Abstract
Objective
To determine if post-menopausal status is associated with self-reported limitations in physical function.
Methods
SWAN is a multi-site, multi-ethnic, longitudinal study of midlife women. Women aged 45–57 years (N=2,566) completed the Medical Outcomes Study Short-Form Physical Function Scale at visit 4 (2000–2001); scores created a 3-category variable of physical function limitations: none (86–100), moderate (51–85) and substantial (0–50). Menopausal status in SWAN is a 5-category list variable based on menstrual bleeding patterns and gynecological surgery. Pre-and peri-menopausal women using hormones (n=284) or missing physical function scores (n=46) were excluded. Multinomial logistic regression was used to relate physical function and menopausal status adjusting for age, ethnicity, site, education, body mass index (BMI), self-reported diabetes, hypertension, arthritis, depressive symptoms, smoking and hormone use among postmenopausal women.
Results
Of 2,236 women, 8% were pre-, 51% early peri-, 12% late peri-, 24% natural post-, and 5% surgical post-menopausal status. In the full model, substantial limitations in physical function were higher in post-menopausal compared to pre-menopausal women whether it occurred naturally (OR 3.82; 95% CI: 1.46–10.0) or surgically (OR 3.54; 95% CI: 1.15–10.84). These associations were attenuated by higher BMI and depressive symptoms, but remained significant. Moderate limitations in physical function were not significantly related to menopausal status.
Conclusion
Women with surgical or naturally occurring post-menopause reported greater limitations in physical function than pre-menopausal women, independent of age, only partly explained by higher BMI and depressive symptoms. This suggests that physiologic changes of menopause could contribute directly to limitations in physical function.
Keywords: Physical functioning, Functional limitations, Menopause, Menopausal status, SF-36
INTRODUCTION
Health-related quality of life (QOL) is an important mediator of and surrogate marker for future disease, disability, and mortality (1). Physical function, a component of health-related QOL, can be an indicator of the aging process (2, 3) and used as a surrogate marker to measure the overall impact of disease and environment (4). An estimated 54.4 million (18.7%) people living in the U.S. have some level of disability and 35 million (12%) have severe disability (5). The impact of limitations in physical function has been extensively studied in older adults ≥ 65 years (6–10).
Of women aged ≥ 70 years, 42% report one physical function limitation and 20% report ≥ 4 limitations (11). Although physical function limitations in older women are common, these limitations may first present in mid-life rather than later-life. Even at midlife, more women report functional limitations compared with men (8, 14–15). Approximately 20% of women aged 40–55 years in the Study of Women’s Health Across the Nation (SWAN) telephone survey perceived themselves as having any physical function limitations (12–13).
Menopausal factors and related health factors may be associated with the increased prevalence of physical function limitations among women compared with men, independent of aging. This paper aims to explore the association between menopausal status and limitations in physical function.
METHODS
SWAN/Study Participants
SWAN is a prospective, multicenter, multiethnic, multidisciplinary study of the natural history of the menopausal transition with a baseline enrollment of 3,302 women. Recruitment procedures and study design have been previously described (16). Briefly 3,302 women were recruited as the 1996–1997 baseline cohorts from Boston, Massachusetts, Chicago, Illinois, the Detroit, Michiganarea, Los Angeles, California, Newark, New Jersey, Pittsburgh, Pennsylvania, and Oakland, California. Eligibility criteria included being aged 42–52 years, having a uterus and at leastone intact ovary, having amenstrual period, no use of reproductive hormones within the past 3 months, no current pregnancy, and self-identifying as African American, White, Chinese, Hispanic, or Japanese. Institutional review board approval was granted at each study site; informed consent was obtained from each study participant.
2,566 women participated in Visit 4 (2000–2001), the first visit at which physical function scale of the Medical Outcomes Study Short-Form was administered. Women with missing physical function scores (n=46) or undetermined menopausal status due to hormone use in pre-and peri-menopausal periods (n=284) were excluded, this resulted in an analytic sample of n=2,236. Women on post-menopausal hormone replacement therapy were included in the analytic sample. Women exluded from analyses were on average 50.3 years old, more likely to be White, have post-college education, and from the Chicago, Los Angelesor Pittsburgh site than women included in analyses. Physical function scores did not significantly differ between exluded women and included women. Women exluded because of missing physical function score were more likely to be pre-menopausal or late peri-menopausal than women with recorded physical function score. There was no significant difference between women exluded because of undeterined menopausal status due to hormone use than women with known menopausal status.
Menopausal status/Independent variable
Menopausal status was defined with a 5-level categorical variable based on SWAN classification of participant answers to questions on bleeding patterns (16). Categories were as follows: pre-menopausal (menses in past 3 months with no change in bleeding pattern), early peri-menopausal (menses in past 3 months but decreasing predictability between menses), late peri-menopausal (no menses in past 3–11 months), natural post-menopausal (no menses in past 12 or more months), surgical post-menopausal (history of hysterectomy or had two ovaries removed).
Physical Function/Dependent variable
Physical functioning status was assessed by participant self-report to the 10-item physical function subscale of the Medical Outcomes Study Short-Form 36 (SF-36) (17). Participants reported whether they were limited a lot, a little, or not limited in activities including bathing, dressing, carrying groceries, bending, moderate and vigorous athletic activities, walking, and climbing stairs. Participant responses were calculated using the original coding algorithm in which raw scores were transformed to a physical function score between 0–100 with higher scores representing better physical function (18). Studies have reported that scores on the SF-36 are highly skewed and not normally distributed with many respondents scoring 100 (19–21). To address this ceiling effect, SF-36physical function scores in this SWAN sample was categorized into a 3-level using cutoffs as recommended by Rose et al (21). Women with a score below 50 were classified as having substantial limitations. Those with a score of 51–85 were classified as having moderate limitations. Women with a score of 86–100 were considered to have no limitations in physical function.
Potential Confounders
We included variables we determined to be potential confounders based on previous literature (12–13, 22). Age, race/ethnicity, and education were self-reported by participants. Race/ethnicity was self-identified as African American, White, Chinese American, Hispanic, and Japanese American. Education was defined as less than high school degree, high school degree or GED, some college, college graduate, and post-college education. An interviewer-administered questionnaire also assessed physician diagnoses of diabetes, high blood pressure, and arthritis based on participants’ self-reports. Other chronic conditions such as heart attack, angina, osteoporosis, and cancer were excluded from analyses due to small variable frequencies. Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies-Depression Scale (22) with a cut-point ≥16 indicating current presence of depressive symptoms. Other risk factors examined included body mass index (BMI), hormone use in post menopausal women, and smoking. BMI was categorized into four groups: underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥ 30 kg/m2). Current hormone use among post-menopausal woman was determined by an interviewer-administered questionnaire. Smoking status was defined as current smoker.
Statisical Analysis
The frequencies of the categorical variables and means ± SD of continuous variables were tabulated according to the 3-level categorical dependent physical function variable; statistically significant associations (p<0.05) were evaluated using ANOVA or chi-squared test of homogeneity. The assumption of proportionality was violated (p < 0.05) and precluded the use of ordinal logistic regression models. Thus multinomial logistic regression models were used to determine the association between menopause status as an independent variable and self-reported physical function as the dependent variable.
Variables found to be statistically significant in chi-squared analysis were included in final models (Table 1). Although not significant, age and hormone use were included due to scientific plausibility. Modeling was done in three stages, initially forcing menopausal status, age and hormone use into the model (Model 1); the nadding significant potential confounders thereafter; and subsequently adjusting for race/ethnicity, education, and site (Model 2). Within each stage, the contribution of each covariate was evaluated. Associations between physical function limitation and risk factors were expressed using odds ratios (OR) and 95% confidence intervals (95% CI) by making two separate comparisons among the women: moderate compared with no limitation in physical function and substantial compared with no limitation in physical function. Interactions between race/ethnicity and menopausal status, as well as between BMI categories and menopausal status were assessed in the full models and were found to be not significant. Statistical analyses were conducted with SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
Table 1.
Participants characteristics by categories of physical functioning
| Total (n=2236) | No Limitation (n=1328) | Moderate Limitation (n=661) | Substantial Limitation (n=247) | P-valuea | |
|---|---|---|---|---|---|
|
| |||||
| Demographics | |||||
| Age (Mean ± SD years) | 49.9±2.7 | 49.8±2.6 | 50.0±2.7 | 50.1±2.9 | 0.11 |
| Ethnicity (n (%)) | <.0001 | ||||
| Caucasian | 1049 (46.9%) | 654 (49.3%) | 307 (46.4%) | 88 (35.6%) | |
| African American | 591 (26.4%) | 279 (21.0%) | 202 (30.6%) | 110 (44.5%) | |
| Chinese | 202 (9.0%) | 124 (9.3%) | 67 (10.1%) | 11 (4.5%) | |
| Hispanic | 166 (7.4%) | 115 (8.7%) | 20 (3.0%) | 31 (12.6%) | |
| Japanese | 228 (10.2%) | 156 (11.8%) | 65 (9.8%) | 7 (2.8%) | |
| Education (n (%)) | <.0001 | ||||
| <High school | 138 (6.2%) | 67 (5.1%) | 33 (5.0%) | 38 (15.5%) | |
| High school or GED | 369 (16.7%) | 190 (14.5%) | 119 (18.1%) | 60 (24.5%) | |
| Some college | 711 (32.1%) | 390 (29.7%) | 239 (36.4%) | 82 (33.5%) | |
| College | 472 (21.3%) | 321 (24.4%) | 117 (17.8%) | 34 (13.9%) | |
| Post-college | 526 (23.7%) | 346 (26.3%) | 149 (22.7%) | 31 (12.7%) | |
| Health Factors (n (%)) | |||||
| Body Mass Index (kg/m2) | <.0001 | ||||
| BMI < 18.5 | 31(1.5%) | 20 (1.6%) | 6(1.0%) | 5 (2.3%) | |
| 18.5 ≤ BMI < 25 | 728 (32.6%) | 545 (43.2%) | 158 (25.3%) | 25 (11.3%) | |
| 25 ≤ BMI < 30 | 582 (27.6%) | 386 (30.6%) | 146 (23.4%) | 50 (22.5%) | |
| BMI ≥ 30 | 768 (34.4%) | 312 (24.7%) | 314 (50.3%) | 142 (64.0%) | |
| Diabetes | 109 (4.9%) | 31 (2.4%) | 41 (6.2%) | 37 (15.2%) | <.0001 |
| Hypertension | 461 (20.7%) | 197 (14.9%) | 174 (26.4%) | 90 (36.9%) | <.0001 |
| Arthritis | 336 (15.1%) | 114 (8.6%) | 120 (18.2%) | 102 (41.8%) | <.0001 |
| Depression | 418 (18.9%) | 183 (13.9%) | 137 (21.0%) | 98 (40.2%) | <.0001 |
| Hormone user | 200 (9.0%) | 125 (9.5%) | 54 (8.2%) | 21 (8.6%) | 0.6299 |
| Smoker | 324 (14.6%) | 167 (12.6%) | 98 (14.9%) | 59 (24.1%) | <.0001 |
| Site (n (%)) | <.0001 | ||||
| Michigan | 323 (14.5%) | 123 (9.3%) | 129 (19.5%) | 71 (28.7%) | |
| Boston | 339 (15.2%) | 199 (15.0%) | 102 (15.4%) | 38 (15.4%) | |
| Chicago | 267 (11.9%) | 157 (11.8%) | 85 (12.9%) | 25 (10.1%) | |
| Davis | 354 (15.8%) | 225 (16.9%) | 105 (15.9%) | 24 (9.7%) | |
| UCLA | 389 (17.4%) | 261 (19.7%) | 109 (16.5%) | 19 (7.7%) | |
| New Jersey | 249 (11.1%) | 168 (12.7%) | 41 (6.2%) | 40(16.2%) | |
| Pittsburgh | 315 (14.1%) | 195 (14.7%) | 90 (13.6%) | 30 (12.2%) | |
P-value indicates overall difference across groups, using chi-square test for categorical variables and ANOVA for continuous variables.
RESULTS
Descriptive Analysis
The sample included 2,236 women with an average age of 49.9 years (SD 2.7 years). Almost half of themself-identified as White, 26% African American, 7% Hispanic, 9% Chinese, and 10% Japanese. The majority of women were early peri-menopausal (51.1%), followed by 24% naturally post-menopausal women. Pre-menopausal and surgically post-menopausal women each represented less than 10% of the sample. Overall, 59% reported no limitation in physical function, 30% reported moderate limitation, and 11% reported substantial limitation. Table 1 shows other participant characteristics.
Significant bivariate associations between study variables and physical function limitations are also shown in Table 1. No significant difference in age was observed among the three groups (p>0.05). Ethnicity, education and BMI differed significantly across levels of physical function limitation (p<0.0001). Women who were White or Japanese, have a normal BMI and higher education most commonly reported no limitation and were least likely to report substantial limitation. The reverse trend was noted in women who were Black, obese, and less educated such that the proportions of these women increased with higher degrees of physical function limitation. The presence of chronic health conditions, including diabetes, hypertension, arthritis and depressive symptoms differed significantly across the three categories of physical function (p<0.0001). The prevalence of these chronic health conditions was found to increase with higher limitation in physical function. 2–15% of women with no limitation had a chronic health condition, while 6–26% with moderate limitation and 15–42% with substantial limitation had a chronic health condition. No significant difference among physical function limitation groups in post-menopausal use of hormone therapy was observed (p>0.05).
Table 2 shows the distribution of menopausal status by categories of limitation in physical function. A statistically significant difference in menopausal status was observed across the three categories of physical function limitation (p<0.001). Twenty-seven percent women with no limitation, 29% with some limitation, and 40% women with substantial limitation were post-menopausal (naturally or surgically).
Table 2.
Menopausal status by categories of physical functioning
| Total (n=2236) | No Limitation (n=1328) | Moderate Limitation (n=661) | Substantial Limitation (n=247) | P-valuea | |
|---|---|---|---|---|---|
|
| |||||
| Menopausal status (n (%)) | <.0001 | ||||
| Pre-menopausal | 172 (7.7%) | 123 (9.3%) | 40 (6.1%) | 9 (3.7%) | |
| Early peri-menopausal | 1136 (51.1%) | 698 (52.9%) | 335 (50.9%) | 103 (42.2%) | |
| Late peri-menopausal | 268 (12.1%) | 145 (11.0%) | 90 (13.7%) | 33 (13.5%) | |
| Natural post-menopausal | 532 (23.9%) | 288 (21.8%) | 164 (24.9%) | 80 (32.8%) | |
| Surgical post-menopausal | 114 (5.1%) | 66 (5.0%) | 29 (4.4%) | 19 (7.8%) | |
P-value indicates overall difference across groups, using chi-square test for categorical variables and ANOVA for continuous variables.
Substantial limitation compared to no limitationin physical function
In a multinomial logistic regression model including age and hormone use, women who were surgically post-menopausal, naturally post-menopausal, late peri-menopausal, or early peri-menopausal were significantly more likely to report substantial limitation in physical functioning than pre-menopausal women by 6-fold, 5-fold, 3-fold, and 2-fold, respectively (Table 3, Limited Model). In the fully adjusted model surgically and naturally post-menopausal women continued to be at a 3 times greater odds of having substantial limitations (Table 3, Full Model and Figure 1). While the association between post-menopausal status and substantial limitation was attenuated by BMI and the presence of depressive symptoms, the association remained statistically significant. Underweight, overweight or obese women had 3.9-fold, 2.2-fold, and 4.8-fold greater odds, respectively, of reporting substantial limitations, compared to women with normal BMI. Other factors significantly associated with substantial limitation are diabetes, arthritis, depression, and lower education, but not ethnicity.
Table 3.
The association of menopausal status with substantial limitationa in physical functioning
| Limited Modelb | OR (95% CI) | Full Modelc | OR (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Menopause status | ||||
| Pre menopause | 1.00 (reference) | 1.00 (reference) | ||
| Early peri | 2.06 | (1.01, 4.19) | 2.08 | (0.85, 5.12) |
| Late peri | 3.32 | (1.50, 7.34) | 2.45 | (0.91, 6.62) |
| Natural post | 4.92 | (2.30, 10.54) | 3.82 | (1.46, 10.00) |
| Surgical post | 6.13 | (2.51,14.99) | 3.54 | (1.15, 10.84) |
| Demographics | ||||
| Age (years) | 0.98 | (0.92, 1.04) | 0.99 | (0.92, 1.06) |
| Ethnicity | ||||
| Caucasian | 1.00 (reference) | |||
| African American | 1.37 | (0.88, 2.11) | ||
| Chinese | 1.02 | (0.39, 2.71) | ||
| Hispanic | 0.84 | (0.23, 3.05) | ||
| Japanese | 0.62 | (0.21, 1.81) | ||
| Education | ||||
| <High school | 3.84 | (1.82, 8.11) | ||
| High school or GED | 1.83 | (1.05, 3.20) | ||
| Some college | 1.28 | (0.78, 2.12) | ||
| College | 0.94 | (0.53, 1.68) | ||
| Post-college | 1.00 (reference) | |||
| Health Factors | ||||
| BMI (kg/m2) | ||||
| BMI < 18.5 | 3.95 | (1.21, 12.87) | ||
| 18.5 ≤ BMI < 25 | 1.00 (reference) | |||
| 25 ≤ BMI < 30 | 2.22 | (1.28, 3.83) | ||
| BMI ≥ 30 | 4.84 | (2.86, 8.20) | ||
| Diabetes | 3.24 | (1.68, 6.25) | ||
| Hypertension | 1.29 | (0.87, 1.91) | ||
| Arthritis | 4.76 | (3.23, 7.03) | ||
| Depression | 3.35 | (2.32, 4.83) | ||
| Hormone user | 0.47 | (0.271, 0.81) | 0.66 | (0.35, 1.29) |
| Smoker | 1.43 | (0.93, 2.19) | ||
Substantial limitation defined by SF-36 physical function score below 50.
Reduced model adjusted for age and hormone use.
In addition to the variables presented in this table, full model adjusted for study site.
Figure 1.
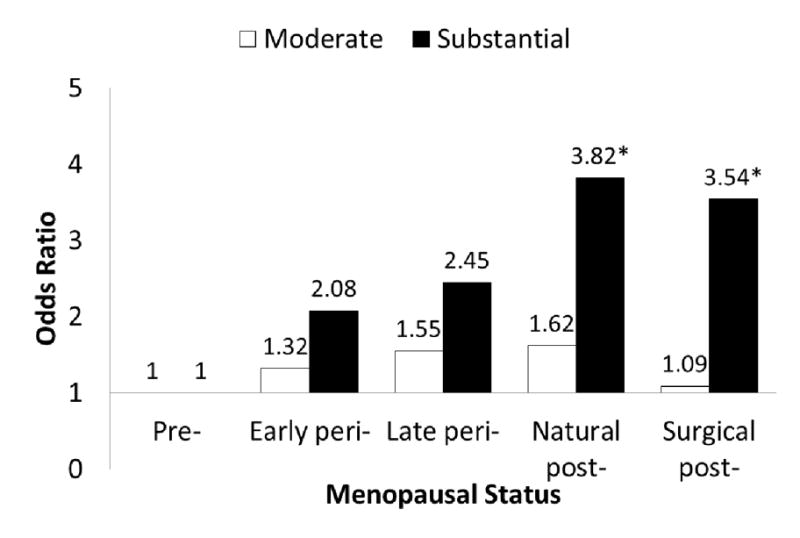
Adjusted odds ratio of reporting moderate or substantial limitation in physical function in early peri-, late peri-, natural post-and surgical post-menopausal women in comparison to pre-menopausal women. Adjusted for age, ethnicity, site, education, body mass index (BMI), self-reported diabetes, hypertension, arthritis, depressive symptoms, smoking and hormone use. *Indicates significant association (p<0.05).
Moderate limitation compared to no limitation in physical function
In a multinomial logistic regression model including age and hormone use women who were naturally post-menopausal and late peri-menopausal were almost twice as likely to report moderate limitation in physical functioning as pre-menopausal women (Table 4, Limited Model). These associations were attenuated by higher BMI. In the fully adjusted model, associations between menopausal status and moderate limitation in physical function were no longer statistically significant (Table 4, Full Model and Figure 1). In comparison to women with normal BMI, those who were over weight were 50% more likely and obese women were over 3 times more likely to report moderate limitations. Other factors including diabetes, hypertension, arthritis, and depressive symptoms were significantly associated with moderate limitations, but not education. Compared to White women, Hispanic women appear to be significantly less likely and Chinese women more likely to report moderate limitations.
Table 4.
The association of menopausal status with moderate limitationa in physical functioning
| Limited Modelb | OR (95% CI) | Full Modelc | OR (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Menopausal status | ||||
| Pre-menopausal | 1.00 (reference) | 1.00 (reference) | ||
| Early peri-menopausal | 1.46 | (1.00, 2.14) | 1.32 | (0.87, 2.02) |
| Late peri-menopausal | 1.86 | (1.17, 2.93) | 1.55 | (0.93, 2.58) |
| Natural post-menopausal | 1.82 | (1.17, 2.85) | 1.62 | (0.99, 2.66) |
| Surgical post-menopausal | 1.59 | (0.86, 2.93) | 1.09 | (0.55, 2.16) |
| Demographics | ||||
| Age | 1.01 | (0.97, 1.05) | 1.01 | (0.97,1.06) |
| Ethnicity | ||||
| Caucasian | 1.00 (reference) | |||
| African American | 0.89 | (0.66, 1.18) | ||
| Chinese | 1.99 | (1.18, 3.37) | ||
| Hispanic | 0.30 | (0.13, 0.73) | ||
| Japanese | 1.15 | (0.70, 1.89) | ||
| Education | ||||
| <High school | 1.71 | (0.96, 3.03) | ||
| High school or GED | 1.33 | (0.94, 1.87) | ||
| Some college | 1.24 | (0.94, 1.65) | ||
| College | 0.89 | (0.62, 1.16) | ||
| Post-college | 1.00 (reference) | |||
| Health Factors | ||||
| Body Mass Index (kg/m2) | ||||
| BMI < 18.5 | 0.90 | (0.35, 2.36) | ||
| 18.5 ≤ BMI < 25 | 1.00 (reference) | |||
| 25 ≤ BMI < 30 | 1.47 | (1.11, 1.94) | ||
| BMI ≥ 30 | 3.35 | (2.51, 4.47) | ||
| Diabetes | 2.05 | (1.16, 3.61) | ||
| Hypertension | 1.35 | (1.02, 1.77) | ||
| Arthritis | 2.10 | (1.53, 2.87) | ||
| Depression | 1.64 | (1.25, 2.16) | ||
| Hormone user | 0.73 | (0.49, 1.09) | 0.79 | (0.51, 1.24) |
| Smoker | 1.08 | (0.78, 1.48) | ||
Moderate limitation defined by SF-36 physical function score of 51–85.
Reduced model adjusted for age and hormone use.
In addition to the variables presented in this table, full model adjusted for study site.
DISCUSSION
Women with surgical or naturally occurring post-menopause are at nearly four-fold higher odds of reporting greater limitations in physical function than pre-menopausal women. Independent risk factors for substantial limitations included BMI, diabetes, arthritis, depressive symptoms and lower education, but not ethnicity. The association between menopausal status and limitations in physical functioning remained significant even after adjusting for age, ethnicity, education and various health factors. This suggests that the physiologic changes of menopause could contribute directly to limitations in physical function.
Our findings are consistent with results from the Michigan Bone Health and Metabolism Study which indicated post-menopausal women had reduced levels of functioning and greater rates of change in performance-based measures (23). The SWAN cross-sectional telephone screening of 14,217 women aged 40–55 years also found an association between menopause and physical functioning, but was limited by using a sample that included women ineligible for SWAN and incomplete data on physical function and chronic health conditions (13). We expanded on these findings by including women enrolled into the SWAN longitudinal cohort. In our analytic sample (Visit 4), the SF-36 was administered to all participating women and included information on diagnosed chronic health conditions.
Based on anextension of the Nagi Model (24–25), we propose a working model of the natural history of physical functioning where them enopausal transition may potentially influence the development of functional limitations and disability. Pathophysiological changes, such as increased ratio of fat mass to lean mass (26), increased visceral fat (27), and bone mass loss (28), that occur during them enopausal transition may result in a cascade of events where active pathology leads to impairment, then functional limitation, and ultimately disability.
A hallmark of the menopausal transition is the dramatic reduction in estrogen, creating a state of “active pathology.” Studies have shown that women have an accelerated decline in strength around the time of menopause, which suggest sex hormones may be the underlying cause of gender differences in muscle strength. For example, men had gradual decreases in knee extensor and handgrip strength between 20 to 80 years of age, whereas women had a steep decline after the age of 55 (29). Another study reported no gender differences in the strength of the adductor pollicis muscle until menopause, when peri-menopausal and post-menopausal women had accelerated strength loss (30). Changes in body composition that occur during the menopause transition may predispose women to the development of physical function limitations and future disability. It may also partly explain why women have an increased prevalence of physical function limitations compared to men. A longitudinal study showed that both chronological age and ovarian age contributed to increased fat mass and loss of skeletal muscle massin women transitioning to menopause (26). The altered relation between muscle and fat may place menopausal women at a biomechanical disadvantage. Increased fat content within skeletal muscle has been shown to influence muscle strength. A study of men and women 70–80 years of age found that reduced muscle density and increased lipid accumulation within muscle were associated with lower muscle strength. They also observed that skeletal muscle lipid content was higher with increasing age, particularly in women (29).
Women with diabetes, hypertension, arthritis and depressive symptoms were at statistically significant 1.3–2.1 greater odds to be moderately limited (Table 4) and 3.2–4.8 times more likely to be substantially limited (Table 3) than women without these conditions, even after adjustment for age, race, education, hormone use, smoking and site. Stronger associations between chronic health conditions and substantial limitations in comparison to moderate limitations in physical function are consistent with what has been shown in the SWAN telephone survey (12). We identified numerous health factors as independent factors for limitation in physical function. High BMI is a well-known predictor of poor physical function. In this sample of women, those with low or high BMI had a significantly greater likelihood of having substantial limitations in physical function. High BMI may place these women at higher risk of developing chronic health conditions such as diabetes, hypertension and arthritis. Conversely, low BMI may indicate the presence of chronic disease as well. Our results show that diabetes, arthritis, and depressive symptoms are significantly associated with both moderate and substantial limitations. Additionally, high blood pressure was significantly associated with moderate limitations. However, these conditions individually could not explain the increased likelihood of post-menopausal women reporting substantial limitations.
This study utilized a sizable and diverse sample of 2,236 mid-life women from five race/ethnic groups. In comparison to White women, Chinese women were more likely and Hispanic women less likely to report moderate limitation, but these trends were not observed among those who reported substantial limitation. Definitive conclusions should be withheld given the small sample size of ethnic groups among physical function categories.
In regards to education, women who completed high school or less, had statistically significant 1.8–3.8 greater odds of reporting substantial limitation (Table 3). This finding suggests the importance of targeting women of lower socioeconomic status when developing interventions to improve health outcomes in aging women.
The findings of our analyses should be interpreted with several factors in mind. First, this cross-sectional analysis cannot establish a cause-effect relationship nor address the temporal relationship between physical function decline and transition through the menopause. Yet, this study confirms the importance of better understanding women with limitations in physical functioning during the menopausal transition. Second, physical function categories were generated from self-reported responses to the SF-36 survey. Objective physical function measures were not available to compare subjective and objective findings in visit 4. However, objective measures of physical function including balance, gait speed and grip strength will be collected beginning with visit 12 (2009–2011). Future evaluation of subjective and objective measures of physical function will be possible. Nonetheless, previous studies indicate that self-reports of functional limitations in both men and women are reproducible (30) and accurate (31). Thus self-reports of physical function limitations in this study are likely a true reflection of physical function disability.
Little information exists on the natural history of physical function limitation. To determine whether changes during the menopausal transition lead to physical function decline needs to be addressed in future longitudinal studies of mid-life women. This study utilized information from visit 4, the earliest visit where self-reported limitation in physical function data within the SWAN longitudinal cohort is available. By demonstrating an association between menopausal status and self-reported limitation in physical function, our study sets the stage for a longitudinal SWAN study to investigate whether physiologic changes of menopause, including altered body composition, could contribute directly to limitations in physical function.
CONCLUSION
Women with surgical or naturally occurring post-menopause are at nearly four-fold higher odds of reporting greater limitations in physical function than pre-menopausal women, independent of age, only partly explained by higher BMI and depressive symptoms. This suggests that physiologic changes of menopause could contribute directly to limitations in physical function.
Acknowledgments
Funding/support: Lisa A. Tseng was supported by a Clinical Research Fellowship from the Doris Duke Charitable Foundation via the University of Pittsburgh School of Medicine and by Pittsburgh Training in Geriatrics and Gerontology Grant #: T32 AG021885. Anne B. Newman was supported by R01-AG-023629 and University of Pittsburgh Claude D. Pepper Older American Independent Center P30-AG-024827.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical centers: University of Michigan, Ann Arbor: MaryFran Sowers, principle investigator (PI); Massachusetts General Hospital, Boston, MA: Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999-present; Rush University, Rush University Medical Center, Chicago, IL: Lynda Powell, PI 1994–2009; Howard Kravitz, PI 2009; University of California, Davis/Kaiser: Ellen Gold, PI; University of California, Los Angeles: Gail Greendale, PI; University of Medicine and Dentistry-New Jersey Medical School, Newark: Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004-present; and the University of Pittsburgh, Pittsburgh, PA: Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD: Marcia Ory 1994–2001; Sherry Sherman 1994-present; National Institute of Nursing Research, Bethesda, MD, Program Officers.
Central Laboratory: University of Michigan, Ann Arbor: Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating center: New England Research Institutes, Watertown, MA: Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA: Kim Sutton-Tyrrell, PI 2001-present.
Steering committee: Chris Gallagher, chair; Susan Johnson, chair.
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Financial disclosure/conflicts of interest: None reported.
References
- 1.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993 Apr 15;137(8):858–869. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM. Assessing physical function in older populations. In: Wallace RBWR, editor. The Epidemiologic Study of the Elderly. New York: Oxford University Press; 1992. [Google Scholar]
- 3.Schultz-Larsen K, Avlund K, Kreiner S. Functional ability of community dwelling elderly. Criterion-related validity of a new measure of functional ability. J Clin Epidemiol. 1992 Nov;45(11):1315–1326. doi: 10.1016/0895-4356(92)90172-j. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM, Kaplan GA. Predictors of healthy aging: prospective evidence from the Alameda County study. Am J Public Health. 1989 Jun;79(6):703–708. doi: 10.2105/ajph.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brault M. In: Americans with disabilities: 2005. Reports CP, editor. Washington, DC: U.S. Department of Commerce, Bureau of the Census; 2008. [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly-Hayes M, Jette AM, Wolf PA, D’Agostino RB, Odell PM. Functional limitations and disability among elders in the Framingham Study. Am J Public Health. 1992 Jun;82(6):841–845. doi: 10.2105/ajph.82.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddox GL, Clark DO. Trajectories of functional impairment in later life. J Health Soc Behav. 1992 Jun;33(2):114–125. [PubMed] [Google Scholar]
- 9.Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. J Gerontol A Biol Sci Med Sci. 1997 Jan;52(1):M19–26. doi: 10.1093/gerona/52a.1.m19. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009 Jan;64(1):76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y, McGee DL, Cao G, Cooper RS. Recent changes in the health status of the older U.S. population: findings from the 1984 and 1994 supplement on aging. J Am Geriatr Soc. 2001 Apr;49(4):443–449. doi: 10.1046/j.1532-5415.2001.49089.x. [DOI] [PubMed] [Google Scholar]
- 12.Pope SK, Sowers MF, Welch GW, Albrecht G. Functional limitations in women at midlife: the role of health conditions, behavioral and environmental factors. Womens Health Issues. 2001 Nov-Dec;11(6):494–502. doi: 10.1016/s1049-3867(01)00089-5. [DOI] [PubMed] [Google Scholar]
- 13.Sowers MF, Pope S, Welch G, Sternfeld B, Albrecht G. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc. 2001;49:1485–1492. doi: 10.1046/j.1532-5415.2001.4911241.x. [DOI] [PubMed] [Google Scholar]
- 14.CDC. Prevalence of disabilities and associated health conditions--United States, 1991–1992. MMWR Morb Mortal Wkly Rep. 1994 Oct 14;43(40):730–731. 737–739. [PubMed] [Google Scholar]
- 15.Ross CE, Bird CE. Sex stratification and health lifestyle: consequences for men’s and women’s perceived health. J Health Soc Behav. 1994 Jun;35(2):161–178. [PubMed] [Google Scholar]
- 16.Sowers MF, Crawford S, Sternfeld B, et al. Design, survey sampling and recruitment methods of SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 17.Ware J. Measures for a New Era of Health Assessment. In: Stewart ALWJ, editor. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. pp. 3–11. [Google Scholar]
- 18.Ware J. Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. SF-36 Health Survey. [Google Scholar]
- 19.Achat H, Kawachi I, Spiro A, 3rd, DeMolles DA, Sparrow D. Optimism and depression as predictors of physical and mental health functioning: the Normative Aging Study. Ann Behav Med. 2000 Spring;22(2):127–130. doi: 10.1007/BF02895776. [DOI] [PubMed] [Google Scholar]
- 20.Michael YL, Colditz GA, Coakley E, Kawachi I. Health behaviors, social networks, and healthy aging: cross-sectional evidence from the Nurses’ Health Study. Qual Life Res. 1999 Dec;8(8):711–722. doi: 10.1023/a:1008949428041. [DOI] [PubMed] [Google Scholar]
- 21.Rose MS, Koshman ML, Spreng S, Sheldon R. Statistical issues encountered in the comparison of health-related quality of life in diseased patients to published general population norms: problems and solutions. J Clin Epidemiol. 1999 May;52(5):405–412. doi: 10.1016/s0895-4356(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 22.Tomey K, Sowers MR, Harlow S, Jannausch M, Zheng H, Bromberger J. Physical functioning among mid-life women: associations with trajectory of depressive symptoms. Soc Sci Med. 2010 Oct;71(7):1259–67. doi: 10.1016/j.socscimed.2010.06.037. Epub 2010 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowers M, Tomey K, Jannausch M, Eyvazzadeh A, Nan B, Randolph J., Jr Physical functioning and menopause status. Obstet Gynecol. 2007;110:1290–6. doi: 10.1097/01.AOG.0000290693.78106.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagi SZ. Disability concepts revisited: implications for prevention. In: Pope AM, Tarlov AR, editors. Disability in America: Toward a National Agenda for Prevention. Division of Health Promotion and Disease Prevention, Institute of Meicine. National Academy Press; Washingon, D.C: 1991. pp. 309–327. [Google Scholar]
- 25.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976 Fall;54(4):439–467. [PubMed] [Google Scholar]
- 26.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007 Mar;92(3):895–901. doi: 10.1210/jc.2006-1393. Epub 2006 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010 Mar;18(3):604–10. doi: 10.1038/oby.2009.251. Epub 2009 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008 Jun;32(2):120–6. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- 29.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000 May;29(3):235–42. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- 30.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993 Jan;84(1):95–8. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001 Jun;90(6):2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 32.Rathouz PJ, Kasper JD, Zeger SL, et al. Short-term consistency in self-reported physical functioning among elderly women: the Women’s Health and Aging Study. Am J Epidemiol. 1998 Apr 15;147(8):764–773. doi: 10.1093/oxfordjournals.aje.a009521. [DOI] [PubMed] [Google Scholar]
- 33.Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. J Gerontol A Biol Sci Med Sci. 1997 Jan;52(1):M19–26. doi: 10.1093/gerona/52a.1.m19. [DOI] [PubMed] [Google Scholar]


