Abstract
Prostate-specific membrane antigen (PSMA), a type II membrane glycoprotein, its high expression is associated with prostate cancer progression, and has been becoming an active target for imaging or therapeutic applications for prostate cancer. On the other hand, streptavidin-biotin system has been successfully employed in pretargeting therapy towards multiple cancers. Herein, we describe the synthesis of bifunctional ligands (biotin-CTT54, biotin-PEG4-CTT54 and biotin-PEG12-CTT54) possessing two functional motifs separated by a length-varied polyethylene glycol (PEG) spacer: one (CTT54) binds tumor-marker PSMA and the other (biotin) binds streptavidin or avidin. All three compounds exhibited high potencies (IC50 values: 1.21, 2.53 and 10 nM, respectively) and irreversibility; but only biotin-PEG12-CTT54 demonstrated specifically labeling PSMA-positive prostate cancer cells in a two-step pretargeting procedure. Additionally, the pre-formulated complex between biotin-PEG12-CTT54 and Cy5-streptavidin displayed the improved inhibitory potency (IC50 = 1.86 nM) and irreversibility against PSMA and rapid uptake of streptavidin conjugate into PSMA-positive prostate cancer cells through PSMA-associated internalization. Together, all these results supported a proof-concept that combination of streptavidin and PSMA’s biotinylated inhibitor may lead to development of a novel strategy of tumor-targeting imaging or drug delivery towards prostate cancer.
Keywords: Streptavidin-biotin system, Biotinylated PSMA inhibitor, Tumor-targeting imaging, Prostate cancer
Prostate cancer is the second leading cause of cancer death in men and accounts for approximately 30% of all new cancer diagnoses in men.1 Prostate-specific membrane antigen (PSMA), also named glutamate carboxypeptidase II (GCPII), is a classic type II membrane glycoprotein and is perhaps the most important enzyme-biomarker and target in prostate cancer research. PSMA is up-regulated and strongly expressed on prostate cancer cells, including those that are metastatic.2 Endothelial-expression of PSMA in the neovasculature of a variety of non-prostatic solid malignancies has also been detected.3, 4 as a classical membrane receptor, PSMA can be induced for internalization by bound antibodies or small-molecule inhibitors.8–10 Its extracellular domain possessing folate hydrolase and N-acetylated-alpha-linked-acidic dipeptidase (NAALADase) activities5–7 can serve as binding target for antibodies, inhibitors and aptamers. As a consequence, these properties enable PSMA to serve as an ideal target for the selective delivery of diagnostic and therapeutic agents. The rapid advance in the resolution of its crystal structure11–13 has accelerated the development of various high-affinity chemical inhibitor scaffolds for this enzyme-biomarker.14–21 The recent successful deployment of PSMA inhibitors as targeting motifs for imaging and therapeutic agents supports their development as formidable pharmacokinetic alternatives to antibodies.9, 22–26
Streptavidin, a bacterium-produced tetrameric protein, is well known for its extremely high affinity for biotin (Kd ≈ 10−15 M)27, 28 and a wide range of applications in biomolecule purification, protein assays, diagnostics, and drug delivery.29–31 In tumor targeting applications, based upon biotin-streptavidin system, pretargeting strategy has been developed in which a monoclonal antibody conjugated with streptavidin or biotin, is first administered to target tumor-associated antigens or receptors followed by the administration of biotin or streptavidin conjugated to imaging or drug agents destined for the pre-targeted antibody.32
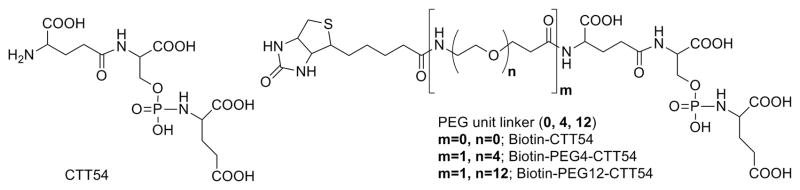
We previously reported that phosphoramidate peptidomimetic PSMA inhibitors were capable of both cell-surface labeling of prostate tumor cells for imaging9, 22, 33 and intracellular delivery for targeted photodynamic therapy.23, 34, 35 We have observed that a suitable-length spacer installed between the PSMA inhibitor core and its diagnostic or therapeutic payload was necessary for ensuring both inhibitory potency against PSMA and positive in vitro performance.36 Our previous work also confirmed that small-molecule inhibitor can induce internalization of PSMA-inhibitor complex9 albeit slower than that reported for antibody-induced rapid internalization.8 In the present study, we examined the feasibility of a macromolecular tumor-targeting scaffold for prostate cancer by employing a biotin-streptavidin coupling system as a model. Biotinylated PSMA inhibitors containing variable polyethylene glycol (PEG) spacers (biotin-CTT54, biotin-PEG4-CTT54, and biotin-PEG12-CTT54, Figure 1) were prepared and initially evaluated for their inhibitory potency against purified PSMA. In a two-step pretargeting study, it was found that only biotin-PEG12-CTT54 could successfully recruit Cy5-streptavidin to the cell surface of PSMA-positive (PSMA+) LNCaP cells. The pre-formulated complex between Cy5-streptavidin and biotin-PEG12-CTT54 not only exhibited enhanced inhibitory potency against purified PSMA compared to biotin-PEG12-CTT54 alone presumably through the multivalent presentation of targeting molecules (CTT54), but also demonstrated considerable cell labeling of PSMA+ cells along with rapid internalization.
Figure 1.
Structures of PSMA inhibitor core CTT54 and its biotinylated conjugates Biotin-PEG12-CTT54, Biotin-PEG4-CTT54, and Biotin-CTT54.
The preparation of the biotinylated inhibitors is described in the Supplementary Data and structures were confirmed by MALDI-HRMS analysis (Figure S1A–C). Based on PSMA inhibition studies (Table S1), biotinylation of CTT549, 23, 37 through various PEG spacer lengths (biotin-CTT54, IC50 = 1.21 nM; biotin-PEG4-CTT54, IC50 = 2.53 nM; biotin-PEG12-CTT54, IC50 = 10 nM) had no adverse effect upon the inhibitory potency of the parent inhibitor core CTT54 (IC50 = 14 nM).9 To further understand the consequence of spacer length on the biotinylated PSMA inhibitors, we examined the enzymatic activity recovery profiles for each conjugate as described previously.20, 22 As was the case for CTT54,36 all biotinylated conjugates exhibited an irreversible mode of binding (Figure S2).
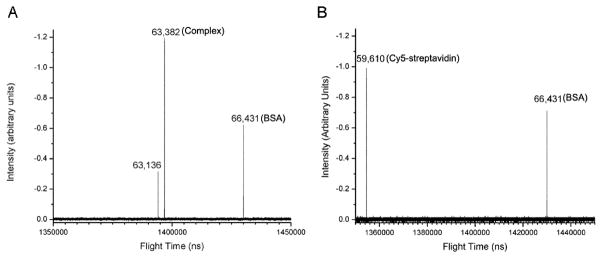
To determine the effect of spacer length between biotin and the PSMA inhibitor core on in vitro imaging of PSMA+ cells, LNCaP cells were treated with each of the biotin conjugates followed by incubation with Cy5-streptavidin. Confocal microscopy imaging revealed that only LNCaP cells treated with biotin-PEG12-CTT54 exhibited a fluorescence signal, albeit weak, of primarily internalized Cy5-streptavidin (Figure S3C). This data suggested that unlike PEG12, shorter spacers between biotin and the PSMA targeting core of biotin-CTT54 and biotin-PEG4-CTT54 precluded the concomitant binding to both streptavidin and PSMA (Figure S3A, B). Based upon this result, Cy5-streptavidin:biotin-PEG12-CTT54 complex was pre-formulated according to the method described in the Supplementary data. The pre-formulated complex was characterized using a newly-developed digitally-operated linear quadrupole ion trap orthogonal acceleration time-of-flight mass spectrometer.38–41 Bovine serum albumin was employed as an internal standard (Figure 2, m/z 66,431), and the pre-formulated complex (Figure 2A, m/z 63,382) was found to contain three biotinylated inhibitors associated with the Cy5-streptavidin tetramer (Figure 2B, m/z 59,610).
Figure 2.
Mass spectra of (A) the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex and (B) Cy5-streptavidin alone. The mass difference between the pre-formulated complex and Cy5-streptavidin is 3.772 Kd; three Biotin-PEG12-CTT54 molecules and two displaced water molecules.
In terms of affinity for PSMA, the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex exhibited the improved inhibitor potency (IC50 = 1.86 nM) over the noncomplexed biotin-PEG12-CTT54 (IC50 = 10 nM) and maintained an irreversible mode of inhibition (Supplementary data, Table S1, Figure S2). These results support the use of a streptavidin tetramer as targeting platform with the advantage of enhanced overall binding affinity compared to monovalent targeting molecules through multivalent binding or statistical rebinding.42
To determine whether the use of the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex was at least comparable in in vitro performance to the pretargeting strategy using monovalent biotin-PEG12-CTT54, cellular specificity and uptake in PSMA+ cells was determined. While LNCaP cells treated with the pre-formulated complex exhibited exceptional fluorescence labeling (Figure 3A), no fluorescence labeling was observed for the PSMA-negative PC-3 cells treated similarly (Figure 3B). Treating LNCaP cells first with nonfluorescent CTT54 effectively blocked the pre-formulated complex from labeling these cells (Figure 3C). No fluorescence was observed on LNCaP cells treated with Cy5-streptavidin only (Figure 3D). These results suggested that the cell labeling with the pre-formulated complex (Figure 3A) was due to the targeting molecule CTT54 and not a result of nonspecific binding. Compared to the pretargeting strategy (Figure S3C), administration of the pre-formulated complex resulted in considerably greater fluorescence consistent with its enhanced affinity for PSMA.
Figure 3.
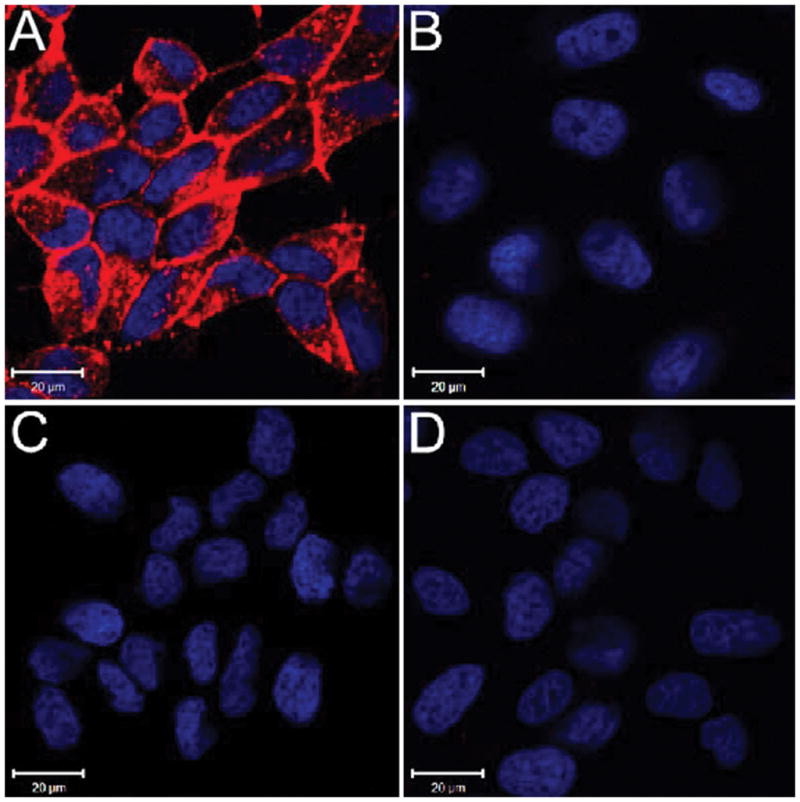
Selective labeling of PSMA+ cells with the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex. (A) LNCaP and (B) PC-3 cells treated with the pre-formulated complex (600 nM) for 1 h at 37 °C. (C) LNCaP cells pretreated (30 min) with nonfluorescent CTT54 (80 μM) prior to treatment with the pre-formulated complex. (D) LNCaP cells treated with Cy5-streptavidin only (600 nM). All cells were fixed and nuclei stained with Hoechst 33342 (blue). Cy5 fluorescence was assigned as pseudocolored red. Distance scale is 20 μm.
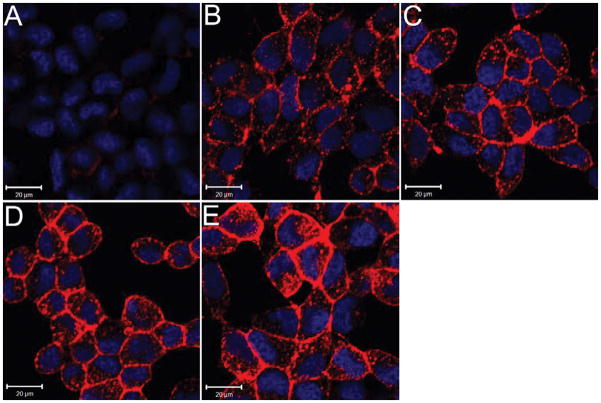
It is known that antibody binding can induce PSMA internalization in PSMA+ cancer cells within 20 min.8 We recently observed that approximately 70% of a 99mTc-radiolabeled conjugate of CTT54 targeted to PSMA+ cells was internalized by 30 min.43 In contrast, the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex exhibited rapid internalization into PSMA+ cells as early as 10 min upon binding PSMA molecules on the cellular surface (Figure 4B). Fluorescence intensity in cytoplasm increased with time (Figure 4C–D) and by 60 min, intense labeling was observed in the perinuclear region of the cell (Figure 4E). Co-localization of internalized Cy5-streptavidin:biotin-PEG12-CTT54 with transferrin-Fluorescein was visualized in merged confocal microscopy images (Figure S4). The pattern of PSMA internalization induced by the pre-formulated complex is consistent with that induced by antibodies or small-molecule inhibitors.8, 9, 20 Therefore, the results presented herein supported a proof-of-concept that pre-formulated complexes of functionalized streptavidin and biotinylated PSMA inhibitors may lead to development of a novel strategy for prostate tumor-targeted imaging or chemotherapy.
Figure 4.
Time-dependent uptake of the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex in PSMA+ cells. Live LNCaP cells were incubated with pre-formulated complex (600 nM) for 5 (A), 10 (B), 15 (C), 30 (D), and 60 (E) min at 37 °C. All cells were fixed and nuclei stained with Hoechst 33342 (blue). Cy5 fluorescence was assigned as pseudocolored red. Distance scale is 20 μm.
In conclusion, the pre-formulated complex between Cy5-streptavidin and biotin-PEG12-CTT54 displayed improved inhibitory potency against purified PSMA compared to the monovalent biotin-PEG12-CTT54 PSMA inhibitor, which is consistent with binding enhancement through multivalency. In addition, the pre-formulated Cy5-streptavidin:biotin-PEG12-CTT54 complex more rapidly and selectively internalized into PSMA+ prostate cancer cells via PSMA-mediated endocytosis. Moreover, the selectivity and uptake of the pre-formulated complex containing Cy5 as a model payload suggests that streptavidin can serve as a carrier platform for clinically-relevant imaging agents or therapeutic drug molecules to target both PSMA+ tumor cells and neovasculature. Therefore, the results presented here suggest that one-step tumor targeting using pre-formulated streptavidin complexes with biotinylated small-molecule PSMA inhibitors may serve as a novel drug delivery platform for PSMA-targeted chemotherapy for both prostate tumors and tumor-associated neovasulature.
Supplementary Material
Acknowledgments
The authors extend their gratitude for technical assistance to G. Helms, W. Hiscox and Gerhard R. Munske at WSU for NMR Spectroscopy and high-resolution mass spectrometry, as well as C. Davitt and V. Lynch-Holm at the WSU Franceschi Microscopy and Imaging Center. This work was supported in part by the Washington State Life Sciences Discovery Fund (LSDF 08-01 2374880) and the National Institutes of Health (5R01CA140617-02).
Footnotes
Supplementary data described experimental details for the synthesis, preparation of pre-formulated complex, PSMA inhibition, MS analysis and cellular imaging.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Cancer. 2004;101:3. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.Bacich DJ, Pinto JT, Tong WP, Heston WD. Mamm Genome. 2001;12:117. doi: 10.1007/s003350010240. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Reuter VE, Heston WD, Gaudin PB. Urology. 2001;57:1179. doi: 10.1016/s0090-4295(01)00983-9. [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Clin Cancer Res. 1999;5:2674. [PubMed] [Google Scholar]
- 5.Grauer LS, Lawler KD, Marignac JL, Kumar A, Goel AS, Wolfert RL. Cancer Res. 1998;58:4787. [PubMed] [Google Scholar]
- 6.Carter RE, Feldman AR, Coyle JT. Proc Natl Acad Sci U S A. 1996;93:749. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, May F, Mukherjee B, Heston WD. Clin Cancer Res. 1996;2:1445. [PubMed] [Google Scholar]
- 8.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, Rahmati R, Bander NH. Cancer Res. 1998;58:4055. [PubMed] [Google Scholar]
- 9.Liu T, Wu LY, Kazak M, Berkman CE. Prostate. 2008;68:955. doi: 10.1002/pros.20753. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Kopeckova P, Buhler P, Wolf P, Pan H, Bauer H, Elsasser-Beile U, Kopecek J. Mol Pharm. 2009;6:959. doi: 10.1021/mp8002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesters JR, Henning K, Hilgenfeld R. Acta Crystallogr D Biol Crystallogr. 2007;63:508. doi: 10.1107/S090744490700902X. [DOI] [PubMed] [Google Scholar]
- 12.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Proc Natl Acad Sci U S A. 2005;102:5981. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R. Embo J. 2006;25:1375. doi: 10.1038/sj.emboj.7600969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding P, Helquist P, Miller MJ. Org Biomol Chem. 2007;5:826. doi: 10.1039/b615603g. [DOI] [PubMed] [Google Scholar]
- 15.Majer P, Hin B, Stoermer D, Adams J, Xu W, Duvall BR, Delahanty G, Liu Q, Stathis MJ, Wozniak KM, Slusher BS, Tsukamoto T. J Med Chem. 2006;49:2876. doi: 10.1021/jm051019l. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Singh P, Topaloglu O, Isaacs JT, Denmeade SR. Cancer Res. 2006;66:9171. doi: 10.1158/0008-5472.CAN-06-1520. [DOI] [PubMed] [Google Scholar]
- 17.Wone DW, Rowley JA, Garofalo AW, Berkman CE. Bioorg Med Chem. 2006;14:67. doi: 10.1016/j.bmc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Neale JH, Pomper MG, Kozikowski AP. Nat Rev Drug Discov. 2005;4:1015. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto T, Wozniak KM, Slusher BS. Drug Discov Today. 2007;12:767. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Toriyabe Y, Kazak M, Berkman CE. Biochemistry. 2008;47:12658. doi: 10.1021/bi801883v. [DOI] [PubMed] [Google Scholar]
- 21.Wu LY, Anderson MO, Toriyabe Y, Maung J, Campbell TY, Tajon C, Kazak M, Moser J, Berkman CE. Bioorg Med Chem. 2007;15:7434. doi: 10.1016/j.bmc.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Wu LY, Hopkins MR, Choi JK, Berkman CE. Bioorg Med Chem Lett. 2010;20:7124. doi: 10.1016/j.bmcl.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Wu LY, Choi JK, Berkman CE. Prostate. 2009;69:585. doi: 10.1002/pros.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. J Nucl Med. 2009;50:2042. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, Zimmerman CN, Barrett JA, Eckelman WC, Pomper MG, Joyal JL, Babich JW. Cancer Res. 2009;69:6932. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kularatne SA, Wang K, Santhapuram HK, Low PS. Mol Pharm. 2009;6:780. doi: 10.1021/mp900069d. [DOI] [PubMed] [Google Scholar]
- 27.Chaiet L, Wolf FJ. Arch Biochem Biophys. 1964;106:1. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- 28.Hendrickson WA, Pahler A, Smith JL, Satow Y, Merritt EA, Phizackerley RP. Proc Natl Acad Sci U S A. 1989;86:2190. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer EA, Wilchek M. J Chromatogr. 1990;510:3. doi: 10.1016/s0021-9673(01)93733-1. [DOI] [PubMed] [Google Scholar]
- 30.Laitinen OH, Nordlund HR, Hytonen VP, Kulomaa MS. Trends Biotechnol. 2007;25:269. doi: 10.1016/j.tibtech.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Lesch HP, Kaikkonen MU, Pikkarainen JT, Yla-Herttuala S. Expert Opin Drug Deliv. 2010;7:551. doi: 10.1517/17425241003677749. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. J Clin Oncol. 2006;24:823. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Jabbes M, Nedrow-Byers JR, Wu LY, Bryan JN, Berkman CE. Int J Oncol. 2011 doi: 10.3892/ijo.2011.946. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Wu LY, Choi JK, Berkman CE. Int J Oncol. 2010;36:777. doi: 10.3892/ijo_00000553. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Wu LY, Berkman CE. Cancer Lett. 2010;296:106. doi: 10.1016/j.canlet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Nedrow-Byers JR, Hopkins MR, Berkman CE. Bioorg Med Chem Lett. 2011;21:7013. doi: 10.1016/j.bmcl.2011.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T, Jabbes M, Nedrow-Byers JR, Wu LY, Bryan JN, Berkman CE. Int J Oncol. 2011;38:1349. doi: 10.3892/ijo.2011.946. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi H, Whitten WB, Reilly PT. J Am Soc Mass Spectrom. 2008;19:1942. doi: 10.1016/j.jasms.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Koizumi H, Wang X, Whitten WB, Reilly PT. Journal of The American Society for Mass Spectrometry. 2010;21:242. doi: 10.1016/j.jasms.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Marino MA, Koizumi H, Reilly PT. Int J Mass Spectrom. 2011;304:36. doi: 10.1016/j.ijms.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Chen H, Liu T, Berkman CE, Reilly PT. Anal Chem. 2011;83:9406. doi: 10.1021/ac202001z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathai M, Seok-Ki C, George MW. Angew Chem Int Ed. 1998;37:2754. [Google Scholar]
- 43.Nedrow-Byers JR, Jabbes M, ewett C, Ganguly T, He H, Liu T, Benny P, Bryan JN, CEB The Prostate. 2011 doi: 10.1002/pros.21493. Epub Oct 5, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Proc Natl Acad Sci U S A. 1999;96:2379. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alon R, Bayer EA, Wilchek M. Eur J Cell Biol. 1993;60:1. [PubMed] [Google Scholar]
- 46.Liu T, Toriyabe Y, Berkman CE. Protein Expr Purif. 2006;49:251. doi: 10.1016/j.pep.2006.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.