Abstract
Background:
This study aimed to determine the association of particulate matters with endothelial function, measured by flow mediated dilation (FMD) of brachial artery, in children with or without exposure to secondhand smoke.
Methods:
This cross-sectional study was conducted from January to March 2011 in Isfahan, which is the second large and air-polluted city in Iran. The areas of the city with lowest and highest air pollution were determined, and in each area, 25 prepubescent boys with or without exposure to daily tobacco smoke in home were selected, i.e. 100 children were studied in total.
Results:
FMD was significantly smaller in those living in high-polluted area and those exposed to secondhand smoke. Multiple linear regression analysis, adjusted for age and body mass index, showed that both passive smoking status and living area in terms of particulate air pollution were effective determinants of the brachial artery diameter. The standardized coefficient of passive smoking status was –0.36 (SD = 0.09, P < 0.0001) showing negative association with percent increase in FMD. Likewise, the percent increase in brachial artery diameter was lower in passive smoker children. Similar relationship was documented for PM10 concentration with a regression coefficient of –0.32 (SD = 0.04, P < 0.0001). Without considering passive smoking variable, PM10 concentration has significant independent effect on FMD level.
Conclusion:
Our findings provide evidence on the association of environmental factors on endothelial dysfunction from early life. Studying such associations among healthy children may help identify the underlying mechanisms. The clinical implications of environmental factors on early stages of atherosclerosis should be confirmed in longitudinal studies.
Keywords: Air pollution, children, endothelium-dependent brachial artery, smoke
INTRODUCTION
There is a growing body of evidence about the potential effects of environmental exposures on the process of atherosclerosis. Air pollution and secondhand smoke are the two most widespread factors in this regard, and share common underlying mechanisms of endothelial dysfunction, arterial stiffness, oxidative stress, inflammation, heart rate variability, platelet aggregation, and energy metabolism.[1,2]
Evidence on harmful effects of secondhand smoke on atherosclerosis is rapidly accumulating.[3,4] Of special concern in this context are the rapid and substantial effects of passive smoking, which are nearly as large as that of long-term active smoking.[5]
Likewise, growing body of knowledge exists on the association of air pollutants, notably particulate matters, with the process of atherosclerosis.[1,6] Such evidence is not limited to adult population, increasing number of reports exist in the pediatric age group.[7,8]
Endothelial dysfunction is a key event and an early feature in the process of atherogenesis. As it precedes clinical vascular events by many years, examining endothelial function may have valuable potential prognostic value for atherosclerotic diseases. Flow-mediated dilation (FMD) of brachial artery is used as a non-invasive measure of endothelial dysfunction.[9]
The association of passive smoking with impaired FMD is well documented in experimental studies,[10] as well as human studies in adult population.[11,12] One of the strongest evidences on the association of secondhand smoke with decreased FMD in young age comes from a cohort of Finnish children, which demonstrated that passive smoking is associated in a dose-dependent manner with significant endothelial dysfunction;[13] frequent exposure to secondhand smoke was independently associated with decreased FMD.[14]
Current research highlights the effects of air pollutants on surrogate biochemical markers of endothelial dysfunction in healthy children and young adults.[15–17] Various results exist on the association of ambient particulate matters with FMD in general adult population.[18–22]
To the best of our knowledge, no previous study has assessed this association in the pediatric age group. This study aimed to determine the association of particulate matters with FMD in children with or without exposure to secondhand smoke.
METHODS
Participants
This cross-sectional study was conducted from January to March 2011 in Isfahan, which is the second large and air-polluted city in Iran. The study was approved in the Research Council and Ethics Committee of the School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran. It was conducted after obtaining written informed consent from the parents and oral assent from participants.
To prevent the confounding effects of gender and puberty, the study was conducted among prepubescent boys, aged 9 to 12 years. The other eligibility criteria were living for at least 6 months in areas of the city with the lowest and highest levels of particulate matter (based on the official reports of the Provincial Environmental Protection Agency), and location of homes and schools in the same area and less than 1 km far from these stations. Those individuals who had a history of chronic disease, long-term medication use, or a history of acute infectious diseases in the past two weeks were not included in the study. In each low- and high-polluted area, equal number of children with or without exposure to daily tobacco smoke in home was selected. By considering a power of 80% and type 1 error (α) of 5%, the sample size was calculated as 25 in each group, i.e. 100 in total.
Study Area
Isfahan is an industrial city with a population of near 1894382, located in the center of Iranian plateau, with an average altitude of 1500 m from the sea level bounded by NW-SE mountain range of 3000 m. The air of this city is predominantly affected by industrial emissions and motor traffic.[23,24]
Study of Arterial Reactivity
The same cardiologist conducted the studies for measurement of brachial arterial reactivity. This non-invasive endothelial function testing was first described in 1992.[25] It involved measuring the diameter of an artery by non-invasive ultrasound before and after increasing shear stress (provided by reactive hyperemia), with the degree of dilatation reflecting (in large part) arterial endothelial NO release. The diameter of the brachial artery was measured from high-resolution B-mode ultrasound images (Aloka, SSD-2200a). It was conducted in 3 steps: first basal brachial artery dimension was detected. In the second step, the cuff was inflated with 200 mmHg in fore arm, and in the third stage the cuff was deflated, and after 30-90 s, the brachial artery dimension in response to reactive hyperemia, endothelium-dependent dilation or flow mediated dilation (FMD) was determined in the previous site. The percent change of FMD was expressed as: (maximum artery diameter after release of a blood pressure cuff inflated above systolic pressure-baseline resting diameter - basal brachial dimension) / basal brachial dimension. [Author: The highlighted text is correct, if it needs reference, #25 can be cited.]
The experiments were conducted in quiet environment, and no significant changes in their heart rate and blood pressure were observed. The cardiologist conducting the sonographic studies was not aware of the group assignment of participants.
Air Pollution Data
Data from air pollution measurement stations were recorded daily for the 7 days prior to blood sampling from participants. Daily data pertaining to particulate matters measuring up to 10 μm (PM10) were recorded. The existing instruments could not determine PM of smaller size. According to the daily information provided by the Isfahan Provincial Directorate of Environmental Protection, Laleh square was considered as the low-polluted area, and Ahmad Abaad square as the high-polluted area of Isfahan city. Similar to our previous studies,[15,26] the mean values of seven 24-h means of PM 10 were considered for statistical analysis.
Statistical Analysis
Student's t- or the Mann–Whitney U tests were used for comparing the PM10 concentrations, when applicable. Student's t- test was used to compare mean FMD levels of children according to living in low-and high-polluted areas, and according to the passive smoking status. Normality, a required assumption for linear regression analyses, was assessed by Shapiro Wilk test. Multiple linear regression analysis was conducted by considering the passive smoking status and the living area in terms of particulate air pollution as independent variables, and brachial artery diameter as dependent variable. In another regression analysis, we considered PM10 concentration as independent variable and the FMD of non-passive smoker children as dependent variable. Both regression analyses were adjusted for children's age and body mass index. Statistical analyses were performed using SPSS 18.0 (SPSS, Chicago, IL., USA) and differences were considered significant when P < 0.05.
RESULTS
Descriptive statistics of PM10 concentrations are presented in Appendix 1. There were statistically significant differences between two monitoring stations according to PM10 concentration (P < 0.001). Further assessments demonstrated there was no statistically significant difference in mean concentrations of PM10 between different months of sampling. Based on separate analysis for each of monitoring stations, the results showed there were statistically significant differences between two months of sampling only for the monitoring station located in the high-polluted area (P < 0.05).
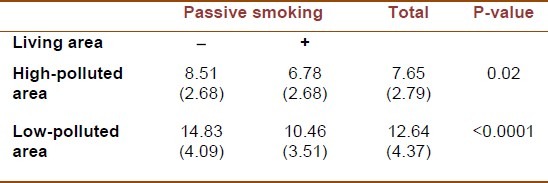
The basal brachial artery diameter was significantly smaller in those living in high-polluted area and those exposed to secondhand smoke [Table 1]. The lowest mean FMD level was documented in passive smoker children living in high-polluted area, whereas non-passive smoker children living in low-polluted area had the highest mean FMD level [Table 2].
Table 1.
Basal brachial artery diameter (mm) according to particulate air pollution and passive smoking
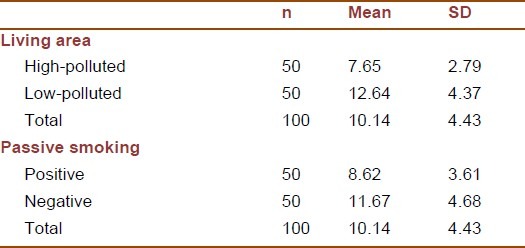
Table 2.
Flow mediated dilation according to particulate air pollution and passive smoking
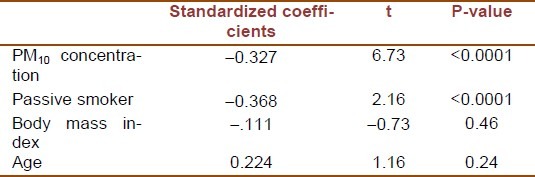
Results of multiple linear regression analysis, adjusted for age and body mass index, are presented in Table 3. Both passive smoking status and living area in terms of particulate air pollution were effective determinants of the brachial artery diameter. The standardized coefficient of passive smoking status was –0.36 (SD = 0.09, P-value < 0.0001) showing negative association with percent increase in brachial artery diameter. Likewise, the percent increase in brachial artery diameter was lower in passive smoker children. Similar relationship was documented for PM10 concentration with a regression coefficient of –0.32 (SD = 0.04, P-value < 0.0001). Comparison between standardized regression coefficients showed that the effect of passive smoking on percent change in Brachial artery diameter was stronger than that of PM10.
Table 3.
Regression analysis of particulate pollution and passive smoking with flow mediated dilation of the brachial artery adjusted for age and body mass index
The regression analysis that considered PM10 concentration as independent variable and the FMD of non-passive smoker children as dependent variable showed that increase in concentration of PM10 was associated with a decrease in FMD measures, the regression coefficient was –0.34 (SD = 0.084, P-value < 0.0001). This result shows that without considering passive smoking variable, PM10 concentration has significant independent effect on FMD level.
DISCUSSION
The findings of this study, which to the best of our knowledge, is the first of its kind in the pediatric age group, revealed significant association of environmental factors on endothelium-dependent brachial artery dilation in young children. Both passive smoking and particulate pollution were associated with attenuated endothelial function and impaired FMD. Those children facing outdoor and indoor pollution had the lowest FMD. Although the effect of passive smoking on FMD was greater than that of particulate pollution, PM10 had an independent inverse association with FMD.
Atherogenesis starts from the fetal life through interrelations of various factors including vascular dysfunction and endothelial biomarkers.[27] The blood vessel endothelium is a sensitive target for environmental factors as air pollutants and secondhand smoke. Studying the associations of environmental factors with early stages of atherosclerosis in childhood can help identify the underlying mechanisms. The relationship of air pollutants with endothelial dysfunction and pro-coagulant state can be an important factor in the development of atherosclerosis from early life.[1,6,15,28]
The findings of the current study on the association of secondhand smoke with reduced endothelial function and impaired FMD are consistent with previous studies on healthy adults.[11,12,29] Our findings are also in line with a longitudinal study, which showed the effect of passive smoking on decreased FMD of 11-year-old Finnish children.[13,14]
The effects of passive smoking on FMD occur shortly after exposure.[30] Trials on healthy volunteers revealed that shortly after exposure, passive smoking impaired FMD in healthy never smoker adults.[31,32]
Nicotine is a pleiotropic agent causing autonomic imbalance and endothelial dysfunction. Nicotinic receptor is stimulated in the autonomic nervous system, and nicotine may promote the oxidative and inflammatory stress to the endothelium. However, nicotine increases myocardial work without impairment of coronary vasodilatation. Therefore, it is proposed that the acute endothelial toxicity of secondhand smoke cannot solely be attributed to a nicotine-dependent mechanism.[33,34] The synergistic effects of air pollution may be one of the factors involved in this regard.
Direct action and independent association of ambient particulates with the vascular endothelium is suggested by various types of study. Comparison of endothelium-dependent brachial artery diameter among 39 healthy volunteers before and after 2 h sitting in bus stops demonstrated that 30 μ/m3 increase in PM2.5 exposure corresponded to a 0.48% decrease in FMD, showing a 5% relative change in the maximum ability to dilate. It is noteworthy to mention that FMD has not been associated with other pollutants and traffic density.[20] Another study among adults with low exposure to secondhand smoke demonstrated a negative association of exposure to ambient PM2.5 with FMD. In this study, brief raises in ambient particulates during usual daily activity were associated with endothelial dysfunction within 2 h of exposure. It is suggested that this rapid effect may be a reflection of acute PM-induced autonomic imbalance.[18]
The acute effects of fine PM on FMD are also documented in double-blind, crossover, controlled exposure studies conducted on adults.[19,22] Likewise, direct action of fine particulates on FMD is reported from a study on sixteen young athletes, 30 min after exercise while inhaling high PM levels, but not after exposure to low levels of PM.[21]
Most studies on healthy adults have addressed the effects of ambient PM2.5 on FMD; our findings on the association of larger particulates, i.e. PM10, on FMD of young children are consistent with a study conducted among diabetic adults.[35]
Given the favorable effects of physical activity[36] and healthy foods[37] on FMD of children and adolescents, encouraging healthy lifestyle may be effective in reducing the harmful effects of outdoor and indoor air pollution.
CONCLUSION
Our findings provide evidence on the association of environmental factors on endothelial dysfunction from early life. Studying such associations among healthy children may help identify the underlying mechanisms. Controlling environmental factors should be considered as an emerging medical and public health problem; as one of the most vulnerable groups, children should be protected against the health hazards of air pollution and secondhand smoke. The clinical implications of environmental factors on early stages of atherosclerosis should be confirmed in longitudinal studies.
ACKNOWLEDGEMENTS
This study was funded as a thesis by Vice Chancellery for Research, Isfahan University of Medical Sciences
Footnotes
Source of Support: This study was funded as a thesis by Vice Chancellery for Research, Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: An overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5:276–92. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- 3.Minicucci MF, Azevedo PS, Paiva SA, Zornoff LA. Cardiovascular remodeling induced by passive smoking. Inflamm Allergy Drug Targets. 2009;8:334–9. doi: 10.2174/1871528110908050334. [DOI] [PubMed] [Google Scholar]
- 4.Vardavas CI, Panagiotakos DB. The causal relationship between passive smoking and inflammation on the development of cardiovascular disease: A review of the evidence. Inflamm Allergy Drug Targets. 2009;8:328–33. doi: 10.2174/1871528110908050328. [DOI] [PubMed] [Google Scholar]
- 5.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation. 2005;111:2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 6.Kelishadi R, Poursafa P. Air Pollution and Non-respiratory Health Hazards for Children. Arch Med Sci. 2010;6:483–95. doi: 10.5114/aoms.2010.14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannuzzi A, Verga MC, Renis M, Schiavo A, Salvatore V, Santoriello C, et al. Air pollution and carotid arterial stiffness in children. Cardiol Young. 2010;20:186–90. doi: 10.1017/S1047951109992010. [DOI] [PubMed] [Google Scholar]
- 8.Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC, et al. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am J Respir Crit Care Med. 2011;184:82–91. doi: 10.1164/rccm.201012-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 10.Fira-Mladinescu O, Noveanu L, Ordodi V, Fira-Mladinescu C, Tudorache V, Mihalaş G. [The effects of chronic exposure to cigarette smoke on vasomotor endothelial function of guinea pig pulmonary arteries] Rev Med Chir Soc Med Nat Iasi. 2008;112:213–9. [PubMed] [Google Scholar]
- 11.Woo KS, Chook P, Leong HC, Huang XS, Celermajer DS. The impact of heavy passive smoking on arterial endothelial function in modernized Chinese. J Am Coll Cardiol. 2000;36:1228–32. doi: 10.1016/s0735-1097(00)00860-3. [DOI] [PubMed] [Google Scholar]
- 12.Thomas GN, Chook P, Yip TW, Kwong SK, Chan TY, Qiao M, et al. Smoking without exception adversely affects vascular structure and function in apparently healthy Chinese: Implications in global atherosclerosis prevention. Int J Cardiol. 2008;128:172–7. doi: 10.1016/j.ijcard.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Kallio K, Jokinen E, Raitakari OT, Hämäläinen M, Siltala M, Volanen I, et al. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–12. doi: 10.1161/CIRCULATIONAHA.106.674804. [DOI] [PubMed] [Google Scholar]
- 14.Kallio K, Jokinen E, Saarinen M, Hämäläinen M, Volanen I, Kaitosaari T, et al. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010;3:196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
- 15.Poursafa P, Kelishadi R, Lahijanzadeh A, Modaresi M, Javanmard SH, Assari R, et al. The relationship of air pollution and surrogate markers of endothelial dysfunction in a population-based sample of children. BMC Public Health. 2011;11:115. doi: 10.1186/1471-2458-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poursafa P, Kelishadi R, Moattar F, Rafiee L, Amin MM, Lahijanzadeh A, et al. Genetic variation in the association of air pollutants with a biomarker of vascular injury in children and adolescents in Isfahan, Iran. J Res Med Sci. 2011;16:733–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 18.Brook RD, Shin HH, Bard RL, Burnett RT, Vette A, Croghan C, et al. Exploration of the rapid effects of personal fine particulate matter exposure on arterial hemodynamics and vascular function during the same day. Environ Health Perspect. 2011;119:688–94. doi: 10.1289/ehp.1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–42. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dales R, Liu L, Szyszkowicz M, Dalipaj M, Willey J, Kulka R, et al. Particulate air pollution and vascular reactivity: The bus stop study. Int Arch Occup Environ Health. 2007;81:159–64. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 21.Rundell KW, Hoffman JR, Caviston R, Bulbulian R, Hollenbach AM. Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal Toxicol. 2007;19:133–40. doi: 10.1080/08958370601051727. [DOI] [PubMed] [Google Scholar]
- 22.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–6. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 23.Talebi SM, Tavakoli T, Ghinani A. Levels of PM10 and its chemical composition in the atmosphere of the city of Isfahan. Iran J Chem Eng. 2008;5:62–7. [Google Scholar]
- 24.Modarres R, Khosravi Dehkordi A. Daily air pollution time series analysis of Isfahan city. Int J Environ Sci Technol. 2005;7:259–62. [Google Scholar]
- 25.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 26.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–9. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Thompson JA, Regnault TR. In utero origins of adult insulin resistance and vascular dysfunction. Semin Reprod Med. 2011;29:211–24. doi: 10.1055/s-0031-1275522. [DOI] [PubMed] [Google Scholar]
- 28.Poursafa P, Kelishadi R. Air pollution, platelet activation and atherosclerosis. Inflamm Allergy Drug Targets. 2010;9:387–92. doi: 10.2174/187152810793937982. [DOI] [PubMed] [Google Scholar]
- 29.Leone A, Balbarini A. Exposure to passive smoking: A test to predict endothelial dysfunction and atherosclerotic lesions. Angiology. 2008;59:220–3. doi: 10.1177/0003319707306300. [DOI] [PubMed] [Google Scholar]
- 30.Bonetti PO, Lardi E, Geissmann C, Kuhn MU, Brüesch H, Reinhart WH. Effect of brief secondhand smoke exposure on endothelial function and circulating markers of inflammation. Atherosclerosis. 2011;215:218–22. doi: 10.1016/j.atherosclerosis.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Giannini D, Leone A, Di Bisceglie D, Nuti M, Strata G, Buttitta F, et al. The effects of acute passive smoke exposure on endothelium-dependent brachial artery dilation in healthy individuals. Angiology. 2007;58:211–7. doi: 10.1177/0003319707300361. [DOI] [PubMed] [Google Scholar]
- 32.Gül I, Karapinar H, Yarlioglues M, Ozdogru I, Kaya MG, Yilmaz A, et al. Acute effects of passive smoking on endothelial function. Angiology. 2011;62:245–7. doi: 10.1177/0003319710377077. [DOI] [PubMed] [Google Scholar]
- 33.Argacha JF, Fontaine D, Adamopoulos D, Ajose A, van de Borne P, Fontaine J, et al. Acute effect of sidestream cigarette smoke extract on vascular endothelial function. J Cardiovasc Pharmacol. 2008;52:262–7. doi: 10.1097/FJC.0b013e318185fa26. [DOI] [PubMed] [Google Scholar]
- 34.Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol. 2008;35:458–63. doi: 10.1111/j.1440-1681.2008.04896.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Ruddy TD, Dalipaj M, Szyszkowicz M, You H, Poon R, et al. Influence of personal exposure to particulate air pollution on cardiovascular physiology and biomarkers of inflammation and oxidative stress in subjects with diabetes. J Occup Environ Med. 2007;49:258–65. doi: 10.1097/JOM.0b013e31803220ef. [DOI] [PubMed] [Google Scholar]
- 36.Kelishadi R, Hashemi M, Mohammadifard N, Asgary S, Khavarian N, et al. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clin Chem. 2008;54:147–53. doi: 10.1373/clinchem.2007.089953. [DOI] [PubMed] [Google Scholar]
- 37.Hashemi M, Kelishadi R, Hashemipour M, Zakerameli A, Khavarian N, Ghatrehsamani S, et al. Acute and long term effects of Grape and Pomegranate Juice Consumption on Vascular Reactivity in Pediatric Metabolic Syndrome. Cardiol Young. 2010;20:73–7. doi: 10.1017/S1047951109990850. [DOI] [PubMed] [Google Scholar]


