Abstract
Background:
Malignancies are primarily environmental diseases mostly attributed to environmental factors. By plotting the prevalence and spatial distribution maps, important differences can be observed in detail. This study aimed to determine the association between map distribution of malignancies and the geological phenomena of lead (Pb) accumulation in soil in the province of Isfahan, Iran.
Methods:
Spatial distribution maps of malignant diseases were plotted by using data recorded during 2007 to 2009 in the Isfahan Cancer Registry Program. Data on Pb accumulation in soil was obtained from the National Geological Survey and Mineral Exploration. Pb concentrations were documented in three parts of agricultural, non-agricultural, urban, and industrial land. The geographical mapping of cancers and soil Pb were then incorporated into a geographic information system (GIS) to create a spatial distribution model.
Results:
The spatial distributions of ten common malignant diseases in the province, i.e. skin cancers, hematological malignancies, and breast cancers, followed by other malignancies were scattered based on Pb distribution. In fact, common cancers were more prevalent in the parts of the province where soil Pb was more abundant.
Conclusion:
The findings of this study underscore the importance of preventing Pb exposure and controlling industrial production of Pb. The data is also important to establish further effects modeling for cancers. Moreover, physicians and health professionals should consider the impact of environmental factors on their patients’ health.
Keywords: Environment, Cancers, Lead, Spatial Distribution, Iran
INTRODUCTION
The global incidence of malignant diseases has significantly increased during the last decades in parallel with the rapid industrialization of most countries. In Iran, as the third cause of death and the second prevalent chronic diseases, cancers pose a significant health burden.[1]
Malignancies are considered primarily as environmental diseases with 90-95% of cases attributed to environmental factors and 5-10% to genetic factors. Common environmental factors that are attributable to cancer death include tobacco use (25-30%), dietary habits and obesity (30-35%), infections (15-20%), radiation (ionizing and non-ionizing, up to 10%), tensions, and environmental pollutants.[2] Environmental pollution is considered as one of the main causes for some types of cancers. For instance, each year nearly one million ton of lead (Pb) is added to the soil at global level. This contains large quantities of atmospheric dust, scattering ash, chemical fertilizers used in agriculture, and industrial and urban wastes.[3] Although inorganic contaminants and extensive distribution of metals are considered as one of the most likely potential exposure ways for populations,[4] still enough attention is not paid to the environmental factors affecting health.
Isfahan, a province with an area of about 107,045 square kilometers, equivalent to 6.3% of the total area of Iran, is located between 30 degrees 43 minutes and 34 degrees 27 minutes north latitude and 49 degrees 38 minutes and 55 degrees 32 minutes east of the Greenwich meridian. The province is 1550 meters above the sea level. With 1650 industrial factories,[5] it is a region prone to industrial pollutants. This province is also considered as one of the major agricultural poles of the country and the usage of chemical fertilizers in agriculture has been added plenty of Pb into the province's soils. Pb is up taken by the plants from contaminated land, thus agricultural products are the most important way to enter this element in the food chain. Pb is not biodegradable, does not undergo dissociation, and cannot be metabolized in human body. Therefore, after entering the body, Pb will be deposited and accumulated in blood, skin, organs, and tissues such as fat, muscles, bones, and joints.[4]
This study aimed to map Pb distribution and the spatial distribution of the ten most common types of cancers in the province.
METHODS
The ten most common cancers in Isfahan were determined by using data recorded from 2007 to 2009 by the Isfahan Cancer Registry Program of the Isfahan Provincial Health Center. We obtained the distribution map of Pb in the province of Isfahan during 2007 to 2009 from the Mineral Exploration Organization, and the information on soil Pb concentration from the Isfahan Agricultural Jihad Organization. The Pb concentration was documented in three fields of agricultural, non-agricultural, and urban and industrial land.
The geographic mapping of cancers and soil Pb was then incorporated using Geographic Information System (GIS) software. After producing the spatial distribution model, the spatial distribution maps of malignant diseases were plotted and compared.[5]
RESULTS
Overall 10,142 medical records of patients with documented pathology report of cancer in 2007 to 2009 were recruited from the Cancer Registry data. The ten most prevalent cancers are presented in Table 1.
Table 1.
Most prevalent cancers in the province of Isfahan during 2007-2009

Spatial distribution of Pb in 2007-2009 is depicted in Figure 1. It shows that Pb was more abundant in south, west, center, north, and east of the province. Mapping the distribution of malignant diseases is presented in Figures 2 to 6. Skin cancer was more prevalent in cities of Isfahan, Najaf Abad, Kashan, Naeen, Ardestan, and Natanz (Figure 2). The rate of skin cancer was correlated with Pb distribution in the province, i.e. it was lower in areas away from Pb-contaminated regions.
Figure 1.
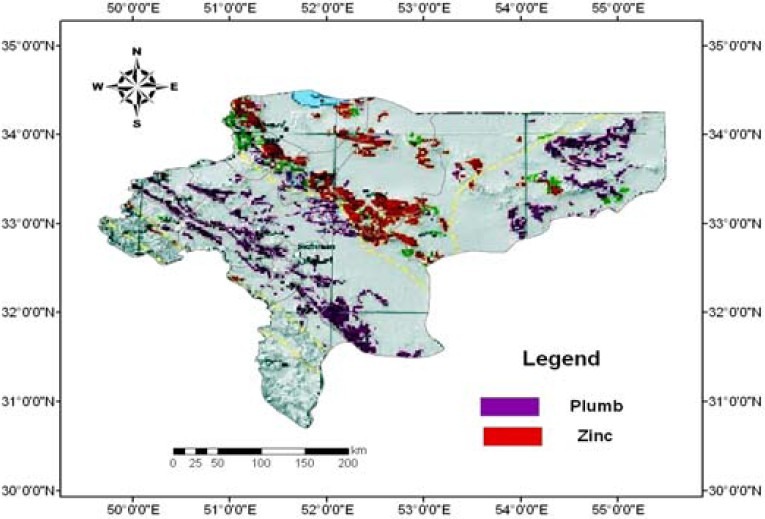
Soil lead distribution map in the province of Isfahan, Iran (Values are presented in Km.)
Figure 2.
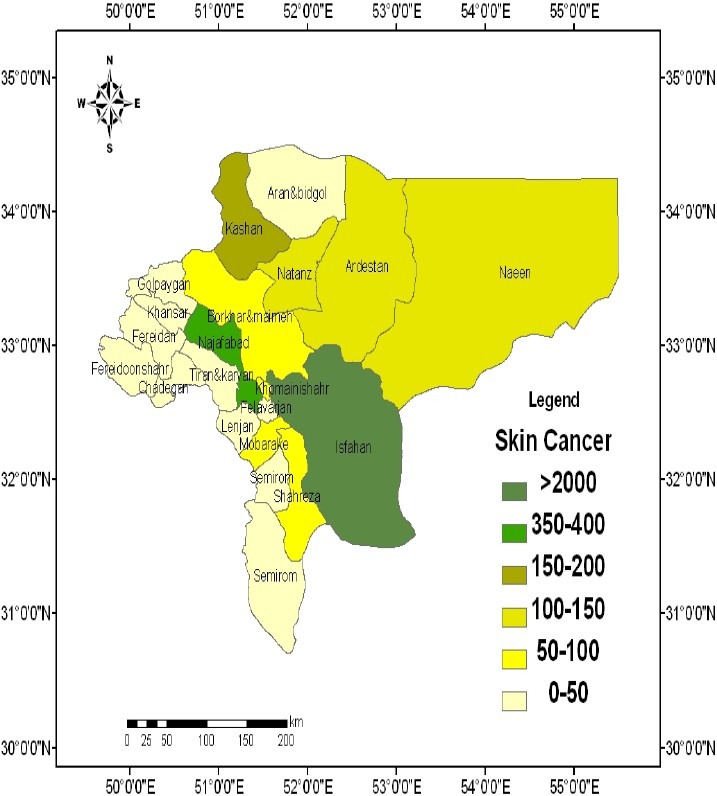
Spatial distribution of skin cancer in the province of Isfahan, Iran (Values are expressed as number of persons.)
Figure 6.
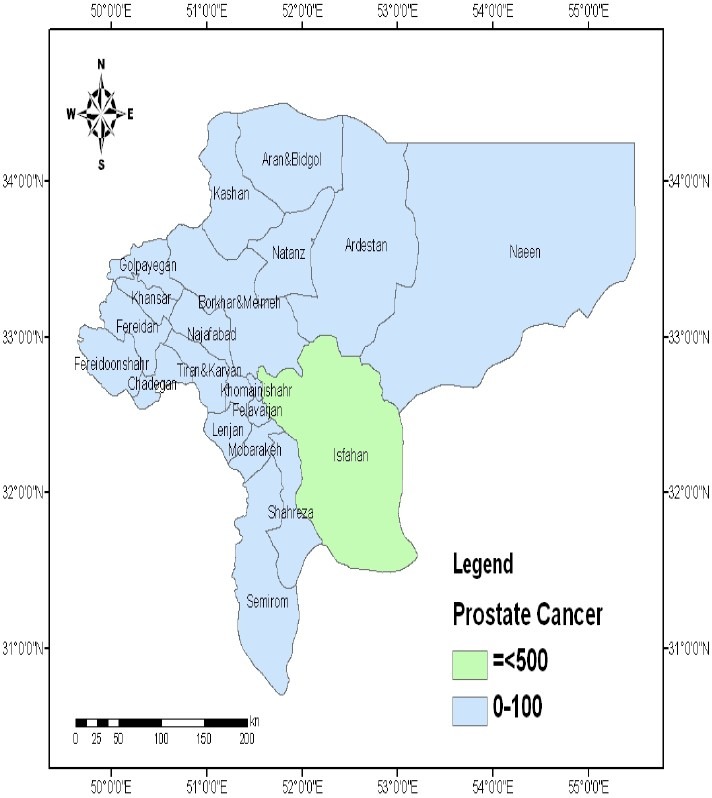
Spatial distribution of prostate cancer in the province of Isfahan, Iran (Values are expressed as number of persons.)
As shown in Figure3, the prevalence of breast cancer was also directly related with the soil Pb concentration. The cities of Isfahan, Najaf Abad, Kashan, Borkhar, Meymeh, Naeen, Ardestan, and Natanz had the highest prevalence of breast cancer (Figure3). The spatial distribution of hematological malignancies was consistent with the distribution of soil Pb throughout the province (Figure4).
Figure 3.
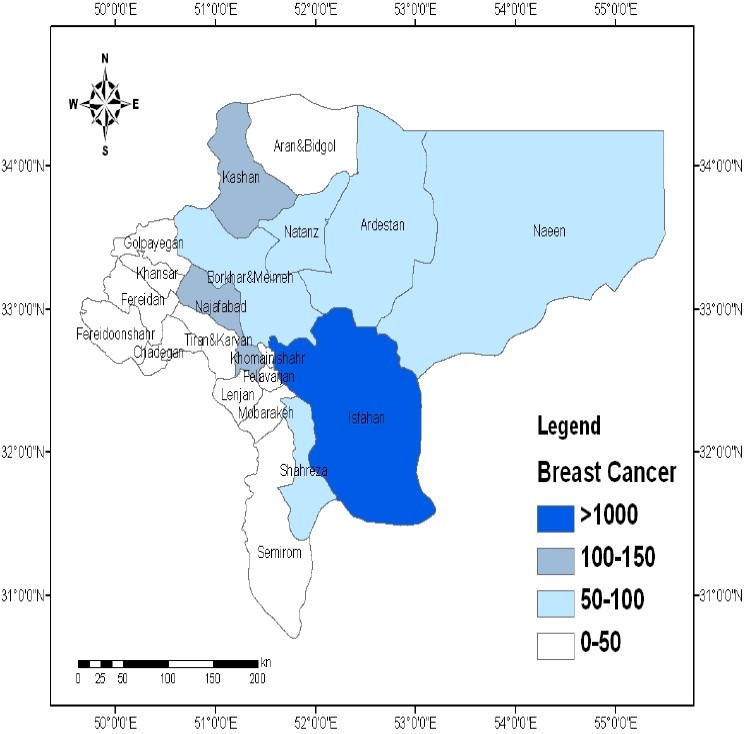
Spatial distribution of breast cancer in the province of Isfahan, Iran (Values are expressed as number of persons.)
Figure 4.
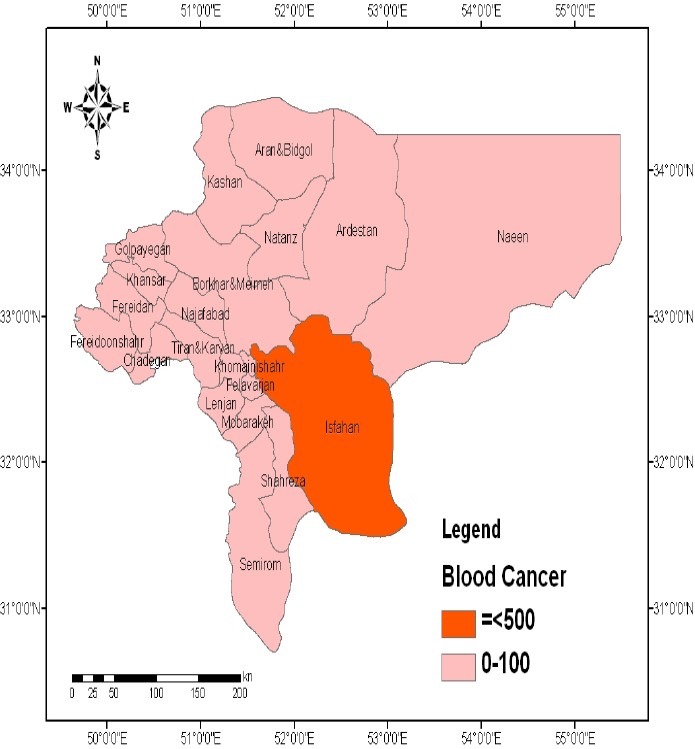
Spatial distribution of hematological malignancies in the province of Isfahan, Iran (Values are expressed as number of persons.)
Mapping of bladder and prostate cancers were not associated with soil Pb distribution (Figures 5 and 6).
Figure 5.
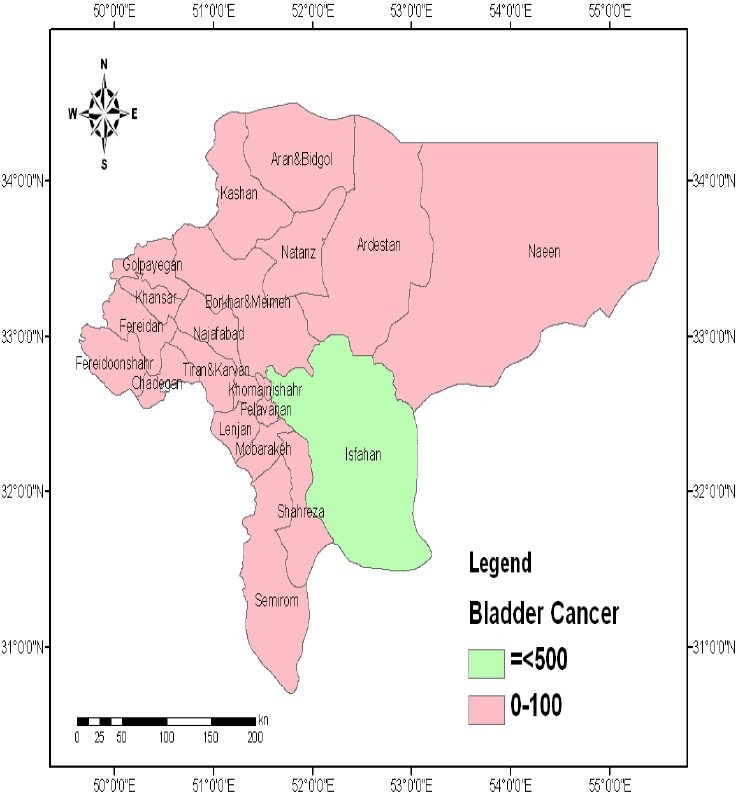
Spatial distribution of bladder cancer in the province of Isfahan, Iran (Values are expressed as number of persons.)
DISCUSION
Plotting the spatial distribution of the most common types of malignant diseases and soil Pb concentration showed significant associations between mapping for skin, breast, and hematological malignancies and Pb distribution in the province of Isfahan. This finding may suggest that soil contamination by Pb, and possibly other industrial metals, may contribute in the incidence of cancers. We should acknowledge that in addition to the possible association of soil Pb with malignancies, the higher prevalence of diagnosed cancers might have been a result of better medical facilities and health centers in larger cities of the province. However, our data comprised the records from the whole province including smaller cities for three years, and earlier diagnosis of malignancies in larger cities would not affect our findings.
Pb pollution has become a worldwide issue. Although Pb naturally exists in the environment, most Pb concentrations found in the environment are accumulated from human activities. The industrial pollutants, fertilizers, and other agricultural items are the main sources of Pb added to the environment by humans. Pb distribution in the areas where the population and human activities are higher is more prominent. The main reason can be contamination by industrial pollution, Pb-acid batteries, electronic components, cable sheathing, ammunition, glass, ceramics, Pb pipes, paints, alloys, connections, and joints in roofs used for protection against rain. The larger Pb particles drop to the ground straightaway and will pollute soils or surface waters, whereas the smaller particles will be transported long distances through air and may remain in the atmosphere. Part of Pb will fall back on ground by raining. This Pb-cycle caused by human production is more extended than the natural Pb-cycle.[6,7]
Although Western lifestyle, obesity, smoking, and epidemiologic transition have crucial roles in the development of non-communicable diseases, including cancers, they cannot solely explain the worldwide rapid increase in the incidence of cancers. Environmental pollution, including soil contamination by toxic metals during industrialization may be an important contributing factor.[8–10] In 1980, Barrett depicted the spatial distribution as the possible etiology of disease, and proposed the importance of medical geography on human health. He suggested for the first time that a geographic area represents the complex physical, biological, and cultural processes. By analyzing the elements and patterns, it is possible to determine and track the spatial distribution of various diseases.[11]
A large body of evidence supports the health hazards of environmental Pb exposure.[12–17] It is estimated that reducing the concentrations of environmental Pb can significantly decrease the standardized death rate.[18] The carcinogenic effects of Pb have been documented in some previous studies. A study in Taiwan revealed that living in high Pb-polluted areas can result in an increased incidence rate of brain cancer.[19] An ecologic study conducted in 2000-2007 among Chinese population living in nine agricultural villages with high environmental pollution showed significant associations between exposure to multiple heavy metals, including Pb, and cancer mortality.[20] Our findings on the probable association of Pb exposure with breast cancer are consistent with some previous studies.[21–23] In addition to ecological studies, such association has also been documented in animal models.[24]
It is suggested that Pb may accelerate tumor growth rates, and may interact with some protective factors against breast cancer development.[23] A growing body of evidence proposed that environmental metal exposure may be associated with changes in epigenetic factors. This association may lead to a possible link between heritable changes in gene expression and disease susceptibility and development.[25–27]
To solve the global problem, soil resources should be tested and evaluated periodically. Preventive measures, such as using filters, lime, and other things to prevent excessive entry of sewage into rivers and freshwater resources and soil, should be considered. Cultivation of plants like borage or oxtongue plant that absorb toxic elements may be beneficial for reducing Pb levels. However, products of such plants should not be consumed in areas with Pb-contaminated soil. An intersectoral collaboration is necessary for reducing environmental Pb production. On the other hand, increasing public knowledge on how to prevent exposure is another important strategy in this regard. Health professionals have a distinctive capacity to increase the knowledge of the whole population through educating their patients.
CONCLUSION
According to the results of this study, not all diseases are caused by inheritance or genetic factors. In fact, environmental factors could also be responsible for some diseases such as malignant. The findings of this study underscore the importance of preventing Pb exposure. Control Pb producing industries to improve work-related environmental health and increasing the knowledge of health professionals and the general population in this regard are also of high importance. Programs aiming at lowering the cancer risk will thus have to consider effective measures to reduce the production of and exposures to Pb and other industrial metals that are currently contaminating the environment.
Footnotes
Source of Support: This paper was derived from a specialty thesis at the Department of Geography Sciences, Isfahan University, Isfahan, Iran.
REFERENCES
- 1.Azizi F. Epidemiology and Control of Common Diseases in Iran. Tehran: Tehran University Press; 2001. [Google Scholar]
- 2.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yavar B, Pour-Ahmad A. Medical geography of esophageal cancer in Iran. Geographical Res. 2001;41(13):24. [Google Scholar]
- 4.Presley SM, Rainwater TR, Austin GP, Platt SG, Zak JC, Cobb GP, et al. Assessment of pathogens and toxicants in New Orleans, LA following Hurricane Katrina. Environ Sci Technol. 2006;40(2):468–74. doi: 10.1021/es052219p. [DOI] [PubMed] [Google Scholar]
- 5.Rashidi M, Ghias M, Rouzbahani R, Rameshat MH, Poursafa P, Gharib H. Relationship between spatial distribution of malignant diseases and plumb element in Isfahan province. Journal of Isfahan Medical School. 2011;29(135):418–25. [Google Scholar]
- 6.Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E. Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol. 2011;213:113–36. doi: 10.1007/978-1-4419-9860-6_4. [DOI] [PubMed] [Google Scholar]
- 7.Bindler R. Contaminated lead environments of man: reviewing the lead isotopic evidence in sediments, peat, and soils for the temporal and spatial patterns of atmospheric lead pollution in Sweden. Environ Geochem Health. 2011;33(4):311–29. doi: 10.1007/s10653-011-9381-7. [DOI] [PubMed] [Google Scholar]
- 8.Skerfving S, Bergdahl IA. Lead. In: Nordberg GF, Nordberg FM, Friberg LT, editors. Handbook on the Toxicology of Metals. 3rd ed. Burlington MA: Elsevier Science Publishing Company; 2007. pp. 599–643. [Google Scholar]
- 9.Koteles F, Simor P. Modern Health Worries, Somatosensory Amplification and Subjective Symptoms: A Longitudinal Study : A Longitudinal Study. Int J Behav Med. 2011 doi: 10.1007/s12529-011-9217-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Iqbal MP. Lead pollution - a risk factor for cardiovascular disease in Asian developing countries. Pak J Pharm Sci. 2012;25(1):289–94. [PubMed] [Google Scholar]
- 11.Kolossa-Gehring M, Becker K, Conrad A, Schroter-Kermani C, Schulz C, Seiwert M. Environmental surveys, specimen bank and health related environmental monitoring in Germany. Int J Hyg Environ Health. 2012;215(2):120–6. doi: 10.1016/j.ijheh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Jakubowski M. Low-level environmental lead exposure and intellectual impairment in children--the current concepts of risk assessment. Int J Occup Med Environ Health. 2011;24(1):1–7. doi: 10.2478/s13382-011-0009-z. [DOI] [PubMed] [Google Scholar]
- 13.Krzywy I, Krzywy E, Pastuszak-Gabinowska M, Brodkiewicz A. Lead--is there something to be afraid of? Ann Acad Med Stetin. 2010;56(2):118–28. [PubMed] [Google Scholar]
- 14.Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12(3):206–23. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- 15.Smith SR. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ Int. 2009;35(1):142–56. doi: 10.1016/j.envint.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Bergdahl IA, Skerfving S. Biomonitoring of lead exposure-alternatives to blood. J Toxicol Environ Health A. 2008;71(18):1235–43. doi: 10.1080/15287390802209525. [DOI] [PubMed] [Google Scholar]
- 17.Alessio L, Campagna M, Lucchini R. From lead to manganese through mercury: mythology, science, and lessons for prevention. Am J Ind Med. 2007;50(11):779–87. doi: 10.1002/ajim.20524. [DOI] [PubMed] [Google Scholar]
- 18.Marchwinska-Wyrwal E, Dziubanek G, Skrzypek M, Hajok I. Study of the health effects of long-term exposure to cadmium and lead in a region of Poland. Int J Environ Health Res. 2010;20(2):81–6. doi: 10.1080/09603120903394656. [DOI] [PubMed] [Google Scholar]
- 19.Wu WT, Lin YJ, Liou SH, Yang CY, Cheng KF, Tsai PJ, et al. Brain cancer associated with environmental lead exposure: evidence from implementation of a National Petrol-Lead Phase-Out Program (PLPOP) in Taiwan between 1979 and 2007. Environ Int. 2012;40:97–101. doi: 10.1016/j.envint.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Song H, Chen WQ, Lu C, Hu Q, Ren Z, et al. Cancer mortality in a Chinese population surrounding a multi-metal sulphide mine in Guangdong province: an ecologic study. BMC Public Health. 2011;11:319. doi: 10.1186/1471-2458-11-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florea AM, Busselberg D. Metals and breast cancer: risk factors or healing agents? J Toxicol. 2011;2011:159619. doi: 10.1155/2011/159619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson LN, Cotterchio M, Cole DE, Knight JA. Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1708–17. doi: 10.1158/1055-9965.EPI-11-0300. [DOI] [PubMed] [Google Scholar]
- 23.Alatise OI, Schrauzer GN. Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol race Elem Res. 2010;136(2):127–39. doi: 10.1007/s12011-010-8608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrauzer GN. Effects of selenium and low levels of lead on mammary tumor development and growth in MMTV-infected female mice. Biol Trace Elem Res. 2008;125(3):268–75. doi: 10.1007/s12011-008-8172-1. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics. 2011;6(7):820–7. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyersmann D. Effects of carcinogenic metals on gene expression. Toxicol Lett. 2002;127(1-3):63–8. doi: 10.1016/s0378-4274(01)00484-2. [DOI] [PubMed] [Google Scholar]
- 27.Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1(3):222–8. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]


