Abstract
Circulation time (the transit time for a bolus of blood through the circulatory system) is a potential index of cardiac dysfunction in chronic heart failure (HF). In healthy subjects, circulation time falls as cardiac output (Q) rises during exercise, however little is known about this index in HF. In this study we examined the relationship between lung-to-lung circulation time (LLCT) during exercise in ten HF (53 ± 14 year, resting ejection fraction = 23 ± 8%) and control subjects (51 ± 18 year). We hypothesized that HF patients would have slower LLCT times during exercise when compared to control subjects. Each subject completed two identical incremental exercise tests during which LLCT was measured in one test and Q measured in the other. Q was measured using the open circuit C2H2 washin technique and circulation time measured using an inert gas technique. In HF patients and control subjects, LLCT decreased and Q increased from rest (HF:LLCT = 53.6 ± 8.2 s, Q = 4.3 ± 1.1 l min−1; control: LLCT = 55.3 ± 10.9 s, Q = 4.5 ± 0.5 l min−1) to peak exercise (HF:LLCT = 20.6 ± 3.9* s, Q = 8.8 ± 2.5* l min−1; control:LLCT = 14.9 ± 2.4 s, Q = 16.5 ± 1.2 l min−1; *P < 0.05 vs control). LLCT was significantly (P < 0.05) slower for the HF group when compared to the control group during submaximal exercise and at peak exercise. However, at a fixed Q the HF subjects had a faster LLCT. We hypothesize that the faster LLCT at a fixed Q for HF patients, may be the result of a more intensive peripheral vasoconstriction of non-active beds and a better redistribution of blood flow.
Keywords: Heart failure, Circulation time, Exercise
Introduction
Circulation time is the time taken for blood to travel through the vascular system. A prolonged or lengthening of the circulation time is an often-cited clinical feature of chronic heart failure (HF; Wolff et al. 2005) and a potential index of worsening disease (Andreas 2000; Caples et al. 2005; Hall et al. 1996; Lanfranchi et al. 1999). Recently we also demonstrated that HF patients with longer resting lung-to-lung circulation times (LLCT, i.e., time taken for blood to travel from the pulmonary circulation, through the vascular system and back again) had poorer peak exercise capacity (VO2peak) and cardiac function (Morris et al. 2007).
Currently little is known of what happens to LLCT during exercise in HF. In healthy individuals, the cardiovascular response to incremental exercise is characterized by an increase in cardiac output (Q) and a redistribution of blood flow away from inactive vascular beds toward exercising muscle, both leading to a progressive shortening of the circulation time (Rowell 1993b; Sowton et al. 1968). In individuals with known cardiac dysfunction such as HF patients one would predict that there would be an attenuated fall in the LLCT time during exercise. However, since these patients also have a more aggressive vasoconstriction (Middlekauff et al. 2001; Momen et al. 2004), the LLCT may be preserved.
Few studies have examined changes in LLCT during exercise. One reason for the paucity of studies published to date, has been the difficulty in measuring changes in circulation time during exercise. We have recently developed a simple, non-invasive method for estimating LLCT using the soluble gas acetylene (C2H2). Using this method, we are able to determine the time taken for a bolus of C2H2 to travel from the lungs, through the systemic circulation and then back to the lungs, i.e., the LLCT. This technique was developed from methods for determining cardiac output (Q) using soluble gases (Triebwasser et al. 1977), where recirculation is a well-described phenomenon.
Therefore, the aim of this study was to compare the changes in LLCT during incremental exercise in a group of HF patients with a group of healthy, age and gender-matched controls. We hypothesized that HF patients would have a prolonged LLCT when compared to healthy controls for a given work load or oxygen consumption. To test this hypothesis we measured LLCT and Q during incremental exercise in ten HF patients and ten healthy controls.
Methods
Subject details
The study was approved by the Mayo Clinic Institutional Review Board and all subjects completed a written consent form prior to commencing. Ten HF patients (three females) and ten healthy control subjects (three females) participated in this study. All HF patients had a history of ischemic or dilated cardiomyopathy, stable HF symptoms (>3 months), duration of HF symptoms >3 months, left ventricular ejection fraction ≤40% and a body mass index <35 kg m−2. The characteristics of the HF and control subjects are outlined in Table 1. Patients were recruited from the Mayo Heart Failure Clinic or the Cardiovascular Health Clinic (a preventive and rehabilitative center). Control subjects had normal pulmonary function, were normotensive and had no signs or symptoms of ischemia during an incremental exercise test to exhaustion.
Table 1.
Subject characteristics for the chronic heart failure and healthy control group
| HF | Control | |
|---|---|---|
| Age (year) | 53 ± 14 | 51 ± 18 |
| Male/female | 7/3 | 7/3 |
| BMI (kg m−2) | 26.0 ± 4.7 | 24.5 ± 2.4 |
| MAP (mmHg) | 76 ± 9.2 | 84 ± 9.2 |
| NYHA functional classes | 2.0 ± 0.9 | |
| Q (l min−1) | 4.3 ± 1.1 | 4.5 ± 0.5 |
| Conductance (ml min−1 mmHg−1) | 54 ± 15 | 53 ± 14 |
| Resting EF (%) | 23 ± 8 | |
| LLCT (s) | 53.6 ± 8.2 | 55.3 ± 10.9 |
| Cardiovascular medications | ||
| ACE/A-II receptor blocker | 10/10 | |
| Digoxin | 6/10 | |
| Beta blocker | 9/10 | |
| Diuretic | 7/10 | |
Results are mean ± SD
HF chronic heart failure group, BMI body mass index, MAP mean arterial pressure, NYHA New York Heart Association Classification, Q cardiac output, EF ejection fraction, LLCT lung-to-lung circulation time, ACE angiotensin-converting enzyme inhibitor, A-II angiotensin II
Study design
After familiarization and consent, each subject undertook two incremental exercise tests on an electronically braked cycle ergometer (Lode, Excalibur Sport, Groningen, the Netherlands). During the first test, classical gas exchange measures were made (e.g., oxygen uptake) along with Q. During the second exercise test LLCT was measured. The two incremental exercise tests were undertaken on separate days at approximately the same time of the day.
Incremental exercise test
Each subject completed a step protocol on the cycle ergometer which took approximately 12 min to complete. Subjects initially commenced unloaded cycling for a warm up period of 3–4 min following which the load was increased by approximately 20–25 W every 3 min for the HF patients and 30–60 W every 3 min for the control subjects. The size of each increment in the load was determined prior to the exercise test and was based on the individual’s previous exercise history and body size with individuals that were more physically active and larger undertaking greater increments.
During the incremental exercise test, oxygen uptake (VO2) was measured breath-by-breath using a metabolic measuring system (CPX-D, Medical Graphics Corporation, St Paul, MN, USA). Heart rate and rhythm were monitored throughout (MedGraphics CardiO2, St Paul, MN, USA) the exercise test. The gas exchange and heart rate data were averaged over 30-s intervals. Peak exercise values were calculated as the average of the two highest consecutive 30-s values obtained prior to termination of exercise. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by auscultation at each stage of exercise with the same investigator measuring blood pressure throughout.
Cardiac output was measured at each incremental exercise stage using the open circuit acetylene washin technique (Johnson et al. 2000). This method has previously been validated with direct FICK method in healthy adults (Johnson et al. 2000). Subjects breathed through a pneumotach connected to a non-rebreathing Y valve (Hans Rudolph, Kansas City, MO, USA). The inspiratory port of the non-rebreathing Y valve was connected to a pneumatic switching valve (Hans Rudolph, Kansas City, MO, USA) that allowed switching between room air and a normoxic test gas mixture reservoir containing 0.65% C2H2, 9% helium. Gases were sampled using a mass spectrometer (Perkins Elma, MGA 1100) integrated with custom designed analysis software for the assessment of Q. At the onset of inspiration, subjects were switched from room air to the test gas mixture for eight to ten breaths to allow for the washin of C2H2 and the estimation of Q.
Circulation time measurement
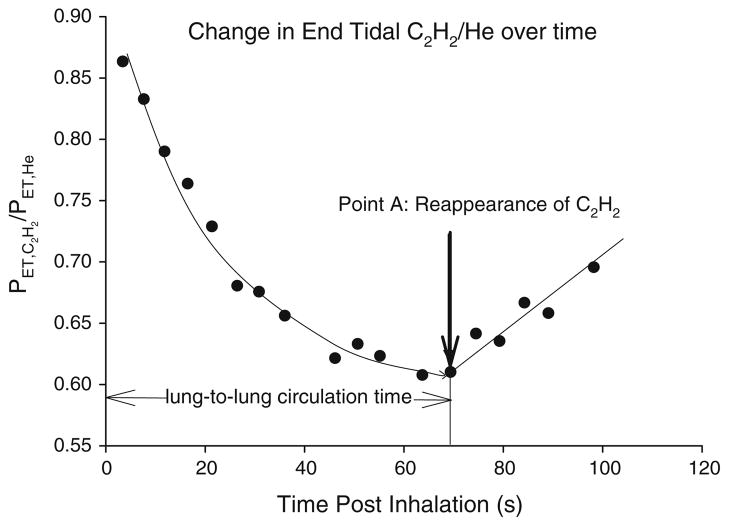
Lung-to-lung circulation time was measured using an acetylene technique which we have previously described (Morris et al. 2007). Briefly, during this procedure, each subject inhaled a single breath of a 0.65% C2H2, 9.0% helium (He), normoxic gas mix. The subject then continued to breathe room air normally. Acetylene is an inert gas that is highly soluble into the blood. Helium is an insoluble tracer gas that is used to account for any breath-by-breath variation in the distribution of C2H2 within the lung. Following inhalation, the PETC2H2/He will fall as a function of the distribution of C2H2 into the pulmonary circulation and subsequently, the systemic circulation. The change in PETC2H2/He following inhalation is shown in Fig. 1. At the point that a significant amount of C2H2 is recirculated, i.e., re-enters the pulmonary circulation from the right side of the heart, PETC2H2/He will start to rise. Using methods described previously (Morris et al. 2007) we defined LLCT from time of the first inhalation of C2H2 to the first point in which PETC2H2/He started to rise again. The intra class correlation coefficients for repeated measures LLCT at rest and during exercise was 0.95 (95% confidence interval 0.82–0.99; Morris et al. 2007).
Fig. 1.
Graphical representative of the change in breath-by-breath end tidal C2H2/He following a single inhalation of 0.9% C2H2, 9% He for a single subject. Following inhalation C2H2/He falls as C2H2 is distributed in the pulmonary blood volume. At the point when a significant volume of C2H2 is returned to the right side of the heart and re-enters the pulmonary circulation, C2H2/He will start to rise. The time taken from inhalation (time 0) until where C2H2/He shows a significant rise and consistent rise was calculated as being the circulation time
Derived measurements
Arterial venous oxygen difference (a-vO2 ) was derived from the measures of oxygen uptake (VO2) and Q using the Fick equation where a-vO2 was equal to the ratio of VO2 to Q. Systemic vascular conductance was determined from the ratio of Q and mean arterial blood pressure (MAP) at rest and each stage of the exercise test. MAP was calculated as 0.33 × (SBP − DBP) + DBP.
Statistical analysis
All data is presented as mean ± SD. Group differences were assessed using independent t tests. Changes in all other dependent measures during incremental exercise were evaluated using a one way repeated measures analysis of variance with the main effects being groups (HF vs control) and time. Bonferroni post hoc tests were used when significant main effects were identified. Relationships between variables were assessed using Pearson correlation coefficients. The statistical significance was set at P < 0.05.
Results
The peak exercise responses for the HF and control subjects are presented in Table 2. At peak exercise, the HF subjects had a significantly lower VO2peak, HRpeak, peak power, peak Q and SVpeak.
Table 2.
Peak exercise responses and chronic heart failure and healthy control group
| HF | Control | |
|---|---|---|
| VO2 (l min−1) | 1.27 ± 0.46* | 2.75 ± 0.88 |
| HR (beats min−1) | 107 ± 16* | 163 ± 14 |
| Power (W) | 88 ± 27* | 212 ± 69 |
| Q (l min−1) | 8.8 ± 2.5* | 16.9 ± 1.3 |
| SV (ml min−1) | 78.1 ± 10.8* | 102.6 ± 27.2 |
| a-vO2 ml dl−1 | 15.3 ± 3.9 | 16.2 ± 2.6 |
| MAP (mmHg) | 92 ± 15 | 108 ± 6.3 |
| Conductance (ml min−1 mmHg−1) | 103 ± 28* | 148 ± 36 |
| LLCT (s) | 20.6 ± 3.9* | 14.9 ± 2.4 |
Results are mean ± SD
HF chronic heart failure group, VO2 oxygen uptake, HR peak heart rate, Q cardiac output, SV stroke volume, a-vO2 arterio-venous oxygen difference, MAP mean arterial pressure, LLCT lung-to-lung circulation time
P < 0.05, HF group less than control group
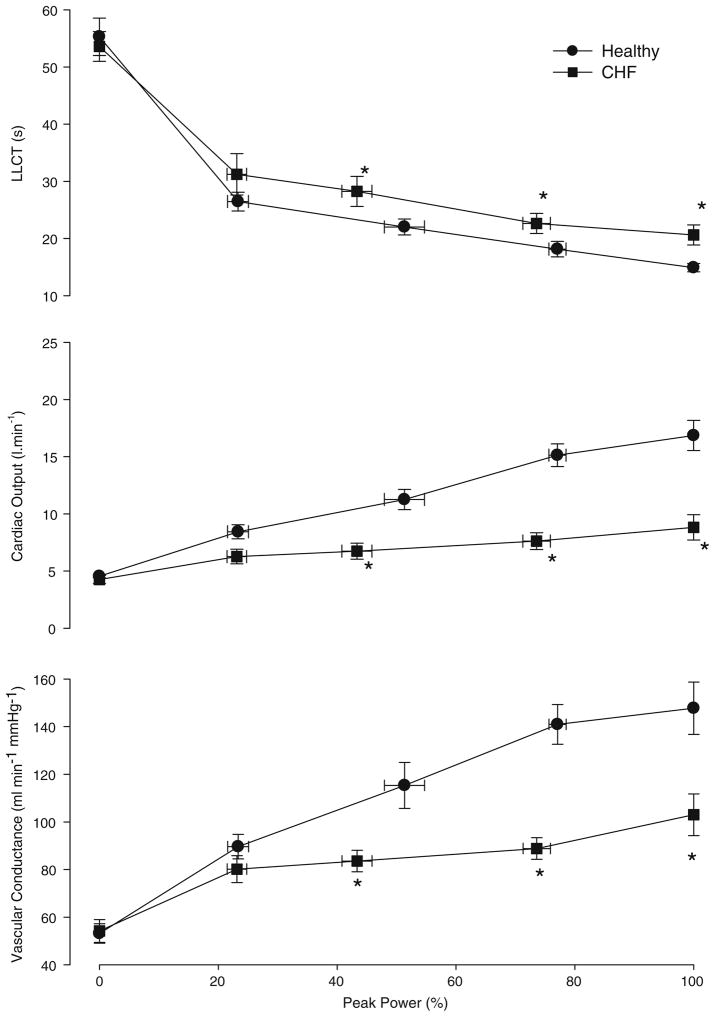
During the incremental exercise test, each subject completed at least three submaximal workload stages and one maximal workload. The changes in LLCT, Q and vascular conductance are shown in Fig. 2a–c and Table 3. For both HF patients and control subjects, LLCT (Fig. 2a) decreased from rest to peak exercise. LLCT was significantly (P < 0.05) longer (slower) for the HF group when compared to the control group during submaximal exercise (stages 2 and 3) and at peak exercise.
Fig. 2.
Changes in dependent measures a function of the percent power in chronic heart failure (HF; filled square) and control (filled circle) subjects. a lung-to-lung circulation time (LLCT), b cardiac output, c vascular conductance. *P < 0.05 HF group significantly different from control group
Table 3.
Hemodynamic changes during submaximal exercise stages in HF and healthy subjects
| Stage | Group | Power (W) | LLCT (s) | Q (l min−1) | Conductance (ml min−1 mmHg−1) |
|---|---|---|---|---|---|
| Rest | HF | 54 ± 3 | 4.3 ± 0.1 | 54 ± 13 | |
| Control | 55 ± 3 | 4.5 ± 0.1 | 53 ± 15 | ||
| Stage 1 | HF | 21 ± 5* | 31 ± 4 | 6.3 ± 0.6 | 80 ± 18 |
| Control | 49 ± 21 | 26 ± 2 | 8.4 ± 0.6 | 90 ± 16 | |
| Stage 2 | HF | 39 ± 11* | 28 ± 3 | 6.7 ± 0.7* | 84 ± 14* |
| Control | 103 ± 38 | 22 ± 1 | 11.3 ± 0.9 | 115 ± 31 | |
| Stage 3 | HF | 69 ± 19* | 23 ± 2 | 7.6 ± 0.7* | 89 ± 14* |
| Control | 164 ± 55 | 18 ± 1 | 15.1 ± 1.0 | 141 ± 26 |
Results are mean ± SD
HF, chronic heart failure group, LLCT lung-to-lung circulation time, Q cardiac output
P < 0.05, HF group less than control group
Q increased significantly from rest to peak exercise for both HF patients and control subjects. During submaximal (stages 2 and 3) and maximal exercise, Q was significantly lower for the HF group when compared to the control group.
For both groups vascular conductance increased from rest and throughout exercise (Fig. 2c). However, for the HF group, the rise in vascular conductance during exercise was significantly less than that for the control group during submaximal exercise (stages 2 and 3) and at peak exercise.
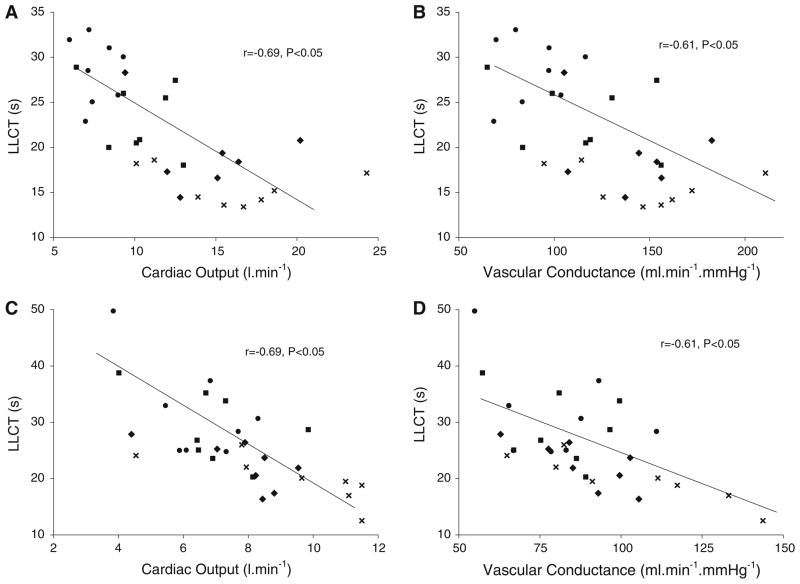
The correlations between LLCT, Q and vascular conductance for the control and HF subjects are shown in Fig. 3. There was a similar and significant correlation between LLCT and Q and between LLCT and vascular conductance for both groups.
Fig. 3.
Correlations between LLCT, Q and vascular conductance during stage 1 to peak exercise for control (a and b) and heart failure (c and d) subjects. (filled circle) Stage 1 exercise, (filled square) stage 2 exercise, (filled diamond) stage 3 exercise, (cross symbol) peak exercise
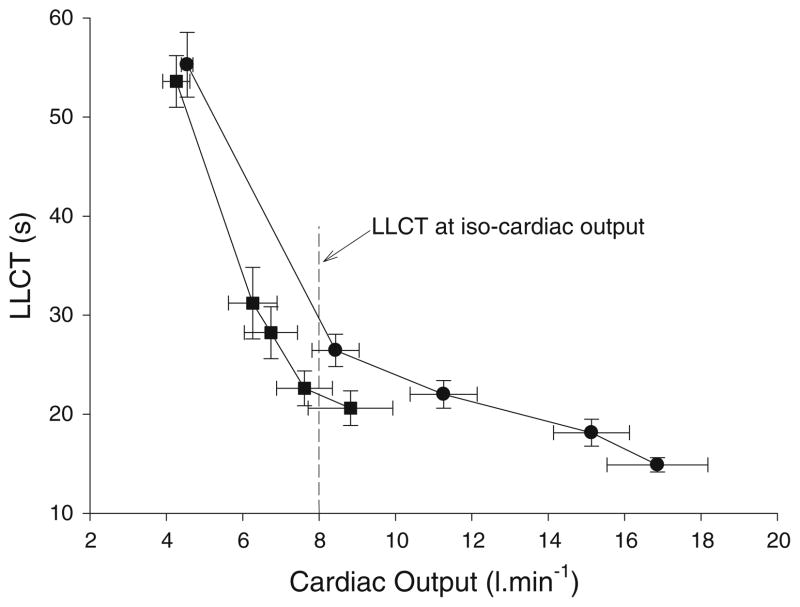
Changes in the LLCT as a function of the rise in Q are shown in Fig. 4. As expected, there was a fall in the LLCT as Q rose during exercise for both groups. However, at a similar Q the HF subjects had a shorter (faster) LLCT. For example, at a Q of 8 l min−1 (iso cardiac output line; Fig. 4), HF patients had a faster LLCT when compared to control subjects.
Fig. 4.
Change in LLCT as function of the change in Q for the heart failure (HF; dark filled square) and control (dark filled circle) groups
Discussion
This study is an extension of our previous work using LLCT as an index of the severity of HF (Morris et al. 2007). In the current study we found that LLCT fell during exercise for both HF and control subjects. We have previously shown that LLCT falls during exercise in healthy subjects (Morris et al. 2007). In a much earlier study, Sowton et al. (1968) reported that recirculation time fell during exercise in a group of patients reported as having ‘myocardial ischemia’. In Sowton et al.’s (1968) study recirculation time was measured using a labeled dye (indocyanine green) introduced into the pulmonary artery. Both lung-to-arterial and total recirculation time were measured. Lung-to-arterial circulation time was determined by taking downstream blood samples from the radial artery while total recirculation time was determined by sampling downstream in the pulmonary artery. While Sowton et al.’s (1968) subjects were not classified as HF, the fact that there was evidence of myocardial ischemia suggests that there may have been some degree of cardiac dysfunction in this group.
Similar to our results, Sowton et al.’s (1968) study found that individuals with myocardial ischemia had longer lung-to-arterial and total circulation times when compared to healthy subjects at rest and during exercise. Hemodynamically, the fall in LLCT for both groups during exercise is due in part to an increase in Q (Morris et al. 2007). LLCT will fall with an increase in Q as a result of an increase blood flow velocity (Rowell 1993a) resulting in faster uptake of C2H2 in the pulmonary circulation and faster transit of C2H2 through the arterial and venous circulations. When exercising at the same relative exercise intensity, we found that HF patients had a lower Q and a prolonged LLCT when compared to healthy subjects. Hence, the lower Q for HF patients during exercise contributes to the longer LLCT.
However, a comparison of the LLCT for the HF and healthy subjects at a fixed Q (Fig. 4) found that HF patients had a faster LLCT. This suggests that when exercising at the same Q, HF patients must redistribute flow so that there is a faster return of blood to the right side of the heart when compared to control subjects.
This result was surprising and we cannot provide a clear explanation for this specific response. Nor was it the aim of this particular study to explore this specific response. However, for the LLCT to be faster during exercise at a similar Q for HF patients, the majority of C2H2 must be distributed into an arterial path with faster transit times and lower vascular resistances and a venous path that favors the return of blood to the right side of the heart. One possible explanation for the faster LLCT is that HF patients aggressively increase the vascular resistance of inactive vascular beds and redirect blood flow away from these beds and toward metabolically active vascular beds—with a lower vascular resistance where there are faster transit times. The redistribution of blood flow away from inactive vascular beds (such as the renal and splanchnic vascular beds) during exercise is well described (Rowell 1993a) and while total systemic conductance increases, this increase is the result of a marked increase in conductance of the active skeletal muscle (Rowell 1993a). Inactive vascular beds have a sympathetically mediated decrease in conductance (increase resistance) resulting in a redirection of blood flow to the exercising skeletal muscle vascular beds (Rowell 1993a). Our results show that HF patients have a smaller rise in vascular conductance during exercise, suggesting that there is a greater general degree of vasoconstriction of vascular beds (Rowell 1993a). This greater vasoconstriction could result in a greater redirection of blood flow to metabolically active beds.
Redistribution of blood flow during exercise appears to be an important mediator of the exercise response in HF, albeit a controversial one. Yamabe et al. (1995) reported that HF was associated with a redistribution of blood flow away from inactive vascular beds to exercising muscles during one-legged incremental exercise. In this study, the ratio of exercising leg blood flow to Q increased during incremental recumbent exercise. Simultaneous measurements of blood flow to the inactive leg showed the ratio of non-exercising leg blood flow to Q decreased during incremental exercise which Yamabe et al. (1995) interpreted as a redistribution of blood flow away from inactive vascular beds.
On the other hand, Sullivan et al. (1989) reported that blood flow to the inactive leg during upright cycling was maintained at the expense of blood flow to the active leg when compared to healthy individuals. As such, these authors concluded that blood flow is not preferentially redistributed during exercise in HF.
While the there is still some debate regarding the presence of a marked redistribution of blood flow during exercise in HF, there is clear evidence that HF patients aggressively vasoconstrict inactive vascular beds during exercise. Recent studies have shown that reflex renal vasoconstriction is exaggerated in both magnitude and duration during dynamic exercise in HF patients (Middlekauff et al. 2001; Momen et al. 2004). In turn there is an increased activation of the renin-angiotensin system (Joyner 2004; Vanhoutte 1996) and an amplification of the already upregulated neurohumoral response seen in HF (Joyner 2004). Sinoway and Li (2005) showed that mechanically and chemically sensitive afferents in the exercising muscle are more likely to fire earlier and more often during dynamic exercise thereby amplifying the sympathetic nervous system response to exercise.
This study is not without limitations due to the non-invasive measures used to determine dependent measures. The open circuit method for measuring Q has been validated in healthy younger and older subjects using direct Fick (Johnson et al. 2000). However the technique has not been validated in HF patients as such this may represent a potential limitation of the project.
In conclusion, this study found that LLCT falls during exercise in both HF and healthy subjects. The fall in LLCT can in part be explained by increase for Q during exercise. However, LLCT at the same Q was faster for HF patients suggesting there was a redistribution of blood flow toward arterial and venous pathways that return blood to the right side of the heart faster. We hypothesize that this may be an example of the greater neurohumoral activation seen in HF patients.
Acknowledgments
The authors of this study would like to thank Kathy O’Malley and Angela Heydmann for their assistance in the data collection and management of this project. This work was supported in part by National Institute of Health grant HL71478, the Heart Foundation Research Centre and Griffith University.
Contributor Information
Norman R. Morris, Email: n.morris@griffith.edu.au, School of Physiotherapy and Exercise Science, Griffith University, Gold Coast Campus, Gold Coast, QLD 4222, Australia
Eric M. Snyder, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
Kenneth C. Beck, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
Bruce D. Johnson, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
References
- Andreas S. Central sleep apnea and chronic heart failure. Sleep. 2000;23(Suppl 4):S220–S223. [PubMed] [Google Scholar]
- Caples SM, Wolk R, Somers VK. Influence of cardiac function and failure on sleep-disordered breathing: evidence for a causative role. J Appl Physiol. 2005;99:2433–2439. doi: 10.1152/japplphysiol.00676.2005. [DOI] [PubMed] [Google Scholar]
- Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1063/1.373866. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Congestive heart failure: more bad news from exercising muscle? Circulation. 2004;110:2978–2979. doi: 10.1161/01.CIR.0000148051.30882.6B. [DOI] [PubMed] [Google Scholar]
- Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol. 2001;90:1714–1719. doi: 10.1152/jappl.2001.90.5.1714. [DOI] [PubMed] [Google Scholar]
- Momen A, Bower D, Boehmer J, Kunselman AR, Leuenberger UA, Sinoway LI. Renal blood flow in heart failure patients during exercise. Am J Physiol Heart Circ Physiol. 2004;287:H2834–H2839. doi: 10.1152/ajpheart.00394.2004. [DOI] [PubMed] [Google Scholar]
- Morris NR, Snyder EM, Beck KC, Haseler LJ, Olson LJ, Johnson BD. The relationship between resting lung-to-lung circulation time and peak exercise capacity in chronic heart failure patients. J Card Fail. 2007;13:389–394. doi: 10.1016/j.cardfail.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Circulatory adjustments to dynamic exercise and heat stress: competing controls. In: Rowell LB, editor. Human cardiovascular control. Oxford University Press; Oxford: 1993a. pp. 363–406. [Google Scholar]
- Rowell LB. Human cardiovascular control. Oxford University Press; Oxford: 1993b. [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- Sowton E, Bloomfield D, Jones NL, Higgs BE, Campbell EJ. Recirculation time during exercise. Cardiovasc Res. 1968;2:341–345. doi: 10.1093/cvr/2.4.341. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RLJ, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium-dependent responses and inhibition of angiotensin-converting enzyme. Clin Exp Pharmacol Physiol. 1996;23:S23–S29. doi: 10.1111/j.1440-1681.1996.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Wolff CB, Checkley SK, Bhageerutty G, Bhatt H, Johnston A, Collier DJ, Tachtsidis I, Garvie N, Rosenberg ME, Benjamin N. Circulation time in man from lung to periphery as an indirect index of cardiac output. Adv Exp Med Biol. 2005;566:311–316. doi: 10.1007/0-387-26206-7_41. [DOI] [PubMed] [Google Scholar]
- Yamabe H, Itoh K, Yasaka Y, Takata T, Yokoyama M. The role of cardiac output response in blood flow distribution during exercise in patients with chronic heart failure. Eur Heart J. 1995;16:951–960. doi: 10.1093/oxfordjournals.eurheartj.a061030. [DOI] [PubMed] [Google Scholar]