, Izquierdo G, Rodriguez-Bano J, Borobio MV. Genotypic identification of an undescribed spotted fever group rickettsia in Ixodes ricinus from southwestern Spain. Am J Trop Med Hyg. 1998;58:570–577. doi: 10.4269/ajtmh.1998.58.570. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Ficher ER, Porcella SF, Schwan TG. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl Environ Microbiol. 1997;63:3933–3940. doi: 10.1128/aem.63.10.3933-3940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K, Pahlson C, Lukinius A, Eriksson L, Nilsson L, Lindquist O. Presence of Rickettsia helvetica in granulomatous tissue from patients with sarcoidosis. J Infect Dis. 2002;9:592–595. doi: 10.1086/339962. [DOI] [PubMed] [Google Scholar]
- Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco RC, Moraes-Filho J, Nava S, Brandao PE, Richtzenhain LJ, Labruna MB. Detection of a novel spotted fever group rickettsia in Amblyomma parvum ticks (Acari, Ixodidae) from Argentina. Exp Appl Acarol. 2007;43:63–71. doi: 10.1007/s10493-007-9099-5. [DOI] [PubMed] [Google Scholar]
- Parola P, Beati L, Cambon M, Raoult D. First isolation of Rickettsia helvetica from Ixodes ricinus ticks in France. Eur J Clin Microbiol Infect Dis. 1998;17:95–100. doi: 10.1007/BF01682163. [DOI] [PubMed] [Google Scholar]
- Phan JN, Lu CR, Bender WG, Smoak RM, 3rd, Zhong J. Molecular detection and identification of Rickettsia species in Ixodes pacificus in California. Vector Borne Zoonotic Dis. 2011;11:957–961. doi: 10.1089/vbz.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic Z, Chochlakis D, Tomanovic S, Milutinovic M, Tselentis Y, Psaroulaki A. First detection of spotted fever group Rickettsiae in ticks in Serbia. Vector Borne Zoonotic Dis. 2011;11:111–115. doi: 10.1089/vbz.2009.0254. [DOI] [PubMed] [Google Scholar]
- Regnery RL, Spurill CL, Pilikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, Whitt DD. Bacterial Pathogenesis: A Molecular Approach. 2nd edition. AMS Press; Washington D.C: 2002. p. 537. [Google Scholar]
- Sexton DJ, Muniz M, Corey GR, Breitschwerdt EB, Hegarty BC, Dumler S, Walker DH, Pecanha PM, Dietze R. Brazilian spotted fever in Espírito Santo, Brazil: Description of a focus of infection in a new endemic region. Am J Trop Med Hyg. 1993;49:222–226. doi: 10.4269/ajtmh.1993.49.222. [DOI] [PubMed] [Google Scholar]
- Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TG, Munderloh UG. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microbiol. 2002;67:546–552. doi: 10.1128/AEM.68.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh ST, Priya NG, Kumar J, Rana VS, Ellango R, Joshi A, Priyadarshini G, Asokan R, Rajagopal R. Diversity and phylogenetic analysis of endosymbiotic bacteria from field caught Bemisia tabaci from different locations of north India based on 16S rDNA library screening. Infect Genet Evol. 2012;12:411–9. doi: 10.1016/j.meegid.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Stothard DR, Fuerst PA. Evolutionary analysis of the spotted fever and typhus group rickettsiae using 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:52–61. [Google Scholar]
- Stromdahl EY, Vince MA, Billingsley PM, Dobbs NA, Williamson PC. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15–24. doi: 10.1089/vbz.2007.0138. [DOI] [PubMed] [Google Scholar]
- Wallet F, Blondiaux N, Foy CL, Loiez C, Armand S, Pagniez D, Courcol RJ. Paracoccus yeei: A new unusual opportunistic bacterium in ambulatory peritoneal dialysis. Int J Infect Dis. 2010;14:173–174. doi: 10.1016/j.ijid.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Walker HD, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am. 2008;92:1345–1361. doi: 10.1016/j.mcna.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Williams SG, Sacci JB, Schriefer ME, Anderson EM, Fujioka KK, Sorvillo FJ, Barr AR, Azad AF. Typhus and thyphus like Rickettsiae associated with oposums and their fleas in Los Angeles country, California. J Clin Microbiol. 1992;30:1752–1762. doi: 10.1128/jcm.30.7.1758-1762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yparraguirre LA, Machado-Ferreira E, Ulmmann AJ, Piesman J, Zeidner NS, Soares CAG. A hard tick relapsing fever group spirochete in a Brazilian Rhipicephalus (Boophilus) microplus. Vector Borne Zoonotic Dis. 2007;7:717–721. doi: 10.1089/vbz.2007.0144. [DOI] [PubMed] [Google Scholar]
- Zou Y, Wang Q, Fu Z, Liu P, Jin H, Yang H, Gao H, Xi Z, Liu Q, Chen L. Detection of spotted fever group Rickettsia in Haemaphysalis longicornis from Hebei Province, China. J Parasitol. 2011;97:960–962. doi: 10.1645/GE-2751.1. [DOI] [PubMed] [Google Scholar]