Abstract
Background:
More than one third of energy intake in Iran is provided from bread. Therefore, improving bread mineral bioavailability through dephytinization can play an important role in decreasing the prevalence of many mineral deficiencies. In this study, effect of phytase supplementation on zinc, iron and calcium status in growing rats fed with a diet containing high phytate Iranian bread (Sangak) was assessed.
Methods:
Thirty weanling Wistar male rats were assigned to phytase (Aspergillus niger) or control group for 6 weeks. The diet was designed based on Iranian's food pattern and 34.2% of the energy was supplied from Sangak bread. Food intake, body and organ weight and body height were measured. Zinc was measured in blood, liver and femur. Iron was assessed in blood and liver and calcium was titrated from femur bone. Statistical analyses were performed using SPSS software. Paired sample t-test, Wilcoxon signed-rank test and repeated measurement ANOVA were used for proper analysis of data.
Results:
Although weekly weight gain was not different between groups, final weight was in favor of control group. Food intakes, liver and femur bone weight did not differ between the two groups. However, the blood zinc was higher in the phytase group (26.2 ± 7.4 vs. 19.2 ± 5.2, P = 0.03). Thus positive effects of phytase supplementation on zinc, independent of growth was found. Other variables did not show any differences between groups.
Conclusion:
Addition of phytase to diet containing high phytate Iranian bread can improve blood zinc status in growing rats.
Keywords: Bread, calcium, iron, phytase, phytic acid, zinc
INTRODUCTION
In 1961, eleven cases of dwarfism, hypogonadism, iron-deficiency anemia and geophagia were reported in Iran.[1] Investigation of similar cases from Egypt showed this syndrome to be associated with zinc deficiency.[2,3] Further studies revealed that mineral deficiencies occur despite intakes of calcium, iron and zinc that equal or exceed those of the population of western countries[4,5] and also that restricted availability caused by phytic acid must be responsible for this deficiency.[4–6]
myo-Inositol hexaphosphate (InsP6), also known as phytic acid, has been recognized as a strong chelator with essential dietary minerals such as calcium, magnesium, zinc and iron to form insoluble salts in the small intestine and therefore acts as an inhibitor of absorption of these minerals.[7–10] Among affected minerals, zinc bioavailability is more diminished by phytate.[11–13] It has been shown that phytate to mineral molar ratio less than 1 for iron and less than 10 for zinc is required.[14] Diets containing negligible animal protein with more than 50% of energy from high phytate food are characterized as low bioavailable in many essential dietary minerals.[15] According to the last national study in Iran, 51.8% of energy is supplied from grains (bread, rice, pasta and other cereals), which are the richest sources of phytate in food groups, and only 11% of energy is obtained from animal proteins (5% from fish, meat and poultry).[16] This dietary pattern is typically low available at least with regard to zinc. Furthermore, many studies have shown prevalent iron deficiency in different vulnerable groups in Iran.[17–20] Although results of nutritional surveys on iron intake are contradictory, low bioavailability has been brought up as a probable cause.[21]
Currently, since more than one third of energy intake in the entire country is still provided from bread,[16] it is assumed that improving bread mineral bioavailability can play an important role in decreasing the prevalence of many mineral deficiencies. No study so far has investigated the effect of low cost, highly active phytase enzyme on Iranian mineral bread absorption. As the interaction between mineral absorption inhibitors and enhancers in different dietary patterns decide to what extent the element is likely to be absorbed, response to added phytase may not be linear and similar. Even though human trials are the most accurate model for studying mineral absorption, they are very expensive and difficult to perform. Therefore, this study is undertaken to assess whether addition of phytase can improve zinc, iron and calcium status in Wistar rats fed with a diet containing Iranian high phytate bread.
METHODS
Animals and Study Design
This matched case-control study was carried out with thirty male weanling Wistar rats (age: 21 ± 5 day) which were housed individually in polycarbonate cages in a 12-h light: dark cycle animal room with maintained temperature at 20-25°C and 40-50% humidity. All rats were given free access to food and tap water. This experiment was implemented in S. Beheshti University of Medical Sciences at winter 2010 and approved by Ethics Committee of National Nutrition and Food Technology Research Institute by the number of 037371.
During the first week, all rats were fed with the designed diet with no phytase added (acclimation period). After this period they were sorted by weight and assigned to phytase or control group alternatively (starting from a random number) yielding a number of 15 rats in each group. They were fed with one of the two, phytase or control diets ad libitum for 6 weeks. At the end of the study, theoretically their growth period was known to be terminated and the results would reveal effects during growth.
Diet Design
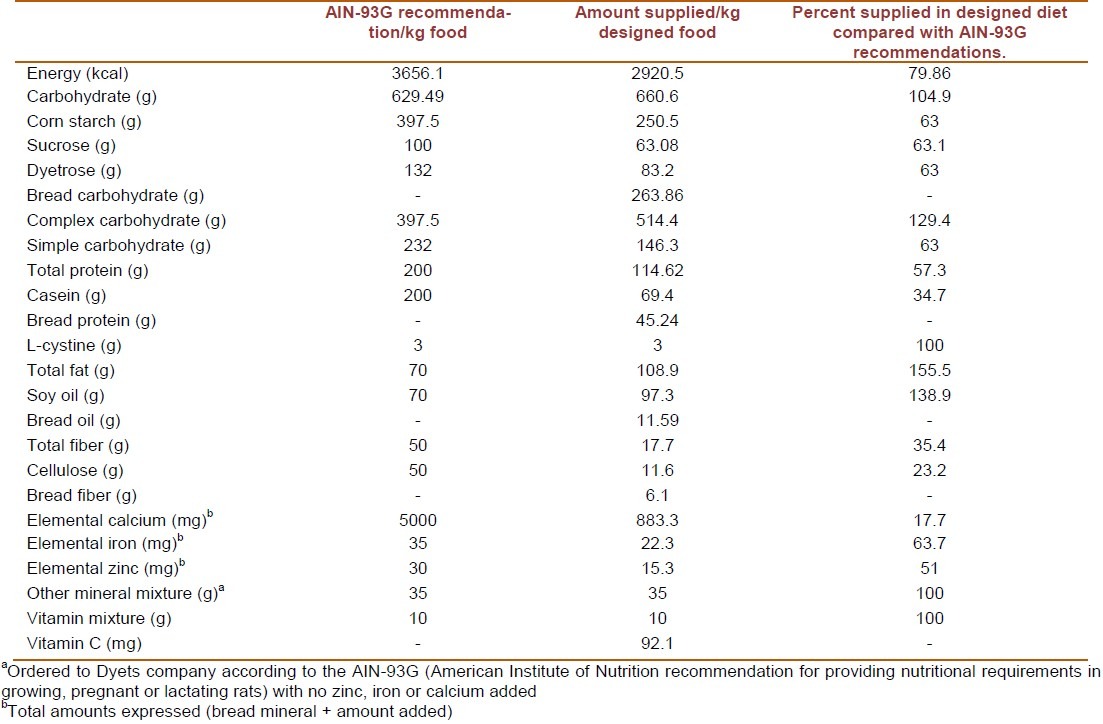
We designed a diet similar to Iranian's mean fiber, macro- and target micro-nutrients (calcium, iron and zinc) intake to make it compatible with Iranian's food pattern. Sixty four percent, 11% and 25% of energy was supplied from carbohydrate, protein and fat respectively on the basis of “The National Comprehensive Study on Household Food Consumption Pattern and Nutritional Status”.[16] According to the above mentioned study, 34.2% of the energy was intended to be provided by high phytate bread. For creating this diet, a homogenized sample of dried bread was analyzed for the macronutrients, fiber, calcium, iron and zinc content. To obtain 64% of energy intake from carbohydrate, corn starch, sucrose and dyetrose (equal to AIN-93G recommended proportions which provides sufficient amounts for growing rats) were added to the diet after subtracting the carbohydrate provided by bread. Similarly the remaining parts of the protein, fat, fiber, zinc, iron and calcium were provided by casein, soy-bean oil, cellulose, zinc carbonate, ferric citrate and calcium carbonate to meet the mean Iranian intake respectively. Keeping in mind that vitamin C improves iron absorption, it was decided to add vitamin C to match the Iranian diet. Other minerals and vitamins were provided on the basis of AIN-93G recommendations. All of the calculated ingredients were first determined on the basis of mean Iranian's daily energy intake (2636 kcal/day) and then adjusted to amount (g) per kg food. All of the ingredients used for preparing the diet were purchased from Dyets company (Bethlehem, PA, USA) except soy-bean oil (Behshahr Company, Karaj, Iran) and bread. The created diet was tested during a pilot study on 6 rats for a period of 6 weeks. The results showed relative palatability of the diet for the rats.
Bread Making Procedure and Analysis
Choosing Sangak was based on the knowledge that this bread has the highest phytate content among most widely consumed breads in Iran. This bread had phytate to zinc and iron molar ratios above those considered for negatively influenced absorption of these minerals. It was assumed that if this bread consumer can benefit from phytase supplementation, other bread consumers (with lower phytate content) may probably benefit from it as well.
Commercial Sangak (an Iranian traditional flat bread) wheat flour (93% extraction rate) was used in this study. The dough was prepared in one batch. In brief, 39.19 kg of Sangak flour, about 60 l of lukewarm water and 350 g salt were mixed for 12 min. After 5 min, 150 g instant yeast (Saccharomyces cervisiae, Dez-Maye, Iran) and 17.35 kg of sourdough (12 hrs aged) were added and mixed for another 2 min. Fermented dough was left for 50 min for proofing at room temperature (23°C). This dough was manually molded (900-950g each), sheeted and baked in a traditional stove for about 6 min at 350°C. Baked breads were cooled and stored at -20°C and were gradually air-dried at room temperature for about 24 h and completely dried in a vacuum oven at 70°C for 24 h to constant weight. Dried breads were then finely ground and stored at 4°C in airtight containers for diet preparation.
A homogenized sample of dried bread was analyzed for the macronutrients, fiber, calcium, iron and zinc amounts. The nitrogen content of bread was determined by the Kjeldahl method and total protein was calculated by multiplying the nitrogen content by 5.7. AOAC methods were used for analyzing the other nutrients and fiber in the bread {carbohydrate (3203051), fat (32/3/04), calcium (32/1/09), iron [944/02B (32/1/9)], zinc (9/1/09) and fiber (4/6/01)}.[22]
Phytic Acid Determination
Freeze-dried ground sample of bread (0.5 g) was extracted with 0.5 M HCl (20 ml) for 3 h under agitation. The extracts were then centrifuged and supernatant decanted, frozen over night and centrifuged again for phytate determination. Carlsson et al.[23] method was used for phytic acid determination. Briefly, the chromatograph consisted of a biocompatible (PEEK) HPLC pump (Waters model 626) equipped with a PA-100 guard column (Dionex Corp., Sunnyvale CA) and an HPIC CarboPac PA-100 analytical column (Dionex Corp.). The InsP6 was eluted with an isocratic eluent consisting of 80% HC (1M) and 20% H2O. The eluents (0.8 ml/min) were mixed with 0.1% Fe(NO3).9H2O in a 2% solution of HCIO4 in a postcolumn reactor pump (HPIC pump, K500, Knauer, Berlin, Germany). InsP6 was detected after postcolumn reaction using UV detection (Waters 486, tunable absorbance detector). The total run time was 6 min and absorbance was monitored at 290 nm. The InsP6 was integrated using the software Empower (Waters Associated, Mileford. MA) and the concentration of InsP6 in the sample was calculated using a standard curve.
Phytase
The phytase used was produced by a genetically modified strain of Aspergillus niger (Natuphos; 10,000 G, BASF, AG. Ludwigshafen, Germany). This product was kindly provided by “Kian Daneh Pars Company” (Tehran, Iran) in granule form. One phytase unit (FTU) is defined as the amount of enzyme necessary to liberate 1nmol of inorganic phosphorous from sodium phytate per minute at pH 5 and 37°C. Phytase activity was 10,000 FTU/g and its activity was confirmed after turning it into a fine ground[24] to be mixed with the diet in the form of a dry powder.
The amount of InsP6 was 224 mg/100g of bread (db). Since each kg of designed diet contained 367.8 g of dried bread, it was calculated that 0.125 g phytase was needed to degrade InsP6 in the mixed diet. To ensure maximal InsP6 degradation, about 0.5 g phytase was added per kg of the designed diet.
Data and Sample Collection
The body weight of the rats and their food intake were measured weekly. Heights were collected (nose to base of tail) at both the onset and completion time of the experiment. At the termination of experiment, rats were euthanized under carbon dioxide and blood was collected from the orbital sinus. Their livers were excised and frozen (-33°C) for later analysis. Both legs were dissected away from the hip joint and adherent tissues were cleaned carefully and femur bones were stored at 4 °C.
Sample Preparation and Analytical Procedures
Whole samples including blood, liver and femur bones were charred and then ashed at 550°C for 6 h. The ash was dissolved with 1% v/v nitric acid and water and filtered. Prepared samples were analyzed for mineral content using flameless atomic absorption spectrophotometer (Rayleigh, WFX-210 AA Spectrophotometer). For measuring calcium in femur bone, a solution of ashed sample, water, HCl and sodium hydroxide was titrated with EDTA using Blue Naphthyl as an indicator. All glasswares used were washed in 5% nitric acid solution for 24 h and rinsed 3 times with double-deionized water.
Statistical Analysis
All biochemical analysis was done in triplicates and mean of each observation was considered as the sample mineral content. Statistical analyses were performed using SPSS software (version 16). Since there were 2 matched rat groups, paired sample t-test was used for comparing variables between groups and the Wilcoxon signed-rank test was used for analyzing variables where normal assumptions were not met. Repeated measurement analysis of variance was used for comparing weights and food intake measured in 7 weeks. P-value <0.05 was considered as the level of significance.
RESULTS
Biochemical analysis of the Sangak showed that this bread had 71.74% carbohydrate, 12.3% protein, 3.15% fat and 1.65% fiber. This bread also had 1.66 mg/100g zinc, 1.2 mg/100g iron and 80.05 mg/100g calcium. Calculating phytate to zinc and iron molar ratio resulted in 13.4 and 15.8 respectively, which both express low bioavailability of the bread. Comparisons between the designed diet and AIN-93G recommendations are shown in Table 1. It reveals that the designed diet is almost similar in total carbohydrate content to AIN-93G, but it has 43% less protein and 55.5% more fat. Moreover, as was expected, this diet is very poor regarding calcium, iron and zinc content.
Table 1.
Comparison between designed diet and AIN-93G recommendations
There were no significant differences in total and weekly measured food intake between groups (total mean food intake per rat was 97.08 ± 38.58 and 100.87 ± 38.38 g/day, P = 0.74 for phytase and control group respectively, weekly results are not shown).
Two rats died during the study (one from each group/analyses are performed with 14 rats in each group) causing a significant difference in group's initial mean weight (initial weight in the control and phytase groups was 42.35 ± 6.7 and 41.39 ± 6.1 respectively). Moreover, significant difference between group's mean weight was observed from week 6 to the end of study (terminal weight in the control and phytase group was 72.75 ± 19.79 and 60.16 ± 16.67 respectively). Results from repeated measurement analysis of variance showed significant effect of time (P < 0.001), but interaction effect of time and group was not significant (P = 0.164). Results regarding heights and weight gain are in favor of the control group although they are not statistically significant [Table 2]. Moreover, this table shows no significant differences between liver and femur weight between groups.
Table 2.
Growth measurements: weekly weight gain#, weight gain to feed intake ratio at the end of study, height, liver weight and femur weight in phytase and control group
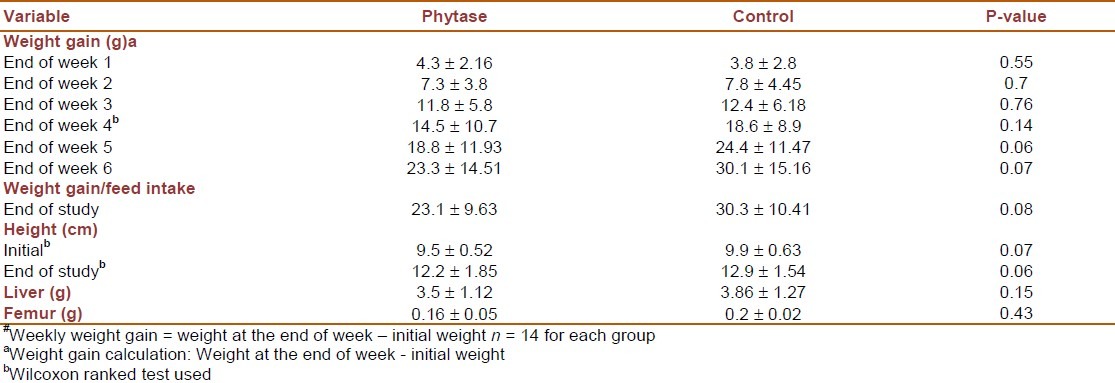
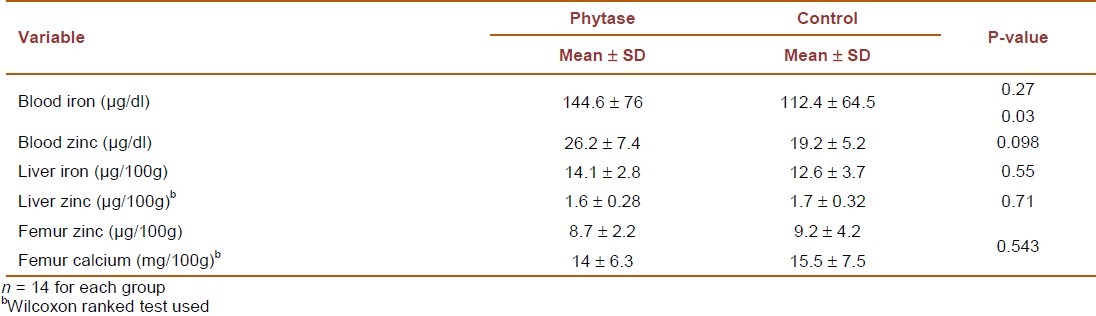
Table 3 shows mineral status in the tissues dry weight. Our results indicated a higher blood zinc in the phytase supplemented group, whereas no differences were seen in liver or femur bone zinc
Table 3.
Mineral content in blood, liver and femur bone in phytase and control groups at the end of study
DISCUSSION
Our results show that phytase supplementation can improve the blood zinc of Wistar rats fed with a diet containing high phytate Iranian bread.
As mineral bioavailability is a multi-factorial matter and several factors other than phytate could also impact their absorption (e.g., protein, fiber and divalent mineral interactions[15] ), we created diet conditions resembling those of Iranian mean intakes.
Although plasma zinc has commonly been assessed in previous studies, we decided to measure whole blood zinc based on the fact that blood zinc is 70-80% cellular.[25] McClung et al.[26] reported improvement in both plasma and bone (femur and tibia) zinc in rats fed with a low-zinc diet supplemented with phytase for 8 weeks. Moreover Scrimgeour et al.[27] showed similar results in serum and tibia zinc concentrations in rats fed with a low-zinc diet with phytase supplementation. In both studies, higher growth in the phytase groups was observed which was not found in this study. In the present study, the lower mean weight of the phytase group at the beginning (caused by the death of two rats in the middle of the study) might be the reason for their lower terminal growth. Regardless of the reason, this suggests that the positive effect of phytase on blood zinc is specific to phytase itself and independent of growth. Liver zinc did not differ in response to phytase supplementation which is in agreement with Rimbach et al.,[28] Roth and Kirchgessner[29] and Rimbach and Paullof,[30] illustrating that zinc concentration in liver remains largely unaffected by improving zinc supplied. Bone has been utilized as a more reliable index of zinc status in animal studies.[31] In contrast to the studies of McClung et al.,[26] Rimbach et al.,[28] and Scirmgeour et al.,[27] no difference was found in femur bone zinc of our study. This could be due to different diet formulation (maize-soy diet in the former study and purified diet with added synthetic phytic acid in the latter one) or the earlier discussion on higher growth performance in the control group which might cause an interaction effect on bone zinc. In another study of Rimbach and Paullof,[32] zinc status measured by plasma, femur, testes, liver and kidney zinc concentration remained unchanged by adding no or different doses of synthetic phytic acid to a high zinc diet in growing rats. It can be concluded that zinc status response is not linear in different doses of diet phytic acid and zinc. This can also be noted as a reason why simulating a dietary pattern in our study was important.
Phytase addition did not change calcium or iron status significantly. These findings are in agreement with similar animal studies[26,27] and in contrast with human studies on iron published by some researchers.[33–36] However, it is difficult to compare our study results directly with other studies because of different study designs, doses and food vehicle of minerals. Growing rats need substantially more iron and calcium than what was provided by the designed diet. As we intended to imitate the Iranian dietary pattern, the large difference between these mineral requirements along with differences in mineral metabolism makes the results difficult to compare to humans. Reddy and Cook (1991) used a dual radioiron method to determine the extent to which iron absorption measurements in the rat can predict nonheme iron bioavailability in humans. They discovered that meat, ascorbic acid, bran and soy protein, which are known to affect iron absorption in humans, affected iron absorption very little in rats. They concluded that rats are far less sensitive than humans to dietary influences on nonheme iron absorption and are of limited value in assessing this aspect of human nutrition.[37]
Many review articles have concluded that whole grain products exhibit beneficial health effects against many civilization diseases, namely some cancers, coronary heart disease and diabetes mellitus.[38,39] Recent evidences on Iranian patients with type 2 diabetes also show the importance of consuming diets full of whole grain products and its beneficial effects.[40] Keeping in mind that in low-income population, meat as a source of zinc and iron is unaffordable, and therefore recommendations should be given carefully to avoid pessimistic views about whole grain breads which are in accordance with higher phytate intake. Collectively, enzymatic dephytinization which occurs without decreasing whole grain bread intake seems to be theoretically the best solution for such countries.
Turk and Sandberg[41] have shown that the addition of Aspergillus niger phytase to the dough during bread making significantly increases phytate hydrolysis. Use of this highly active low cost microbial phytase seems to be a feasible and promising way for improving the bioavailability of several essential dietary minerals at the same time in Iran. As this enzyme has not received GRAS status (generally regarded as safe) for human consumption yet, further studies on adding this enzyme to Iranian bread dough for measuring to what extent phytase remains intact in the high temperature of the Iranian traditional stove (300-350°C) is recommended. If this enzyme brakes down, these studies collectively will have implications for using phytase as an alternative for mineral supplementation especially zinc in Iranian high phytate flour in the future.
Similar to many previous studies, growing rats were used as a valuable alternative for human studies. Despite efforts that were made to simulate a typical Iranian dietary pattern in this animal model, results cannot necessarily be extrapolated to humans. Therefore, further human studies are necessary to support these results in Iranian adolescents and adults.
CONCLUSION
The source of phytate used in the present study makes it unique and it is hard to find similar studies for comparison. Thus, more efforts should be devoted to low bioavailable dietary patterns used in developing countries. In any event, the results of this study show that phytase can improve blood zinc even under low zinc diet condition in growing rats fed with high phytate Iranian bread.
ACKNOWLEDGEMENTS
We are thankful to Professor Ann-Sofie Sandberg for her valuable scientific comments. We are also especially grateful to Annette Almgren and Nils-Gunnar Carlsson for kindly measuring phytic acid in bread. We also owe thanks to Sara Atef-vahid for devoting her time in providing astute comments and to Rozita Komeili for her help in drying breads.
Footnotes
Source of Support: This study was financially supported by National Nutrition and Food Technology Research Institute as Ph.D. dissertation of Soodeh Shockravi (number of research contract: P/25/47/3259)
Conflict of Interest: None declared.
REFERENCES
- 1.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532–46. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 2.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR, Darby WJ. Biochemical studies on dwarfism, hypogonadism, and anemia. Arch Intern Med. 1963;111:407–28. doi: 10.1001/archinte.1963.03620280007003. [DOI] [PubMed] [Google Scholar]
- 3.Sandstead HH, Prasad AS, Schulert AR, Farid Z, Miale A, Jr, Bassilly S, et al. Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr. 1967;20:422–42. doi: 10.1093/ajcn/20.5.422. [DOI] [PubMed] [Google Scholar]
- 4.Reinhold JG. High phytate content of rural Iranian bread: A possible cause of human zinc deficiency. Am J Clin Nutr. 1971;24:1204–6. doi: 10.1093/ajcn/24.10.1204. [DOI] [PubMed] [Google Scholar]
- 5.Reinhold JG. Phytate concentration of leavened and unleavened Iranian breads. Ecol Food Nutr. 1972;1:187–92. [Google Scholar]
- 6.Mahloudji M, Reinhold JG, Haghshenass M, Ronaghy HA, Fox MR, Halsted JA. Combined zinc and iron compared with iron supplementation of diets of 6- to 12-year old village schoolchildren in southern Iran. Am J Clin Nutr. 1975;28:721–5. doi: 10.1093/ajcn/28.7.721. [DOI] [PubMed] [Google Scholar]
- 7.Fredlund K, Isaksson M, Rossander-Hulthen L, Almgren A, Sandberg AS. Absorption of zinc and retention of calcium: Dose-dependent inhibition by phytate. J Trace Elem Med Biol. 2006;20:49–57. doi: 10.1016/j.jtemb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Erdman J. Phytic acid in health and disease. Crit Rev Food Sci Nutr. 1995;35:495–508. doi: 10.1080/10408399509527712. [DOI] [PubMed] [Google Scholar]
- 9.Torre M, Rodriguez A, Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit Rev Food Sci Nutr. 1991;30:1–22. doi: 10.1080/10408399109527539. [DOI] [PubMed] [Google Scholar]
- 10.Lopez HW, Leenhardt F, Coudray C, Remesy C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int J Food Sci Technol. 2002;37:727–39. [Google Scholar]
- 11.Lonnerdal B. Dietary Factors Influencing Zinc Absorption. J Nutr. 2000;130:1378S–83S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 12.Wise A. Phytate and zinc bioavailability. Int J Food Sci Nutr. 1995;46:53–63. doi: 10.3109/09637489509003386. [DOI] [PubMed] [Google Scholar]
- 13.Oberleas D, Harland BF. Phytate content of foods: Effect on dietary zinc bioavailability. J Am Diet Assoc. 1981;79:433–6. [PubMed] [Google Scholar]
- 14.Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74:445–52. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- 15.Hallberg L, Sandstrom B, Ralph A, Arthur J. Iron, zinc and other trace elements. In: Garrow J, James W, Ralph A, editors. Human nutrition and dietetics. 10th ed. London: Churchill livingstone; 2000. pp. 177–209. [Google Scholar]
- 16.Kalantari N, Ghaffarpour M, HoushiarRad A, Abdollahi M, Kianfar H, Bondarianzadeh D. National comprehensive study on household food consumption pattern and nutritional status IR IRAN, 2001–2003. Tehran: Nutrition Research Department, National Nutrition and Food Technology Research Institute; 2005. [Google Scholar]
- 17.Javadzadeh Shahshahani H, Attar M, Taher Yavari M. A study of the prevalence of iron deficiency and its related factors in blood donors of Yazd, Iran, 2003. Transfus Med. 2005;15:287–93. doi: 10.1111/j.0958-7578.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 18.Kadivar M, Yarmohammadi H, Mirahmadizadeh A, Vakili M, Karimi M. Prevalence of iron deficiency anemia in 6 months to 5 years old children in Fars, Southern Iran. Med Sci Monit. 2003;9:100–4. [PubMed] [Google Scholar]
- 19.Sarraf Z, Goldberg D, Shahbazi M, Arbuckle K, Salehi M. Nutritional status of schoolchildren in rural Iran. Br J Nutr. 2005;94:390–6. doi: 10.1079/bjn20051487. [DOI] [PubMed] [Google Scholar]
- 20.Keikhaei B, Zandian K, Ghasemi A, Tabibi R. Iron-deficiency anemia among children in southwest Iran. Food Nutr Bull. 2007;28:406–11. doi: 10.1177/156482650702800405. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann M, Hurrell R. Improving iron, zinc and vitamin A nutrition through plant biotechnology. Curr Opin Biotechnol. 2002;13:142–5. doi: 10.1016/s0958-1669(02)00304-x. [DOI] [PubMed] [Google Scholar]
- 22.Cunniff P. Official methods of analysis of AOAC International. 16th ed. Gaithersburg, MD: AOAC Int; 1995. [Google Scholar]
- 23.Carlsson NG, Bergman EL, Skoglund E, Hasselblad K, Sandberg AS. Rapid analysis of inositol phosphates. J Agric Food Chem. 2001;49:1695–701. doi: 10.1021/jf000861r. [DOI] [PubMed] [Google Scholar]
- 24.Engelen A, Van der Heeft F, Randsdorp P, Smit E. Simple and rapid determination of phytase activity. J AOAC Int. 1994;77:760–4. [PubMed] [Google Scholar]
- 25.Milne DB, Ralston NV, Wallwork JC. Zinc content of cellular components of blood: Methods for cell separation and analysis evaluated. Clin Chem. 1985;31:65–9. [PubMed] [Google Scholar]
- 26.McClung JP, Stahl CH, Marchitelli LJ, Morales-Martinez N, Mackin KM, Young AJ, et al. Effects of dietary phytase on body weight gain, body composition and bone strength in growing rats fed a low-zinc diet. J Nutr Biochem. 2006;17:190–6. doi: 10.1016/j.jnutbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Scrimgeour A, Marchitelli L, Whicker J, Song Y, Ho E, Young A. Phytase supplementation increases bone mineral density, lean body mass and voluntary physical activity in rats fed a low-zinc diet. J Nutr Biochem. 2010;21:653–8. doi: 10.1016/j.jnutbio.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Rimbach G, Walter A, Most E, Pallauf J. Effect of microbial phytase on zinc bioavailability and cadmium and lead accumulation in growing rats. Food Chem Toxicol. 1998;36:7–12. doi: 10.1016/s0278-6915(97)00117-8. [DOI] [PubMed] [Google Scholar]
- 29.Roth HP, Kirchgessner M. [De- and repletion study of zinc in the bones and liver of growing rats] Arch Tierernahr. 1974;24:283–98. doi: 10.1080/17450397409424270. [DOI] [PubMed] [Google Scholar]
- 30.Rimbach G, Pallauf J. Enhancement of zinc utilization from phytate-rich soy protein isolate by microbial phytase. Z Ernahrungswiss. 1993;32:308–15. doi: 10.1007/BF01611169. [DOI] [PubMed] [Google Scholar]
- 31.Bobilya DJ, Johanning GL, Veum TL, O’Dell BL. Chronological loss of bone zinc during dietary zinc deprivation in neonatal pigs. Am J Clin Nutr. 1994;59:649–53. doi: 10.1093/ajcn/59.3.649. [DOI] [PubMed] [Google Scholar]
- 32.Rimbach G, Pallauf J. Cadmium accumulation, zinc status, and mineral bioavailability of growing rats fed diets high in zinc with increasing amounts of phytic acid. Biol Trace Elem Res. 1997;57:59–70. doi: 10.1007/BF02803870. [DOI] [PubMed] [Google Scholar]
- 33.Troesch B, Egli I, Zeder C, Hurrell RF, de Pee S, Zimmermann MB. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am J Clin Nutr. 2009;89:539–44. doi: 10.3945/ajcn.2008.27026. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Önning G, Öste R, Gramatkovski E, Hulthen L. Improved iron bioavailability in an oat-based beverage: The combined effect of citric acid addition, dephytinization and iron supplementation. Eur J Nutr. 2007;46:95–102. doi: 10.1007/s00394-006-0637-4. [DOI] [PubMed] [Google Scholar]
- 35.Sandberg AS, Hulthen LR, Turk M. Dietary Aspergillus niger phytase increases iron absorption in humans. J Nutr. 1996;126:476–80. doi: 10.1093/jn/126.2.476. [DOI] [PubMed] [Google Scholar]
- 36.Sandberg AS, Brune M, Carlsson NG, Hallberg L, Skoglund E, Rossander-Hulthen L. Inositol phosphates with different numbers of phosphate groups influence iron absorption in humans. Am J Clin Nutr. 1999;70:240–6. doi: 10.1093/ajcn.70.2.240. [DOI] [PubMed] [Google Scholar]
- 37.Reddy MB, Cook JD. Assessment of dietary determinants of nonheme-iron absorption in humans and rats. Am J Clin Nutr. 1991;54:723–8. doi: 10.1093/ajcn/54.4.723. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Sinha A, Makkar H, Becker K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010;120:945–59. [Google Scholar]
- 39.Das A, Raychaudhuri U, Chakraborty R. Cereal based functional food of Indian subcontinent: A review. J Food Sci Technol. 2011;48:1–8. doi: 10.1007/s13197-011-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension Eating Plan Affects C-Reactive Protein, Coagulation Abnormalities, and Hepatic Function Tests among Type 2 Diabetic Patients. J Nutr. 2011;141:1083–8. doi: 10.3945/jn.110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turk M, Sandberg AS. Phytate degradation during breadmaking: Effect of phytase addition. J Cereal Sci. 1992;15:281–94. [Google Scholar]


