Abstract
The objective of this study was to evaluate the reproductive risk associated with exposure of adult male Fisher-344 rats to inhaled benzo(a)pyrene (BaP). Rats were assigned randomly to a treatment or control group. Treatment consisted of sub-chronic exposure of rats via inhalation to 75μg BaP/m3, 4 hours daily for 60 days, while control animals were unexposed (UNC). Blood samples were collected immediately after the cessation of exposures (time 0) and subsequently at 24, 48, and 72 hrs, to assess the effect of bioavailable BaP on plasma testosterone and luteinizing hormone (LH) concentrations. Rats were sacrificed after the last blood collection. Testes were harvested, weighed and prepared for histology and morphometric analysis, and cauda epididymides were isolated for the determination of progressive motility and density of stored spermatozoa. BaP exposure reduced testis weight compared with UNC (Mean ± SE; 2.01 ± 0.11 vs. 3.04 ± 0.16 g; P< 0.025), and caused significant reductions in the components of the steroidogenic and spermatogenic compartments of the testis. Progressive motility and mean density of stored spermatozoa were reduced (P< 0.05). Plasma testosterone concentrations were decreased by two-thirds in BaP-exposed rats throughout the time periods studied compared with those of their UNC counterparts (P< 0.05), concomitant with increased concentrations of LH in BaP-exposed rats (P< 0.05). These data suggest that sub-chronic exposure to inhaled BaP contribute to reduced testicular and epididymal function in exposed rats.
Keywords: Polycyclic aromatic hydrocarbons, Benzo(a)pyrene, Inhalation, Sperm motility, Testosterone, Luteinizing hormone, Epididymis
1. Introduction
In roughly 50% of infertile couples, the male factor is partially responsible for the failure to conceive (Jarow and Zirkin, 2005). Of the known causes of male infertility, 50% are potentially correctable with therapy (Greenberg et al., 1978). The remaining known causes such as genetic/chromosomal abnormalities, gonadotoxin exposure, and testicular trauma are not amenable to therapy. In approximately 25% of men evaluated, no identifiable cause of their abnormal semen analyses can be found; hence the diagnosis of idiopathic male factor infertility (Jarow and Zirkin, 2005) and could be secondary to exposure(s) to environmental/occupational reproductive endocrine disruptors. Reproductive endocrine disruptors can alter the hypothalamo/hypophyseal and testicular hormones that regulate spermatogenesis and epididymal sperm maturation. One such endocrine disruptor that is prevalent in the environment and reduces plasma testosterone concentrations and epididymal function is benzo(a)pyrene (BaP; Inyang et al., 2003). Benzo(a) pyrene is a high molecular weight, semi volatile, lipophilic compound that belongs to the polycyclic aromatic hydrocarbon (PAH) family. This prototypical representative of PAHs is ubiquitous in the environment, hazardous waste sites (around 600 on the National Priority List for cleanup; ATSDR, 1995) and in foodstuffs due to their formation and subsequent deposition by the incomplete combustion of fossil fuels, wood and other organic matter. Inhalation, consumption of contaminated foods and water are the relevant routes of exposure of humans to PAHs (Ramesh et al., 2004).
Polycyclic Aromatic Hydrocarbons cause toxicity in several organs including gonads (Inyang et al., 2003, Knuckles et al., 2001, Archibong et al., 2002) after metabolic activation to electrophilic intermediates. These intermediates are capable of covalent binding to cellular macromolecules such as DNA, RNA, or proteins before their deleterious effects ensue (Baird and Ralston, 1997). The first step in metabolic activation of PAHs occurs via microsomal cytochrome P450-dependent monooxygenase. The epoxides formed may either spontaneously rearrange, undergo hydrolysis by epoxide hydrolase, or be conjugated by related enzymes to benzo(a)pyrene 7,8-dihydrodiol, 9,10-epoxide (BPDE), which require recycling of PAH-diols through the microsomal monooxygenase system (Baird and Ralston, 1997). Ramesh et al. (2002) demonstrated significant levels of BaP metabolites in the testis of exposed rats. The substantial presence of BaP and some of its metabolites in the male gonads even after 8 hours (oral), or 4 hours (inhalation) post-exposure (Ramesh et al., 2002), suggests their incorporation into the Leydig lipogenic tissue and possible alteration of normal steroidogenic and spermatogenic function. We have shown that sub-acute exposures of adult male rats to BaP results in reduced plasma total testosterone concentrations and stored sperm motility among BaP-exposed compared with control rats (Inyang et al., 2003). The lack of effect on testis weight and stored sperm density observed in the latter study, suggests inadequate duration of exposure of rats to BaP to effect observable decline in testicular exocrine function.
The objective of this study was to assess the effect of sub-chronic exposure of adult male rats (60-days, a period encompassing one sperm cycle in the aforementioned species; (Working and Chellman, 1993) to BaP on their indices of fertility.
2. Materials and methods
Animals and exposure
Adult male Fisher-344 rats, approximately 12–13 weeks of age and weighing approximately 340–360g, were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were housed in pairs, in polyethylene cages and allowed to acclimatize to the animal care facilities for one week prior to initiation of studies. Rats were maintained in an environmentally controlled room with a 14 hour light: 10 hour dark cycle (lights on at 6.0 AM.), 22°C and humidity range of 50–60% and allowed ad libitum access to commercial rat chow (5001 Lab meal, Ralston Purina Co., MO, USA) and water. Before initiation of animal exposures to BaP via inhalation, all rats were acclimated to 52 port Cannon nose-only exposure chambers, 4 hrs a day for 3 days. Subsequently, rats were randomly assigned to a treatment and a control group (N = 10 per group). The group size was selected on the basis of consensus-study protocols for toxicity testing for environmental agents developed or codified by several organizations, including United States Environmental Protection Agency (USEPA) and the Organization for Economic Co-operation and Development (OECD; NRC, 2006). Treatment consisted of sub-chronic exposure of rats to 75 μg BaP/m3 via nose-only inhalation, 4 hrs daily for 60 days, using a State-Of-The-Art dual-component aerosol generator developed in our laboratory (Hood et al., 2000). Carbon black (CB) was used as a carrier for BaP because it adsorbs and strongly binds to PAHs (Accardi-Dey and Gschwend, 2003) and was not known to be mutagenic or carcinogenic in mammalian systems (Anon, 2004). Rats in the control group served as unexposed controls (UNC). We did not control for the carrier of BaP (CB) because of the lack of effect of sub-acute (Archibong et al., 2002, Inyang et al., 2003) and sub-chronic exposures to CB (Anon, 2004; unpublished data) on the endocrine and reproductive characteristics of rats. Even though rats in the UNC group were unexposed, they were subjected to conditions of restraint similar to those imposed on rats exposed to BaP via inhalation. In short, the UNC animals were placed in the same nose-only exposure tubes and the tubes placed in sham exposure units. This setup ensured that both the controls and BaP-exposed animals were subjected to identical exposure conditions. This study was approved by the Animal Care and Use Committee of Meharry Medical College, Nashville, TN and conforms to the guidelines of the U.S. EPA (1989) and the European Union (1992) for conducting inhalation exposures. Details on the design, fabrication, installation, and characterization of the exposure system are reported in Hood et al. (2000). The methods for aerosol generation, preparation of carbon black cakes and impactor substrates as well as the characterization and quantitation of BaP aerosol and substrate extraction, analysis and quality assurance/control are detailed in our previous study (Inyang et al., 2003).
Control and BaP-exposed rats were weighed prior to initiation of exposures, followed subsequently by weekly weight (BW) monitoring throughout the duration of the study to determine whether BaP affected thriftiness among treated animals.
Post-exposure processing of tissue samples
Blood samples were collected via sinus orbital puncture using heparinized pulled Pasteur pipettes, between 17:00 and 18:00 h immediately following the last exposure (on day 60; 0 hr) and at 24, 48 and 72 hrs post initial sampling into heparinized polyethylene tubes. Subsequently, plasma was harvested post centrifugation at 2000-x g at 4°C for 10 minutes from each sample and stored at −20°C until assayed for testosterone and luteinizing hormone (LH). Following the last blood sampling, animals were sacrificed by CO2 asphyxiation, testes harvested and weighed. Other organs of interest in this study (lungs, liver and epididymides) were also harvested. The right testis, lungs and liver were used for the determination of BaP metabolite concentrations and aryl hydrocarbon hydroxylase (AHH) activity, while the left testes were processed for histologies and morphometric analysis according to the method of Lunstra et al. (1986). Briefly, fixed testis pieces in 3.8% formaldehyde in 1% phosphate-buffered saline (PBS, pH 7.4; Protocol fixative solution, Fisher Scientific Inc.) at room temperature. Following embedding in paraffin wax, serial sections (5 μm) were cut from the middle of each testis preparation. Sections were dried overnight onto glass slides at 37°C and stored at room temperature (26 ± 2°C) until processed for histology. For staining and morphometric evaluation, sections were deparaffinized in Microclear (2 × 5min; Micron Environmental Industries, Fairfax, VA) and rehydrated through graded ethanol (2 × 100%, 2 × 95%, 1 × 70%). Sections were rinsed thoroughly in water, stained with haematoxylin, dehydrated, cleared in Microclear and mounted using DPX mounting media (Fluka). Stained sections were stored at room temperature until morphometric analysis was conducted. Stored spermatozoa were recovered from the cauda epididymides of each animal for the determination of sperm motility and density as described below.
Morphometry & Histopathology
Stained sections were evaluated at 50× magnification using a Zeiss Axioplan-2 Photomicroscope equipped with planaphotochromatic objectives for differential interference contrast microscopy coupled with a computerized morphometric planimetry system (Bioquant Nova 2000 Advanced Image Analysis, R&M Biometrics, Nashville, TN) to obtain tubule diameters and area percentages (volume percentages) occupied by seminiferous tubules and interstitium. Two full cross-sections from near the middle of each testis were used in these evaluations. Four areas randomly selected to represent each quadrant on each section were evaluated. Briefly, images from an Optronics DEI-750 triple-CCD color camera attached to the microscope were displayed on a high resolution (1600 lines/inch) color video monitor and the tubular components of the parenchyma were measured by tracing outlines of whole seminiferous tubules (about 60 tubules per rat) using a computer mouse connected to a high-resolution digital pad. Thus, approximately 10 × 106 μm2 was evaluated per testis (approximately 1.2 × 106 μm2 evaluated per quadrant). For all morphometric calculations, specific gravity of the testicular tissue was assumed to be 1.0, and area percentages were assumed equal to volume percentages (Weibel, 1973). No correction factor for shrinkage was applied, since the samples used for comparison were fixed and processed into paraffin blocks using identical conditions. Morphometric assessments included the evaluation of the percentage tubular volume, percentage interstitial volume, average tubule diameter, and percentage tubules displaying elongated spermatids (i.e., full spermatogenesis), were conducted as described previously (Jiménez-Severiano et al., 2005; Okwun et al., 1996). The volume percentage of seminiferous tubules was multiplied by testicular volume per paired testes to obtain total tubular volume, and then divided by average area per round tubule profile, using the formula for volume of a cylinder, to obtain total length of seminiferous tubules per paired testes (Okwun et al., 1996).
Extraction and analysis of tissues for benzo(a)pyrene metabolites
Right testes, lungs and liver samples retrieved from BaP-exposed and UNC rats were subjected to liquid-liquid extraction with deionized water, chloroform and methanol. Subsequently, the metabolites from the extracts were identified and measured by a reverse-phase HPLC equipped with a UV and a fluorescence detector as detailed in Ramesh et al. (2001).
Aryl hydrocarbon hydroxylase (AHH) assay
Microsomes were isolated from lung, liver and testis homogenates as outlined in Ramesh et al. (2000) and expressed as nMol 3(OH) BaP/min/mg of microsomal protein. Microsomal protein concentrations were determined according to the method of Bradford (1976). The AHH activity of the sub-cellular preparations was assayed according to the method of Yang et al. (1978).
Recovery of stored spermatozoa and the determination of sperm progressive motility and sperm density
Highly motile spermatozoa were harvested from excised cauda epididymides per rat according to the method of Slott and Perreault (1993). Briefly, both ends of excised cauda epididymides were clamped to increase pressure within the tubules, following which, epididymal tubules were punctured in blood capillary-free zones. Exuding spermatozoa were scooped with sterile Pasteur pipette and placed in 2 ml of pre-equilibrated Armstrong’s medium (Evans and Armstrong, 1984) at 37°C in an atmosphere of 5% CO2 in air in a 35 × 10 mm Petri dish (dish A; Becton Dickinson, Franklin Lakes, NJ). Remnant cauda epididymides were minced in 2 ml of pre-equilibrated Armstrong’s medium (at conditions mentioned above) in a 35 × 10 mm Petri dish (Becton Dickinson, Franklin Lakes, NJ) and allowed to incubate for 5 minutes to permit spermatozoa to swim out of the epididymal tubules (dish B) following which, cauda epididymal fragments were removed. Percentage sperm progressive motility was determined in each dish A by phase contrast microscopy. Spermatozoa were considered progressively motile if they moved from one point to another. In order to determine stored sperm density per paired epididymides, sperm preparations in dishes A and B were pooled per rat and sperm density was determined as previously described by hemocytometric counting.
Determination of epididymal sperm morphology
Morphologies of recovered stored spermatozoa were assessed with a light microscope on 100 cells according to the criteria of Filler (1993).
Plasma testosterone and LH determination
Plasma samples from UNC and BaP-exposed rats were analyzed for testosterone and LH by radioimmunoassay methods previously validated in our laboratory (Archibong et al., 1987; Inyang et al., 2003). The sensitivity of testosterone assay was 2 pg/tube and the intra-assay coefficient of variation (CV) was 8%. The inter-assay CV for this assay is not available because testosterone concentrations in all samples were determined in a single assay. The sensitivity of LH assay was 0.08 ng/tube and the intra- and inter-assay CVs were 6.5 and 11.0%, respectively.
Statistical analyses
Data on weekly body weights, testosterone and LH concentrations were analyzed by ANOVA with repeated measures and the differences among means were tested with orthogonal contrasts. Those on testis weight, density of stored spermatozoa, sperm morphological forms, BaP metabolite concentrations and AHH activity were compared by unpaired t-test. Data on percentage progressive sperm motility and morphometric assessments of testicular histologies were analyzed by Chi-square and the GLM procedure of SAS Version 6.12 (SAS/STAT, 1990) respectively.
Results
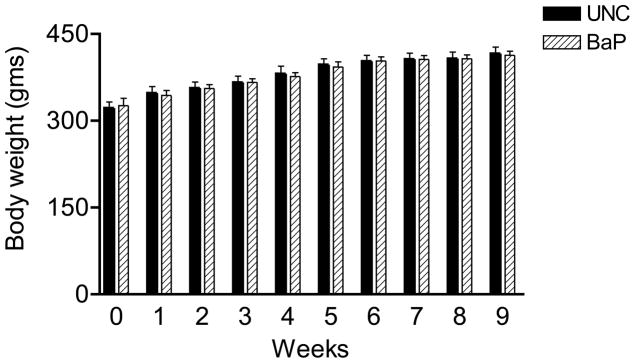
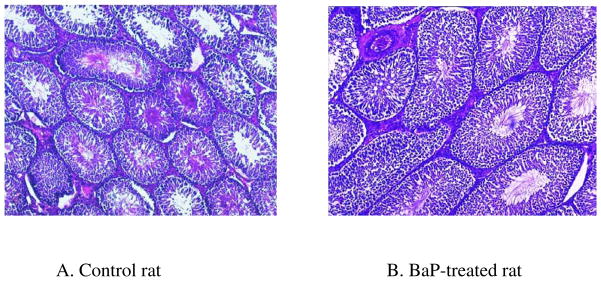
Weekly body weights of UNC and BaP-exposed rats were similar during the nine weeks of this study (Fig. 1). Table 1 depicts the morphometric characteristics rat testis exposed to BaP via inhalation. Testes from BaP-exposed rats weighed 34% less (P< 0.025) than those recovered from their UNC counterparts. Tubule diameter (μM) and percentage of tubules exhibiting elongated spermatids were similar between UNC and BaP-exposed rats. However exposure to BaP caused a reduction in total tubular volume (P<0.002), total weight of tubules (P<0.002) and total tubular length (P<0.01) per paired testes compared with those of their control counterparts. Similarly, both total volume and total weight of interstitium per paired testes were reduced by approximately 12% by exposure of rats to BaP compared with those of testes recovered from control rats (P<0.05). While the shrinkage of seminiferous tubules due to formalin preservation is not discounted, testicular tissue samples from BaP-treated and control rats were left in neutral-buffered formalin for a shorter duration (less than 48 hours) to avoid hardening and ease of sectioning. Short term formalin fixation followed by processing into paraffin wax provided better preservation of structures. Under these conditions, the observed shrinkage was less than 3% in both controls and treated animals compared to rat testes preserved in Bouin’s solution. Thus, the formalin-caused shrinkage artifacts did not impair the functional value of the data considerably. The formalin-caused shrinkage notwithstanding, these morphometric data collectively suggest that exposure of rats to BaP caused reduced testis weight. Some of the above mentioned pathological changes are depicted in the photomicrographs of BaP and UNC testis histologies (Fig. 2).
FIGURE 1.
Weekly weight profile of control and BaP exposed male rats.
Table 1.
Morphometric analysis of testicular parameters in rats inhalationally exposed to 75μg BaP/m3 BaP.
| Parameter | Control | BaP-exposed |
|---|---|---|
| Testis weight (gm/paired testis) | 3.0 ± 0.16 | 2.0 ± 0.11*1 |
| Tubule diameter (μm) | 250 ± 8.5 | 230 ± 8.2*2 |
| Tubules with elongated spermatids (%) | 99 ± 1.0 | 99 ± 1.0 |
| Tubular volume (X 109 μm3) | 2.0 ± 0.7 | 1.6 ± 0.004*3 |
| Total weight of tubules (gm) | 2.20 ± 0.7 | 1.6 ± 0.004 |
| Total tubular length (μm) | 55 ± 1.0 | 33 ± 1.0*5 |
| Total volume of interstitium per paired testis (μm3) | 0.43 ± 0.01 | 0.38 ± 0.01 |
| Total weight of interstitium per paired testis (gm) | 0.45 ± 0.01 | 0.37 ± 0.01*4 |
p < 0.025,
p < 0.09,
p < 0.002,
p < 0.05,
p < 0.01
FIGURE 2.
Photomicrographs of testes histologies from A) unexposed control F-344 rat; B) F-344 rat exposed to sub-chronic concentration of 75 μg benzo(a)pyrene /m3. Magnification bar = 250 μm. Though the seminiferous tubules appear qualitatively similar, the size of tubular lumens and length decreased in BaP-exposed rats, compared with controls. This indicates a reduction in spermatogenic activity and loss of fluid in the seminiferous tubules due to decreased testosterone production, thus the decreased testis size.
The concentrations of BaP metabolites of toxicological interest ([7,8-dihydrodiol; precursor for 7,8-diol 9,10-epoxide] and 3,6-dione) were elevated in the testicular, liver and lungs tissues of BaP-exposed rats relative to the aforementioned tissues recovered from UNC rats. The concentrations of these metabolites also showed a similar trend in liver and lung tissues of BaP-exposed rats compared to those of UNC rats. Furthermore, AHH activity was higher in testicular, liver and lung tissues recovered from BaP-exposed rats compared with that measured in corresponding tissues from UNC rats (Table 2).
Table 2.
Benzo(a)pyrene metabolite concentrations and AHH activities in rats inhalationally exposed to 75μg BaP/m3 BaP.
| BaP 7,8-diol+ (pmol/gm tissue) | BaP 3,6-dione+ (pmol/gm tissue) | AHH activity (nmol 3(OH) BaP/min/mg protein) | |
|---|---|---|---|
| Testis
|
|||
| Control | 0.3 ± 0.02 | 0.5 ± 0.04 | 0.015 ± 0.004 |
| BaP exposed | 17 ± 1.2* | 8.0 ± 0.9* | 0.19 ± 0.04* |
| Lung
|
|||
| Control | 0.1 ± 0.02 | 0.05 ± 0.01 | 0.023 ± 0.005 |
| BaP exposed | 1.0 ± 0.09* | 1.0 ± 0.01* | 0.61 ± 0.05* |
| Liver
|
|||
| Control | 0.3 ± 0.02 | 0.3 ± 0.04 | 0.017 ± 0.004 |
| BaP exposed | 4.0 ± 0.2 * | 4.0 ± 0.4* | 0.21 ± 0.02* |
p < 0.05
It is not unusual to have background levels of BaP metabolites in controls as some of these rats may have been inadvertently exposed to carbon black vapor, the vehicle for BaP and which may contain residual levels of BaP. However, the background levels seen in controls were low, and the metabolite data for BaP-treated rats were corrected for control values.
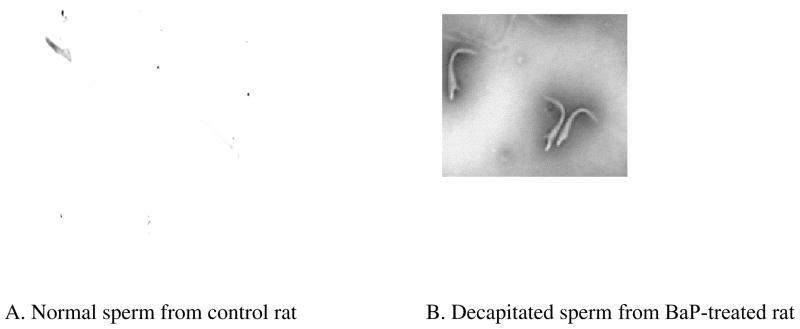
The percentage of progressively motile stored spermatozoa and stored sperm density were lower among BaP-exposed rats than their UNC counterparts (Table 3). Rats exposed to BaP disproportionately produced fewer morphologically normal sperm than their controls counterparts. The mean percentage of the prevalent abnormal morphologic form of spermatozoa present in the cauda epididymides of rats exposed to BaP compared with those of UNC rats (Table 3) was decapitated spermatozoa (Fig. 3). The mean percentage of tail abnormalities was similar between sperm recovered from BaP-exposed and UNC rats (Table 3).
Table 3.
Sperm characteristics in rats inhalationally exposed to 75μg BaP/m3 BaP.
| Characteristic | Control | BaP-treated |
|---|---|---|
| Sperm progressive motility (%) | 85 ± 5.0 | 23 ± 2.0*1 |
| Density of stored spermatozoa (X 106) | 81 ± 4.0 | 25 ± 6.0*1 |
| Morphologically normal sperm (%) | 67 ± 4.0 | 13 ± 3.9*2 |
| Decapitated spermatozoa (%) | 7.2 ± 2.3 | 60 ± 11*3 |
| Sperm with abnormal tail (%) | 26 ± 3.0 | 28 ± 7.0 |
p < 0.05;
p < 0.001;
p < 0.01
FIGURE 3.
Photomicrograph showing A) a normal spermatozoan taken from unexposed F-344 rat; B) spermatozoa with decapitated heads taken from a F-344 rat exposed to sub-chronic exposure concentration of 75 μg benzo(a)pyrene /m3.
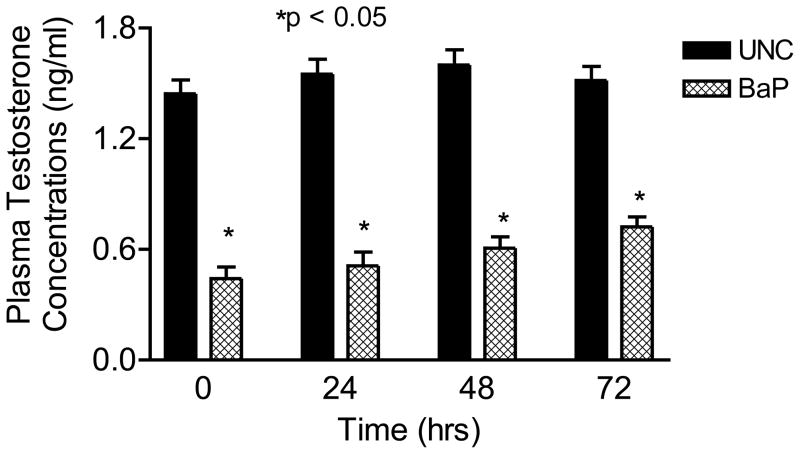
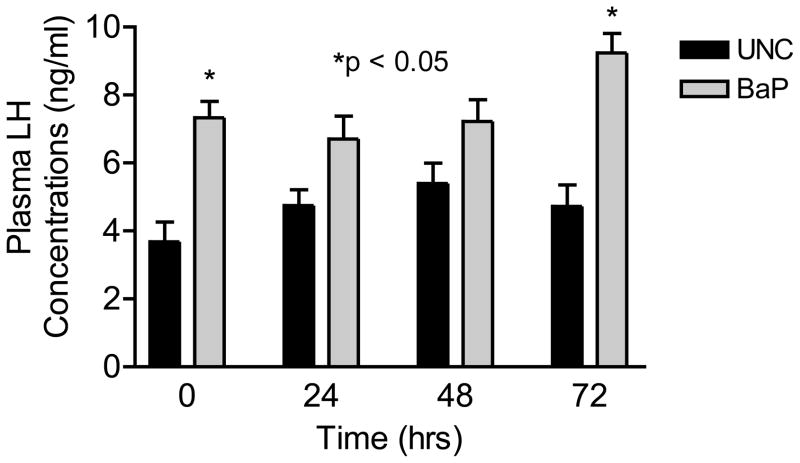
Concomitant with the reduction in the above-mentioned reproductive indices among BaP-exposed rats, plasma testosterone concentrations were also reduced (P<0.05) to approximately one-third of those observed in UNC rats (Fig. 4). On the contrary, LH concentrations were elevated in BaP-exposed rats (P<0.05; Fig. 5) compared with rats in UNC group, throughout the time periods studied.
FIGURE 4.
Effect of inhaled benzo(a)pyrene on plasma testosterone concentrations in F-344 male rats exposed to sub-chronic exposure concentrations of 75 μg BaP/m3; n = 10 per treatment or control group. Results are expressed as mean ± SE. UNC-unexposed control; CB-carbon black control; BaP-exposed rats. Asterisks indicate a significant difference from controls (P < 0.05).
FIGURE 5.
Effect of inhaled benzo(a)pyrene on plasma luteinizing hormone concentrations in F-344 male rats exposed to sub-chronic exposure concentrations of 75 μg BaP /m3; n = 10 per treatment or control group. Results are expressed as mean ± SE. UNC-unexposed control; BaP-benzo(a)pyrene inhaled rats. Asterisks indicate a significant difference from controls (P < 0.05).
Discussion
The exposure concentration of BaP used in this study was based on earlier studies in our laboratory in which a sub-acute exposure concentration of 75μg/m3 reduced circulating gonadal steroid concentrations and sperm motility (Archibong et al., 2002; Inyang et al., 2003). The exposure concentration of BaP used in this study is within the range (10μg–2mg/m3) present in the environment from sources such as: aluminum smelter and coke oven battery industries (WHO, 1998); ambient air of highly polluted industrial cities (Chorazy et al., 1994); cooking oil and wood combustion fumes (Viau et al., 2000); home heating with coal-gas (WHO, 1998). Furthermore, the exposure concentration of BaP used in this study is close to the legally enforceable limit of 100μg/m3 established for PAHs by the Occupational Safety and Health Administration (OSHA, 1998).
The exposure concentration of BaP used in this study did not affect weekly weight gains in BaP-exposed versus UNC rats. These data suggest that inhaled BaP, at the exposure concentration administered and the daily duration of exposures as well as the manner in which animals were restrained, did not impose undue stress on the rats, hence the identical weight gains between BaP-exposed and UNC rats.
We observed a significant reduction in mean testis weight among BaP-exposed rats compared with their UNC counterparts, an observation similar to that made by Blazak et al. (Blazak et al., 1985) for adult rats and by Singh and Tate (Singh and Tate, 1981) in adult hamsters, suggesting toxicity of BaP to the testis (Lipshultz and Corriere, 1977). It is apparent from our testicular morphometric data that BaP exposure reduced components of both the steroidogenic and spermatogenic compartments of the testis but more so the latter compartment compared with controls (12% reduction in both total volume and total weight of interstitium per paired testis [steroidogenic compartment] versus 20% reduction in both tubular volume and tubular weight and 40% reduction in tubular length [spermatogenic compartment]). This is not surprising in as much as the testis bulk (85%; Lipshultz and Witt, 1993) is involved in sperm production; consequently, when seminiferous tubular cells such as Sertoli cells undergo apoptosis due to exposure to BaP (Raychoudhury and Kubinski, 2003), paracrine mediators involved in the regulation of spermatogenesis (Ku and Chapin, 1993) are reduced, leading to a reduction in spermatogenetic support and testicular mass.
The presence of significant concentrations BaP metabolites in the testis, lung and liver of exposed rats compared with controls is similar to our previous findings (Ramesh et al., 2001 and 2002) and those of other investigators (Lee and Nagayama, 1980) in the aforementioned organs of exposed rats. These findings indicate the presence of constitutive and inducible metabolic enzymes for this PAH in the aforementioned organs. Furthermore, the higher activity of Aryl hydrocarbon hydrolase (AHH; a marker for CYP1A1 and CYP1B1 enzymatic activity; Ou and Ramos, 1992) in the above mentioned organs confirms the inherent ability of these organs to metabolize BaP upon exposure. This metabolizing enzyme has been shown to be regulated by the aryl hydrocarbon receptor (AhR) and the AhR nuclear translocator (ARNT) complex (Nebert et al., 2000). The metabolism of BaP by this system induces the formation of reactive metabolites such as BaP diol epoxide, 3,6-dione and 7,8-dione and ultimately, the production of reactive oxygen species (ROS; Senft et al., 2002). It is therefore conceivable that prolonged induction of AHH is associated with the production of high levels of ROS, enough to overwhelm the antioxidant defense systems and consequently, lead to the alteration of endocrine milieu required for normal organ function(s) and in this instance, testosterone regulated epididymal function (Inyang et al., 2003).
Additionally, we observed a significant decrease in mean stored sperm density among BaP-exposed versus UNC rats. This observation is most likely due to BaP-induced reduction in sperm numbers (Tate and Singh, 1981; Revel et al., 2001) transported into the epididymides post spermeation. According to Plant and Marshall (2001), high intratesticular testosterone concentration is required for the regulation of spermatogenesis, the reduction of which results in fewer matured sperm produced by the testis. Furthermore, the reduced concentrations of plasma testosterone among BaP-exposed rats compared with UNC may have caused a rapid regression in the epididymal epithelium, especially in the initial segment and the proximal caput epididymal region (Jones, 2004). As a result, epididymal sperm maturation and survival were adversely affected and during the ensuing weeks, only a few degenerated sperm became present in the cauda epididymides and vas deferens (Jones, 2004). Raychoudhury and Kubinski (2003) reported that in vitro exposure of isolated rat Sertoli cells to BaP resulted in cellular changes characteristic of apoptosis. Although Sertoli cells were not enumerated in this study, the dramatic reduction (39%) of total tubular length in BaP-treated rats implies that a significant reduction in Sertoli cell numbers also occurred during BaP exposure. Sertoli cells initiate, support, maintain and regulate spermatogenesis in mammals by (1) providing structural and functional barrier that regulate the movement of extratubular blood-borne components into the seminiferous tubular environment; (2) synthesizing and secreting various nutrients utilized by developing germ cells; and (3) synthesizing and secreting paracrine mediators proposed to be involved in the regulation of spermatogenesis (Ku and Chapin, 1993). Thus, the reduced stored sperm density observed in this study may have been due to reduced spermatogenesis resulting from BaP-induced reduction in testosterone synthesis and release via ROS-induced oxidative damage to Leydig cells (Senft et al., 2002), DNA damage and apoptosis in spermatozoa and Sertoli cells (Raychoudhury and Kubinski, 2003; Revel et al., 2001).
Interestingly, exposure of rats to inhaled BaP significantly reduced progressive motility among stored sperm compared with that of their control counterparts. The present observation is in agreement with our previous data in which rats were exposed sub-acutely to BaP (Inyang et al., 2003) and to those of Selevan et al. (1995) and Sram et al. (1999) who observed poor semen quality among young men in Czech Republic exposed to air highly polluted with PAHs. The similarity in this important sperm motion characteristic between rats exposed sub-acutely and sub-chronically to BaP, suggests that the reduction in motility of stored sperm by BaP ensued before the 11th day of exposure. This assumption is based on the significant decline in sperm motility among rats similarly exposed to BaP for duration of 10 days (Inyang et al., 2003). Sperm progressive motility is positively correlated with fertilization of oocytes (Donnelly et al., 1998) and pregnancy rates (Jouannet et al., 1988), the decline of which is one of the markers of male reproductive toxicity and also an indicator for the onset of infertility (Perreault, 1997). Post exposures, PAHs undergo an AHH-mediated transformation into reactive metabolites (Ou and Ramos, 1992; Nebert et al., 2000) which subsequently induce ROS production. Reactive oxygen species have been detected in the epididymis (Taylor, 2001) and have been shown to contribute to poor sperm motility (Aitken et al., 1993).
Mature spermatozoa acquire the ability to move progressively during epididymal transit, a process that is regulated by testosterone and dihydrotestosterone (DHT; Klinefelter, 1997). In this study, we observed a significant reduction in total testosterone in BaP-exposed rats compared with controls. This observation is significant because it suggests that the reduced sperm motility observed among treated rats is secondary to reduced epididymal exposure to physiological concentrations of androgens. According to Vinggaard et al. (2000), Kizu et al. (2003), and Charles et al. (2005), PAHs including BaP and/or their metabolites perturb the androgen-dependent processes in target tissues by acting as antiandrogens. Benzo(a)pyrene may interrupt epididymal function by competing for androgen receptors in this organ (Mahony and Hodgen, 1995). Besides androgens, estrogens are also vital for male reproductive function via its receptors, whose synthesis it mediates. Our studies (Archibong et al., 2002) and others (Raj and Katz, 1984) indicate that BaP reduces the synthesis and release of estradiol 17β and as a consequence, a reduction in its receptor population in target organs. Therefore it is conceivable that BaP contributes to testicular and epididymal dysfunction by reducing circulating concentrations of testicular steroids.
In addition to sperm motility, spermatozoa with abnormal morphologies were disproportionately represented in samples of stored spermatozoa from BaP-exposed rats than their control counterparts. This observation is similar to that made by Singh and Tate (1981) and Raj and Katz (1984) in mice exposed to BaP and dimethylbenz(a)anthracene, respectively. In human studies, Sram et al. (1999) demonstrated a significant relationship between air contaminated with PAHs and the production of spermatozoa with abnormal morphologies in the Czech Republic. The reduction in the percentage of normal morphologic forms of spermatozoa may be related to BaP-induced reduction of testosterone synthesis and release. If BaP reduces intra-testicular steroidogenesis substantially during sub-chronic exposure, the level of testosterone reaching the epididymides from general circulation and testicular fluid (Jones, 2004) may not be sufficient to optimally regulate the epididymides. This scenario could lead to a significant reduction in sperm maturation, survival and ultimately to the presence of degenerated spermatozoa in the cauda epididymides and vas deferens (Jones, 2004). The increased percentage of decapitated spermatozoa in the cauda epididymides of BaP-exposed rats compared with UNC could be likened to the degenerating conditions of stored spermatozoa due to chronic deprivation of the epididymides of adequate testosterone. This is comparable to the situation found in seasonal-breeding animals at the time of testicular regression, when large numbers of semi-condensed spermatids and decapitated spermatozoa are present in the epididymis (Millar, 1972).
The circulating testosterone concentrations in control rats in this study are similar to those reported by Laurenzana et al. (Laurenzana et al., 2002; Inyang et al. (2003). The profile of this testicular androgen remained virtually unchanged during the four time periods studied (time 0 to 72 hours post cessation of exposures). On the contrary, circulating testosterone in BaP-exposed rats decreased significantly throughout the above-mentioned time periods and may have resulted from the exposure of Leydig cells to sequestered BaP in high-density lipoproteins that are essential for steroid hormone biosynthesis (Polyakov et al., 1996). Mandal et al. (2001) have demonstrated that DMBA inhibits the ability of Leydig cells to synthesize and release testosterone.
Although the specific mechanism involved in the regulation of this steroid by BaP is not examined in the present study, it could be surmised that the metabolism of BaP by Leydig cells or the BaP metabolites produced elsewhere in the body and transported to the testis may have adversely affected these cells. In this context it is worth mentioning that the tissues in the male reproductive organs and the Leydig cells in particular extensively metabolize PAHs including BaP (Ramesh et al., 2000, 2002; Lee and Nagayama, 1980; Williams et al., 2000). The metabolites of inhaled BaP may have significantly reduced the ability of the Leydig cell to synthesize and release testosterone due to ROS-induced aging (Senft et al., 2002; Mandal et al., 2001). Peltola et al. (1996) have shown that ROS can damage critical components of the steroidogenetic pathway in Leydig cells, including steroidogenic acute regulatory (StAR) protein (Diemer et al., 2003). The reduction in the synthesis and release of testosterone could be exacerbated by a reduction in the population of Leydig cells via apoptosis as exemplified in data on Leydig cells from rats exposed to cigarette smoke (Yardimci et al., 1997). Our data show a decrease in the interstitial volume and weight in BaP-exposed versus UNC rat testis histological preparations, suggesting the loss of Leydig cells. Also, the reactive metabolites of BaP may have disrupted androgen function by reducing androgen receptor levels in testes (Plant and Marshall, 2001), particularly in Sertoli cells. Such a decrease could result in decreased intra-testicular androgen binding proteins. Androgen binding proteins are required for the maintenance of high concentrations of testosterone in the testis, a requirement for the maintenance of optimal spermatogenesis after its initiation by FSH (Plant and Marshall, 2001). A reduction in intra-testicular testosterone normally results in reduced spermatogenesis (Jeyaraj, 2005) and could explain the reduced density of stored spermatozoa among BaP-exposed rats in this study compared with controls. Furthermore, decreased testosterone levels similar to that observed in the present study upon prolonged exposure to BaP may have implications on successful mating of females by BaP-exposed males (Karabelyos and Csaba, 1996). Additionally, it is very likely that the reduction in the circulating testosterone concentrations by BaP may be secondary to the induction of the liver cytochrome P450s that are necessary for detoxification of BaP (Baird and Ralston, 1997).
Decreased plasma concentrations of testosterone in BaP-exposed rats were accompanied by concomitant increases in plasma LH concentrations throughout the time periods studied, indicating that BaP did not have a direct effect on this pituitary gonadotropin, but rather a reflection of BaP-induced Leydig cell failure to respond to LH. The observed elevated plasma concentrations of LH among BaP-exposed versus UNC rats could result from reduced aromatizable androgens to estrogen and as a consequence, the abrogation of estrogen regulated negative feedback on GnRH-induced LH synthesis and release (Rochira et al., 2006). Furthermore, BaP metabolites can induce antiestrogenic condition in the hypothalamus by binding to estrogen receptors and preventing estrogen receptor recycling at the level of the hypothalamus (Arcaro et al., 1999; Hirose et al., 2001). This triggers the nullification of the steroid induced negative feedback on GnRH synthesis and release, concomitant with decreased plasma testosterone during the time periods studied.
The findings of our study strongly suggest that sub-chronic exposure of rats to BaP contributes to impaired testicular endocrine function, altered microenvironment in the epididymis and consequently, a reduction in stored sperm density and sperm motility. Data presented in this study should raise awareness among humans who fall into “at risk” categories for exposure to this PAH, on the ability of BaP to induce adverse endocrine and reproductive outcomes.
Acknowledgments
This work was supported in part by PHS grants No. U50ATU3989-48-06 (Meharry), 2SO6GMO8037-28 & -32, G12RRO3032 (AR, AA, DBH), 1U54HD0431501-09, RO1 HD020419-19S1 (AA), 1R15ES012168 and S11ES014156-01 (AR), NS41070 and S11ES014156-01 (DBH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accardi-Dey A, Gschwend PM. Reinterpreting literature sorption data considering both absorption into organic carbon and adsorption onto black carbon. Environ Sci Technol. 2003;37:99–106. doi: 10.1021/es020569v. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs) US Department of Health & Human Service; Atlanta, Georgia: 1995. p. 458. [PubMed] [Google Scholar]
- Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalyzed lipid peroxidation and human sperm function. J Reprod Fertil. 1993;98:257–265. doi: 10.1530/jrf.0.0980257. [DOI] [PubMed] [Google Scholar]
- Anonymous. Carbon black users guide. Safety, health and environmental information. International Carbon Black Association; 2004. [Google Scholar]
- Arcaro KF, O’Keefe PW, Yang Y, Clayton W, Gierthy JF. Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology. 1999;133:115–127. doi: 10.1016/s0300-483x(99)00018-9. [DOI] [PubMed] [Google Scholar]
- Archibong AE, England DC, Stormshak F. Factors contributing to embryonic mortality in gilts bred at pubertal estrus. J Anim Sci. 1987;64:474–478. doi: 10.2527/jas1987.642474x. [DOI] [PubMed] [Google Scholar]
- Archibong AE, Inyang F, Ramesh A, Greenwood M, Nayyar T, Kopsombut P, et al. Alteration of pregnancy related hormones and fetal survival in F-344 rats by inhaled benzo(a)pyrene. Reprod Toxicol. 2002;16:801–808. doi: 10.1016/s0890-6238(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Baird WM, Ralston SL. Carcinogenic polycyclic aromatic hydrocarbons. In: Bowden GT, Fischer S, editors. Comprehensive Toxicology, vol. 12. Chemical Carcinogens and Anticarcinogens. New York: 2004. pp. 171–200. [Google Scholar]
- Blazak WF, Ernst TL, Stewart BE. Potential indicators of reproductive toxicity: testicular sperm production and epididymal sperm number, transit time, and motility in Fischer 344 rats. Fundam Appl Toxicol. 1985;5:1097–1103. doi: 10.1016/0272-0590(85)90145-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein, utilizing the principle of dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Charles GD, Kan HL, Schisler MR, Gollapudi BB, Marty MS. A comparison of in vitro and in vivo EDSTAC test battery results for detecting antiandrogenic activity. Toxicol Appl Pharmacol. 2005;202:108–120. doi: 10.1016/j.taap.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Chorazy M, Szeliga J, Strozyk M, Cimander B. Ambient air pollutants in upper Silesia: partial chemical composition and biological activity. Environ Health Perspect. 1994;102(Suppl 4):61–66. doi: 10.1289/ehp.94102s461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- Donnelly ET, Lewis SE, McNally JA, Thompson W. In vitro fertilization and pregnancy rates: The influence of sperm motility and morphology on IVF outcome. Fertil Steril. 1998;70:305–314. doi: 10.1016/s0015-0282(98)00146-0. [DOI] [PubMed] [Google Scholar]
- European Union Guideline 92/69/ECC. Acute toxicity inhalation. Office J Eur Comm Legal Spec L383A. 1992;35:117. [Google Scholar]
- Evans G, Armstrong DT. Reduction in fertilization rate in vitro of oocytes from immature rats induced to super ovulate. J Reprod Fertil. 1984;70:131–135. doi: 10.1530/jrf.0.0700131. [DOI] [PubMed] [Google Scholar]
- Filler R. Methods for evaluation of rat epididymal sperm morphology. In: Heindel J, Chapin RE, editors. Male Reproductive Toxicology, Part A. Academic Press; New York: 1993. pp. 334–343. [Google Scholar]
- Greenberg SH, Lipshult LI, Wein RR. Experience with 425 subfertile male patients. J Urol. 1978;119:507–510. doi: 10.1016/s0022-5347(17)57531-x. [DOI] [PubMed] [Google Scholar]
- Hirose L, Morito K, Kizu R, Toriba A, Hayakawa K, Ogawa S, et al. Estrogenic/antiestrogenic activities of benzo(a)pyrene monohydroxy derivatives. J Health Sci. 2001;47:552–558. [Google Scholar]
- Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo(a)pyrene following maternal inhalation. Inhal Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- Inyang F, Ramesh A, Kopsombut P, Niaz MS, Hood DB, Nyanda AM, et al. Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod Toxicol. 2003;17:527–537. doi: 10.1016/s0890-6238(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Jarow JP, Zirkin B. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann NY Sci. 2005;1061:208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- Jeyaraj DA, Grossman G, Petrusz P. Altered bioavailability of testosterone in androgen-binding protein-transgenic mice. Steroids. 2005;70:704–714. doi: 10.1016/j.steroids.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Jiménez-Severiano H, Mussard ML, Fitzpatrick LA, D’Occhio MJ, Ford JJ, Lunstra DD, et al. Testicular development of Zebu bulls after chronic treatment with a gonadotropin-releasing hormone agonist. J Anim Sci. 2005;83:2111–2122. doi: 10.2527/2005.8392111x. [DOI] [PubMed] [Google Scholar]
- Jones R. Sperm survival versus degradation in the mammalian epididymis: a hypothesis. Biol Reprod. 2004;71:1405–1411. doi: 10.1095/biolreprod.104.031252. [DOI] [PubMed] [Google Scholar]
- Jouannet P, Ducot B, Feneux D, Spira A. Male factors and the likelihood of pregnancy in infertile couples. I. Study of sperm characteristics. Int J Androl. 1988;11:379–384. doi: 10.1111/j.1365-2605.1988.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Karabelyos CS, Csaba G. Benzpyrene treatment decreases the sexual activity of adult rats, what is reversed in neonatally allylestrenol treated animals. Acta Physiol Hung. 1996;84:131–137. [PubMed] [Google Scholar]
- Kizu R, Okamura K, Toriba A, Kakishima H, Mizokami A, Burnstein KL, et al. A role of aryl hydrocarbon receptor in the antiandrogenic effects of polycyclic aromatic hydrocarbons in LNCaP human prostate carcinoma cells. Arch Toxicol. 2003;77:335–343. doi: 10.1007/s00204-003-0454-y. [DOI] [PubMed] [Google Scholar]
- Klinefelter GR. The epididymis as a target for toxicants. In: Boekelheide K, Chapin RE, Hoyer PB, Harris C, editors. Comprehensive Toxicology, vol. 10. Reproductive and Endocrine Toxicology. Pergamon Press; New York: 1997. pp. 151–163. [Google Scholar]
- Knuckles ME, Inyang F, Ramesh A. Acute and subchronic oral toxicities of benzo(a)pyrene in F-344 rats. Toxicol Sci. 2001;61:382–388. doi: 10.1093/toxsci/61.2.382. [DOI] [PubMed] [Google Scholar]
- Ku WW, Chapin RE. Preparation and use of Sertoli cell-enriched cultures from 18-day-old rat. In: Chapin RE, Heindel JJ, editors. Male Reproductive Toxicology, Methods in Toxicology. Vol. 3. Academic Press; San Diego: 1993. pp. 210–229. [Google Scholar]
- Laurenzana EM, Balasubramanian G, Weis C, Blaydes B, Newbold RR, Delclos KB. Effect of nonylphenol on serum testosterone and testicular steroidogenic enzyme activity in neonatal, pubertal, and adult rats. Chem-Biol Interact. 2002;139:23–41. doi: 10.1016/s0009-2797(01)00291-5. [DOI] [PubMed] [Google Scholar]
- Lee IP, Nagayama J. Metabolism of benzo(a)pyrene by the isolated perfused rat testis. Cancer Res. 1980;40:3297–3303. [PubMed] [Google Scholar]
- Lipshultz LI, Corriere JN., Jr Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117:175–176. doi: 10.1016/s0022-5347(17)58387-1. [DOI] [PubMed] [Google Scholar]
- Lipshultz LI, Witt MA. Infertility in the male. In: Hammond MG, Talbert LM, editors. Infertility, a practical guide for the physician. Blackwell Scientific Publications; Boston: 1993. pp. 26–55. [Google Scholar]
- Lunstra DD, Ford JJ, Christenson RK, Allrich RD. Changes in Leydig cell ultrastructure and function during pubertal development in the boar. Biol Reprod. 1986;34:145–158. doi: 10.1095/biolreprod34.1.145. [DOI] [PubMed] [Google Scholar]
- Mahony MC, Hodgen GD. Toxic effects on the hypothalamus-anterior pituitary-gonadal axis, control of the male and female reproductive system, and related tissues. In: Witorsch RJ, editor. Reproductive Toxicology. Raven Press Ltd; NewYork: 1995. pp. 195–213. [Google Scholar]
- Mandal PK, McDaniel LR, Prough RA, Clark BJ. 7, 12- Dimethlbenz(a)anthracene inhibition of steroid production in MA-10 mouse Leydig tumor cells is not directly linked to induction of CYP1B1. Toxicol Appl Pharmacol. 2001;175:200–208. doi: 10.1006/taap.2001.9241. [DOI] [PubMed] [Google Scholar]
- Millar RP. Degradation of spermatozoa in the epididymis of a seasonally breeding mammal, the rock hyrax, Procavia capensis. J Reprod Fertil. 1972;30:447–450. doi: 10.1530/jrf.0.0300447. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) Toxicity testing for assessment of environmental agents; interim report. National Academies Press; Washington DC: p. 270pp. [Google Scholar]
- Nebert DW, Roe A, Dieter M. Role of the aromatic hydrocarbon and the Ah gene battery in the oxidative stress response, cell cycle control and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA) Occupational Safety and Health Standards, Toxic and Hazardous Substances. Code of Federal Regulations. 1998;29:CFR 1910.1000. [Google Scholar]
- Okwun OE, Igboeli G, Ford JJ, Lunstra DD, Johnson L. Sertoli cell number and function, spermatogonial number and yield and daily sperm production in three breeds of boars. J Reprod Fertil. 1996;107:137–149. doi: 10.1530/jrf.0.1070137. [DOI] [PubMed] [Google Scholar]
- Ou X, Ramos KS. Modulation of aortic protein phosphorylation by benzo(a)pyrene: implications in PAH-induced atherogenesis. Biochem Toxicol. 1992;7:147–154. doi: 10.1002/jbt.2570070303. [DOI] [PubMed] [Google Scholar]
- Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology. 1996;137:105–112. doi: 10.1210/endo.137.1.8536600. [DOI] [PubMed] [Google Scholar]
- Perreault SD. The mature spermatozoa as a target for reproductive toxicants. In: Boekelheide K, Chapin RE, Hoyer PB, Harris C, editors. Comprehensive Toxicology, vol. 10. Reproductive and Endocrine Toxicology. Pergamon Press; New York: 1997. pp. 165–179. [Google Scholar]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocrine Reviews. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- Polyakov LM, Chasovskikh MI, Panin LE. Binding and transport of benzo(a)pyrene by blood plasma lipoproteins: the possible role of apolipoprotein B in this process. Bioconjugate Chem. 1996;7:396–400. doi: 10.1021/bc960005e. [DOI] [PubMed] [Google Scholar]
- Raj AS, Katz M. Inhibitory effects of alpha- and beta- naphthoflavones on DMBA- induced anomalies in germ cells assessed by sperm normality assay. Mutat Res. 1984;136:81–84. doi: 10.1016/0165-1218(84)90137-x. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Knuckles ME. Aryl hydrocarbon hydroxylase activity in F-344 rats subchronically exposed to benzo(a)pyrene and fluoranthene through diet. J Biochem Mol Toxicol. 2000;14:155–161. doi: 10.1002/(sici)1099-0461(2000)14:3<155::aid-jbt5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckles ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo(a)pyrene in F-344 rats following oral administration. Exp Toxic Pathol. 2001;53:275–290. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Hood DB, Inyang F, Greenwood M, Archibong AE, Knuckles Me, et al. Comparative metabolism, bioavailability and toxicokinetics of benzo(a)pyrene in rats after acute oral, inhalation, and intravenous administration. Polycyclic Aromatic Compounds. 2002;22:969–980. [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider H, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Raychoudhury SS, Kubinski D. Polycyclic aromatic hydrocarbon induced cytotoxicity in cultured rat sertoli cells involves differential apoptotic response. Environ Hlth Perspect. 2003;111:33–38. doi: 10.1289/ehp.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel A, Roanani H, Younglai E, Xu J, Han R, Savouret J-F, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod Toxicol. 2001;15:479–486. doi: 10.1016/s0890-6238(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Rochira V, Zirilli L, Genazzani AD, Balestrieri A, Aranda C, Fabre B, et al. Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. Eur J Endocrinol. 2006;155:513–522. doi: 10.1530/eje.1.02254. [DOI] [PubMed] [Google Scholar]
- SAS/STAT. SAS Institute SAS/STAT Statistical Analysis System User's Guide (Version 6) 5. Cary, North Carolina: Statistical Analysis System Institute, Inc; 1990. pp. 113–709. [Google Scholar]
- Selevan SG, Borkovec L, Zudova Z, Slott V, Hanjova R, Rubes J, et al. Semen quality in young men and air pollution in two Czech communities. Epidemiology. 1995;6(suppl):S85. [Google Scholar]
- Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, et al. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Singh H, Tate F. Antispermatogenic effects of ethylmethanesulfonate and benzo(a)-pyrene in PD4 Lakeview hamsters. J Toxicol Environ Health. 1981;8:929–937. doi: 10.1080/15287398109530127. [DOI] [PubMed] [Google Scholar]
- Slott VL, Perreault SD. Computer-assisted sperm analysis of rodent epididymal sperm motility using the Hamilton-Thorne motility analyzer. In: Chapin RE, Heindel JJ, editors. Male Reproductive Toxicology, Methods in Toxicology. Vol. 3. Academic Press; San Diego: 1993. pp. 219–333. [Google Scholar]
- Sram RJ, Binkova B, Rössner P, Rubeš J, Topinka J, Dejmek J. Adverse reproductive outcomes from exposure to environmental mutagens. Mutat Res. 1999;428:203–215. doi: 10.1016/s1383-5742(99)00048-4. [DOI] [PubMed] [Google Scholar]
- Taylor CT. Antioxidants and reactive oxygen species in human fertility. Environ Toxicol Pharmacol. 2001;10:189–198. doi: 10.1016/s1382-6689(01)00099-0. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Acute Inhalation Toxicity. TSCA. 1989;798:40 CFR 790. [Google Scholar]
- Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health. 2000;73:331–338. doi: 10.1007/s004209900112. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Hnida C, Larsen JC. Environmental polycyclic aromatic hydrocarbons affect androgen receptor activation in vitro. Toxicology. 2000;145:173–183. doi: 10.1016/s0300-483x(00)00143-8. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological techniques for electron microscopic morphometry. In: Hayat MA, editor. Principles and Techniques of Electron Microscopy, Vol 3, Biological Applications. Van Nostrand Reinhold Co; New York: 1973. pp. 237–296. [Google Scholar]
- WHO. Environmental Health Criteria. Vol. 202. World Health Organization; Geneva: 1998. Selected non-heterocyclic polycyclic aromatic hydrocarbons; p. 883. [Google Scholar]
- Williams JA, Martin FC, Muir GH, Hewer A, Grover PL, Phillips DH. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis. 2000;21:1683–1689. doi: 10.1093/carcin/21.9.1683. [DOI] [PubMed] [Google Scholar]
- Working PK, Chellman GJ. The testis spermatogenesis and the excurrent duct system. In: Scialli AR, Zinaman MJ, editors. Reproductive Toxicology and Infertility. McGraw-Hill, Inc; NewYork: 1993. pp. 55–76. [Google Scholar]
- Yang C, Strickhart FS, Kicha LP. Analysis of the aryl hydrocarbon hydroxylase assay. Biochem Pharmacol. 1978;27:2321–2326. doi: 10.1016/0006-2952(78)90138-7. [DOI] [PubMed] [Google Scholar]
- Yardimci S, Atan A, Delibasi T, Sunguroglu K, Guven MC. Long-term effects of cigarette-smoke exposure on plasma testosterone, luteinizing hormone and follicle-stimulating hormone levels in male rats. Br J Urol. 1997;79:66–69. doi: 10.1046/j.1464-410x.1997.28314.x. [DOI] [PubMed] [Google Scholar]