Abstract
The aim of this study was to determine the relationship between airway blood flow (Q̇aw), airway conductance (Gf-aw) and pulmonary function in patients with stable HF. 12 controls (CTRL: age=63±9yr, FVC=98±15%pred, LVEF=61±6%) (all data presented as mean±SD), 16 patients with mild HF (HF-A, NYHA I–II: age=64±9yr, FVC=90±17%pred, LVEF=28±6%), and 14 patients with moderate/severe HF (HF-B, NYHA III–IV: age=65±6yr, FVC=84±12%pred, LVEF=26±6%) were studied. Q̇aw was assessed using soluble gas measurements; perfusion pressure across airway bed (ΔPaw) was estimated from systemic and pulmonary pressure measurements; Gf-aw was calculated as Q̇aw/ΔPaw; PF was assessed by spirometry. While Q̇aw was not significantly different between CTRL (61.3±17.9 μL·min−1·ml−1), HF-A (70.1±26.9 μL·min−1·ml−1) and HF-B (56.2±14.9 μL·min−1·ml−1) groups, Gf-aw, was elevated in HF-A (1.1±0.4 μL·min−1·ml−1·mmHg−1, p<0.03) and tended to be elevated in HF-B (1.2±0.6 μL·min−1·ml−1·mmHg−1, p=0.07) when compared to CTRL (0.8±0.3 μL·min−1·ml−1·mmHg−1). Significant positive correlations were found between Gf-aw and RV/TLC for HF-A (r=0.63, p<0.02) and HF-B (r=0.58, p<0.05). These results support the hypothesis that increased bronchial conductance and bronchial congestion may be related to greater small airway obstruction and as such may play a role in the PF abnormalities and symptoms of congestion commonly observed in HF patients.
Keywords: Congestion, Left Ventricular Dysfunction, Bronchial Blood Flow
1. Introduction
The changes in pulmonary function with chronic heart failure (HF) are well documented, with patients typically presenting with a combined restrictive/obstructive pattern, increased airway resistance and marked ventilatory constraint both at rest and during exercise (Johnson et al. 2000; Johnson et al. 2001). We have previously hypothesized that small changes in the caliber of airway mucosal vasculature could alter the morphometry of compliant distal airways, displacing tissue towards the adjacent lumen airspace and explain in part, the combined obstructive/restrictive and increased airway resistance seen in HF (Ceridon et al. 2009). This engorgement of the bronchial mucosal and change in airway caliber may occur because the bronchioles are highly vascularized and the bronchial circulation lies near the airway lumen surface.
Currently, there is no direct evidence that changes in bronchial circulation are related to measurable changes in pulmonary function in HF patients. However the results of several studies provide indirect evidence that changes in bronchovascular conductance may be related to changes in pulmonary function and symptoms of congestion in HF (Cabanes et al. 1992; Lockhart et al. 1992; Wetzel et al. 1993; Agostoni et al. 1995; McIlveen 2000). In an early study of anesthetized mini-pigs, Wetzel and colleagues (1993) demonstrated that intravascular volume loading resulted in bronchial mucosal engorgement and decreased airway cross sectional area. These authors concluded that this type of mucosal engorgement may be a cause of airway obstruction in HF patients (Wetzel et al. 1993). In a study of HF patients undergoing cardiopulmonary bypass, Agostoni et al. (1995) reported an increased bronchovascular conductance (Gf-aw) in HF patients compared with healthy control subjects. While this study did not examine changes mucosal blood flow or cross sectional area of the airway, these changes in conductance may in turn be consistent with bronchial vascular engorgement postulated to occur in chronic HF. Similarly in a group of HF patients reporting moderate exertional dyspnea, Cabanes and colleagues reported a blunted methacholine-induced bronchial obstruction and improved maximal exercise performance with inhalation of methoxamine, an α1-specific adrenergic receptor agonist known to constrict the bronchial vasculature (Cabanes et al. 1989; Cabanes et al. 1992). These authors concluded that exercise-induced vasodilation (or engorgement) of the bronchial vasculature and subsequent bronchial obstruction may contribute to exertional dyspnea in this group of HF patients.
The regulation of bronchial vascular tone is complex and there are a number of pathophysiological alterations characteristic of HF likely to affect the vascular tone and structure of this circulation. In healthy individuals, the bronchial circulation is under efferent autonomic control and is tonically vasoconstricted (Hennessy et al. 1993; McIlveen et al. 1997). Airway blood flow and conductance are sensitive to changes in pulmonary vascular pressures (Agostoni et al. 1987) and chemical mediators of inflammation (Long et al. 1990), both of which are altered in chronic, stable HF patients. Additionally, it has been shown that the stretch of cardiac tissue alters the hemodynamics in the bronchial circulation via afferent neural pathways (McIlveen 2000; White et al. 2003). Hence it is possible then that the multiple physiological changes that occur with HF may potentially impact on Q̇aw and Gf-aw.
To date there have been no studies directly examining the relationship between Q̇aw and pulmonary function in healthy or heart failure patients. This is primarily because the bronchial circulation is difficult to access and study in humans. The relatively recent development of a modified soluble gas technique to non-invasively measure Q̇aw has provided a means for further study of the bronchial circulation in human subjects (Wanner et al. 2006). This technique, which has been validated against accepted invasive techniques in animals (Scuri et al. 1995) has made the measurement of Q̇aw more practical to perform in clinical populations and to our knowledge, there have been no previous studies conducted that directly measure Q̇aw in a population of stable, ambulatory HF patients.
It is worth noting that the reported measures of airway blood flow in the literature are dependent on the specific methods used and typically reflect one of the following: the total blood inflow from systemic sources into the bronchial circulation (total Q̇aw); the outflow of blood from the bronchial circulation occurring via the bronchopulmonary anastomoses (anastomotic Q̇aw); or blood flow in the bronchial circulation occurring through the vessels within the bronchial mucosa (mucosal Q̇aw). The technique described in this study measures Q̇aw of the bronchial mucosa and thus use of the term Q̇aw from here on will be in reference to mucosal Q̇aw unless otherwise stated.
Therefore the aim of the present study was to firstly quantify Q̇aw and Gf-aw at rest in stable HF patients versus healthy matched controls, and secondly determine if Q̇aw and Gf-aw are related to pulmonary function in HF.
2. Methods
2.1 Study design
Q̇aw was measured in 30 HF patients and in 12 healthy controls using soluble gas techniques. Q̇aw measurements and systemic and cardiac pressure measurements were used to determine Gf-aw. Measures of Q̇aw and Gf-aw were then related to pulmonary function measurements.
2.2. Ethical Information
The protocol was reviewed and approved by the Mayo Clinic Institutional Review Board and all participants provided informed written consent prior to participation. All procedures followed guidelines set forth by Health Insurance Portability and Accountability Act and complied with the standards set in the Declaration of Helsinki.
2.3 Subjects
Patients with HF were recruited from the Mayo Clinic Heart Failure Service and from the Cardiovascular Health Clinic. Inclusion criteria for all HF patients consisted of the following: a primary diagnosis of congestive HF and categorization in the NYHA classification system, stable (no hospital admissions or medication changes within one month of the study), and a left ventricular ejection fraction (EF) < 35%. Exclusion criteria included significant obesity (BMI > 35 kg/m2), smoking history > 15 pack-years or other co-morbidities that could influence study results separate from the effects of HF (i.e. lung disease, diabetes). All patients maintained their optimized medication regimens while participating in the study.
HF patients were separated into two groups: Mild HF-A, patients with symptoms classified as NYHA Class I–II and moderate/severe HF-B patients with symptoms classified as NYHA Class III–IV. A third group of healthy individuals that were age- and sex-matched (approximately) to the patient population were used as a control group (CTRL).
Control subjects were recruited from the surrounding community. Inclusion criteria for CTRL included the following: normal cardiac function (left ventricular EF > 50%), BMI < 35 kg/m2, a non-significant smoking history (< 15 pack-years), and no history of diabetes, lung disease, or coronary artery disease.
2.4 Protocol
Prior to the study all participants underwent a screening visit that included pulmonary function tests, a complete blood count, and pregnancy testing for women of child bearing potential. Subjects with HF were evaluated clinically prior to recruitment. Once participant eligibility was confirmed from the screening visit, subjects reported to the laboratory on two separate days. Day 1 data collection included measures of resting pulmonary function, hemodynamics, oxygen saturation, cardiac output (Q̇), lung membrane diffusing capacity (DM), diffusing capacity for carbon monoxide (DLCO), diffusing capacity of nitric oxide (DLNO) and pulmonary capillary blood volume (VC). Echocardiographic and Q̇aw measures were made on Day 2.
2.5 Pulmonary Function Measurements
Participants underwent pulmonary function testing which consisted of spirometry and body plethysmography (Medgraphics, Elite Series Plethysmograph, St Paul, MN, USA). Measures obtained included forced vital capacity (FVC), forced expiratory volume in one second (FEV1), mean maximal expiratory flows between 25 and 75% of FVC (FEF25–75), total lung capacity (TLC) and residual volume (RV). All measures were collected in accordance with American Thoracic Society (ATS) guidelines (Miller et al. 2005).
2.6 Resting Hemodynamics and Lung Diffusing Capacity
Heart rate (HR) and arterial oxygen saturation (%SaO2) were continuously monitored using a pulse oximeter (Nellcor, N 595, Dublin, Ireland). Blood pressure (BP) was assessed by sphygmomanometer and was taken by the same technician throughout the entire study.
Rebreathe techniques measuring the simultaneous disappearance of acetylene, carbon monoxide (CO) and nitric oxide (NO) were used to non-invasively estimate, DM, and VC as described previously by our laboratory (Snyder et al. 2006). Briefly, A 5-liter rebreathe bag was filled with 0.3% carbon monoxide (C18O), 0.7% acetylene, 9% helium, 35% O2, 40 parts per million (ppm) NO in an N2 balance. Gases were sampled by mass spectrometer (Perkin-Elmer, MGA 1100, St Louis, MO, USA) and nitric oxide analyzer (Sievers Instruments, Boulder, CO, USA) and analysis was performed using a custom software package (Snyder et al. 2007). The volume of gas used to fill the rebreathe bag was determined by the tidal volume of the subject. At the end of a normal expiration, subjects were switched into the rebreathe bag and instructed to nearly empty the bag with each breath for 10 consecutive breaths. Following each diffusing capacity maneuver, the rebreathe bag was emptied with a suction device and refilled immediately before the next maneuver. All measures of Q̇, DM and VC were repeated triplicate with the mean value being reported.
Serial measurements of gas concentrations enabled calculation of the ratios of carbon monoxide/helium and acetylene/helium to estimate the diffusing capacity for carbon monoxide (DLCO) and Q̇ as previously described (Snyder et al. 2007). Calculations of DM and VC were based on the solubility of CO and NO and determined according to the Roughton-Forster equation (Roughton et al. 1957): 1/DLCO = 1/DMCO + 1/(ΘCO × Vc), where 1/θ = (0.73 + 0058 PAO2) × 14.6/Hgb, and DMCO is determined from the ratio DLNO/DMCO = DMNO/DMCO = αNO/αCO ×√(MWco/MWNO) which yields a value of 1.99 (Meyer et al. 1990). Additionally, because each maneuver is performed with less than 30 seconds of rebreathing, the recirculation of acetylene, CO, and NO are considered to be negligible (Morris et al. 2007). For our laboratory, the coefficients of variation are 7.2% for DM and 6.4% for VC.
2.7 Echocardiographic Measures to Estimate of Mean Left Atrial Pressure
Echocardiographic images were acquired using a 4MHz matrix array cardiac transducer (Vivid 7, General Electric, Milwaukee, WI, USA). All images were acquired with the subject was in a semi-recumbent position from either the apical or parasternal position. Doppler echocardiography and tissue Doppler imaging techniques as described by (Oh et al. 2006) were used to determine early diastolic mitral inflow velocity (E) and medial mitral annulus velocity (e′). The ratio of the inflow velocity to the annulus velocity (E/e′) was then used to non-invasively estimate pulmonary capillary wedge pressure (PCWP) as described by (Nagueh et al. 1997).
2.8 Estimation of Bronchovascular Perfusion Pressure
Because the bronchial circulation originates from a systemic source and largely drains into left atria via the pulmonary vasculature, flow in the bronchial vascular bed is affected by both upstream systemic pressures and by downstream pulmonary pressures (Wagner et al. 1990). An estimate of the perfusion pressure across the airway circulatory bed (ΔPaw) was calculated as the difference between systemic mean arterial pressure (MAP) and the estimated PCWP i.e. MAP–PCWP.
2.9 Measurement of Airway Blood Flow
Q̇aw was measured non-invasively using a modified soluble gas technique (Wanner et al. 2006) as previously described by our laboratory (Morris et al. 2008). Briefly, subjects breathed on a mouthpiece connected to a pneumotachometer and a sliding valve system. Gas concentrations for N2 and dimethyl ether (DME) were continuously sampled using a mass spectrometer (MGA 1100, Perkins Elmer, St. Louis, MO). Flow, volume, and gas concentration signals were recorded throughout the breathing maneuvers and analyzed using custom analysis software (Morris et al. 2008).
Subjects were coached through the following breath-hold maneuver. In rapid succession, subjects inhaled to total lung capacity, expired slowly until ~500 ml of air had been exhaled, and inhaled the test gas mix consisting of 9% DME, 91% N2 (Praxair, Danbury, CT) back to TLC. Subjects were then coached to hold their breath with an open glottis for a specified time period. A final exhalation was then performed through a critical flow orifice that controlled expiratory flow rate at ~0.25 liters per second. Breath-hold times were set at 5, 8, 10, and 13 seconds and subjects performed a minimum of two maneuvers at each breath-hold time. In our laboratory, the test-retest reliability for determining resting Q̇aw is 0.98 with a coefficient of variance of 3.8% (Morris et al. 2008).
2.10 Calculation of Bronchovascular Conductance and Bronchovascular Resistance
Bronchovascular conductance was calculated as Q̇aw divided by ΔPaw.
2.11 Data Analysis
All statistical comparisons were made using the SPSS statistical software package (Version 12.0, Chicago, IL). One-way ANOVA testing was used to compare variables between the three groups (HF-A, HF-B, and CTRL). Levene’s test was applied to each variable to determine the equality of variance between groups. Bonferroni or Tamhane’s T2 post hoc analyses were applied when comparing variables of equal or unequal variance, respectively. Fisher’s exact test was used to examine the difference in medication usage and other categorical variables between the three groups. Correlation and covariance were determined with Pearson Mean Correlation Coefficients. Significance was set at α=0.05. Values are reported as mean ± (SD unless otherwise noted.
3. Results
Forty-two subjects completed testing: 16 in HF-A (NYHA Class I-II), 14 in HF-B (NYHA Class III-IV), and 12 age- and sex-matched healthy controls (CTRL). Subject characteristics and medications are summarized in Table I. There were no significant differences in age, height and body weight between groups, however BMI was significantly higher in the mild HF-A group when compared to CTRL (p<0.05) and tended to be higher for the moderate/severe HF-B group. LVEF was significantly reduced in both HF groups when compared to CTRL (p<0.05).
Table I.
Subject Characteristics and Medications
| Characteristic | Control | HF Group A (NYHA Class I–II) | HF Group B (NYHA Class III–IV) |
|---|---|---|---|
| N (female) | 12 (2) | 16 (5) | 14 (3) |
| Age (years) | 63±9 | 64±9 | 65±6 |
| Body Mass Index (kg/m2) | 25.5±3.5 | 30.6±5.3 * | 29.1±4.2 |
| Body Surface Area (m2) | 2.0±0.2 | 2.1±0.3 | 2.0±0.2 |
| Ejection Fraction (%) | 60.7±6.3 | 28.0±5.5 * | 25.9±5.5 * |
| Medications | |||
| Aspirin | 25% (3) | 69% (11) * | 93% (13) * |
| ACE-Inhibitor | 0% (0) | 69% (11) * | 79% (11) * |
| Angiotensin II receptor antagonist | 0% (0) | 25% (4) | 29% (4) |
| β-Adrenergic receptor antagonist | 0% (0) | 81% (13) * | 100% (14) * |
| β1-selective antagonist | – | 25% (4) | 43% (6) * |
| Non-selective antagonist | – | 69% (11) * | 64% (9) * |
| Digitalis | 0% (0) | 44% (7) * | 57% (8) * |
| Diuretic | 6% (1) | 75% (12) * | 57% (8) * |
| Statin | 25% (3) | 69% (11) * | 57% (8) |
ACE = angiotensin converting enzyme, data are reported as mean ± SD and % (number).
Significantly different from Control group.
3.1 Hemodynamics and Lung Diffusing Capacity
Hemodynamic and diffusing capacity measures are summarized in Table II. Compared to CTRL, MAP was significantly lower and PCWP significantly higher in both HF groups (p<0.05). As a result, the calculated ΔPaw was significantly lower in both HF groups (CTRL: 81.4±7.8 mmHg, HF-A: 64.4±12.5 mmHg, HF-B: 57.0±16.6 mmHg, p<0.01 when compared to CTRL). VC was lower in mild HF-A when compared to CTRL (p<0.05). Although values tended to be lower in HF patients, no significant differences in DLCO, DLNO, and DM were observed between groups. Similarly there was a tendency for the values for Q̇ and SV, to be lower in both HF groups when compared to CTRL, however these comparisons did not reach significance (p>0.05).
Table II.
Hemodynamics, Lung Diffusing Capacity, Pulmonary Function and Lung Volumes
| Control | HF Group A (NYHA Class I–II) | HF Group B (NYHA Class III–IV) | |
|---|---|---|---|
| Hemodynamics | |||
| HR, beats/min | 66 ± 14 | 58 ± 4 | 68 ± 10 † |
| SBP, mmHg | 125 ± 12 | 115 ± 19 | 106 ± 16 * |
| DBP, mmHg | 79 ± 8 | 71 ± 10 | 68 ± 10 * |
| MAP, mmHg | 94 ± 8 | 83 ± 9 * | 80 ± 10 * |
| Q, L/min | 4.4 ± 1.1 | 3.7 ± 1.3 | 3.4 ± 0.9 |
| SV, ml | 71 ± 9 | 62 ± 25 | 48 ± 10 |
| PCWP, mmHg | 11.3 ± 2.1 | 18.7 ± 7.2* | 22.8 ± 7.7* |
| SaO2, % | 97 ± 29 | 97 ± 2 | 97 ± 2 |
| Diffusing Capacity and Components | |||
| DLCO, ml/min/mmHg | 17.4 ± 5.6 | 14.3 ± 4.8 | 14.7 ± 3.9 |
| DLNO, ml/min/mmHg | 55.5 ± 19.1 | 50.5 ± 19.7 | 50.8 ± 15.4 |
| DM, ml/min/mmHg | 28.8 ± 9.9 | 26.2 ± 10.2 | 26.3 ± 8.0 |
| VC, ml | 89.7 ± 26.8 | 65.9 ± 20.5 * | 73.6 ± 26.7 |
SBP = systolic blood pressure (systemic), DBP = diastolic blood pressure (systemic), MAP = mean arterial pressure (systemic), Q = cardiac output, SV = stroke volume, PCWP: Pulmonary Capillary Wedge Pressure, SaO2 = arterial oxygen saturation, DLCO = diffusing capacity of the lung for CO, DLNO = diffusing capacity of the lung for NO, DM = diffusing capacity for the entire lung membrane, VC = pulmonary capillary blood volume. Data reported as mean ± SD.
p<0.05 when compared to Control.
p<0.05 when compared to HF Group A.
3.2 Pulmonary Function Measurements
Results of pulmonary function testing are shown in Table III. Overall, patients with HF tended to have lower maximal expiratory testing values compared to CTRL with measures of FEV1 (%predicted) and FEF25–75 reaching significance. Pulmonary function values were similar within HF groups (p>0.05).
Table III.
Pulmonary Function Testing and Lung Volumes
| Control | HF Group A (NYHA Class I–II) | HF Group B (NYHA Class III–IV) | |
|---|---|---|---|
| FVC, L | 4.4 ± 1.0 | 3.8 ± 1.2 | 3.6 ± 0.7 |
| %predicted | 98 ± 15 | 90 ± 17 | 84 ± 12 |
| FEV1, L | 3.5 ± 0.9 | 2.7 ± 1.1 | 2.7 ± 0.6 |
| %predicted | 100 ± 14 | 81 ± 19 * | 82 ± 14 * |
| FEV1/FVC | 78 ± 7 | 71 ± 10 | 75 ± 8 |
| %predicted | 98 ± 7 | 90 ± 13 | 96 ± 10 |
| FEF25–75, L/sec | 3.3 ± 1.3 | 2.1 ± 1.3 * | 2.1 ± 0.8 * |
| %predicted | 106 ± 36 | 67 ± 31 * | 75 ± 29 * |
| RV, L | 3.0 ± 0.3 | 2.5 ± 0.7 | 2.5 ± 0.4 |
| %predicted | 127 ± 22 | 120 ± 33 | 120 ± 26 |
| TLC, L | 7.1 ± 0.8 | 6.1 ± 1.8 | 6.2 ± 0.9 |
| %predicted | 105 ± 7 | 98 ± 16 | 97 ± 9 |
| RV/ TLC | 38.6 ± 9.0 | 41.8 ± 8.1 | 41.3 ± 3.7 |
FVC = forced vital capacity, FEV1 = forced expiratory volume in 1 second, FEF25–75 = maximum mid-expiratory flow, RV = residual volume, TLC = total lung capacity. Data reported as mean ± SD.
p<0.05 when compared to Control.
3.3 Measures of Q̇aw and Gf-aw
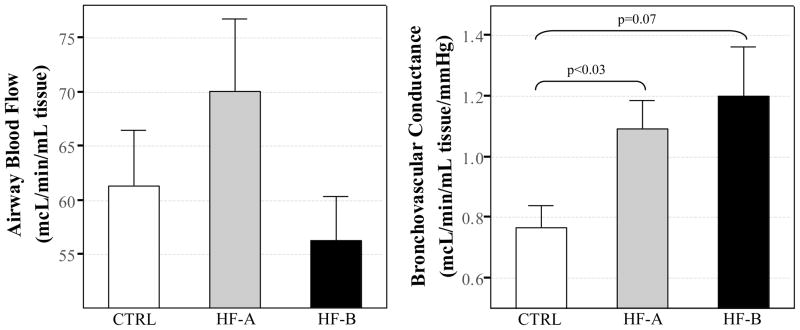
Measured values for Q̇aw and Gf-aw are presented in Figure 1. Q̇aw was not significantly different between CTRL (61.3±17.9 μL·min−1·ml−1), mild HF-A (70.1±26.9 μL·min−1·ml−1), or moderate/severe HF-B (56.2±14.9 μL·min−1·ml−1) (p>0.05 for all comparisons). Airway blood flow conductance, Gf-aw, was elevated in mild HF-A (1.1±0.4 μL·min−1·ml−1·mmHg−1, p<0.03) and tended to be elevated in moderate/severe HF-B (1.2±0.6 μL·min−1·ml−1·mmHg−1, p=0.07) when compared to CTRL (0.8±0.3 μL·min−1·ml−1·mmHg−1). Calculated values of Gf-aw were similar in both HF groups (Figure 1).
Figure 1. Airway blood flow and bronchovascular conductance.
White bars represent control group (CTRL), grey bars represent patients with HF-A (HF-A group, NYHA class I–II), and black bars represent patients with moderate to severe HF (HF-B group, NYHA class III-IV). Data reported as mean ± SE.
3.4 Relationships of Q̇aw and Gf-aw to Pulmonary Function and Hemodynamics
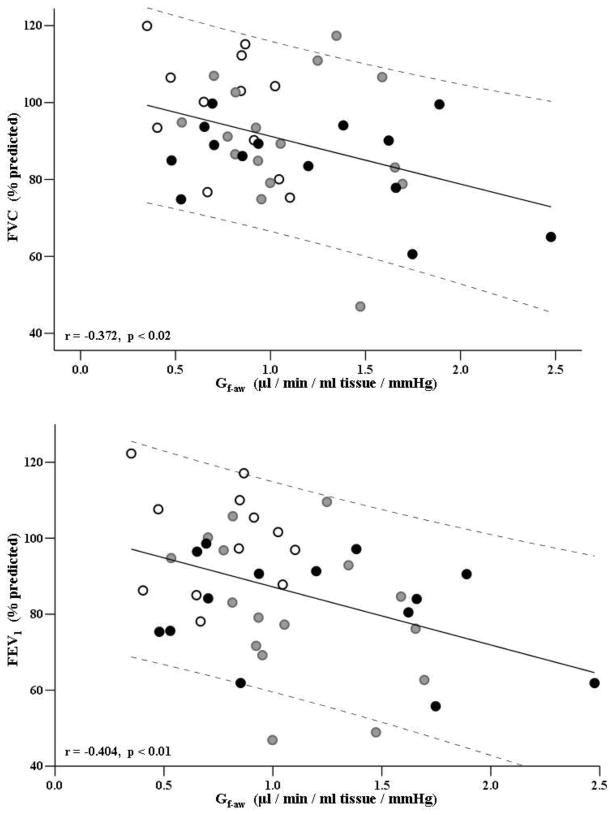
The relationships between pulmonary function variables and Q̇aw and Gf-aw are presented in Figures 2 and 3. As a first pass analysis we examined the relationship between pulmonary function variables presented in Table III and Q̇aw and Gf-aw for the entire group i.e. HF and CTRL combined (Figure 2, Figure 3B). Where there was a significant group effect, i.e. HF subjects behaved differently to CTRL, data for the HF patients is presented separately (Figure 3A, 3C–D). Figure 2 shows the relationships between Gf-aw and %predicted FVC and between Gf-aw and % predicted FEV1 for all subjects. Significant negative correlations were observed between Gf-aw and %predicted FVC and also between Gf-aw and %predicted FEV1 values.
Figure 2. Bronchovascular conductance versus FVC (%predicted, top graph) and FEV1 (%predicted, bottom graph) in all subjects.
FVC=forced vital capacity. FEV1=forced expiratory volume in 1 second. White dots represent control group (CTRL), grey dots represent patients with HF-A (HF-A group, NYHA class I–II), and black dots represent patients with moderate to severe HF (HF-B group, NYHA class III–IV).
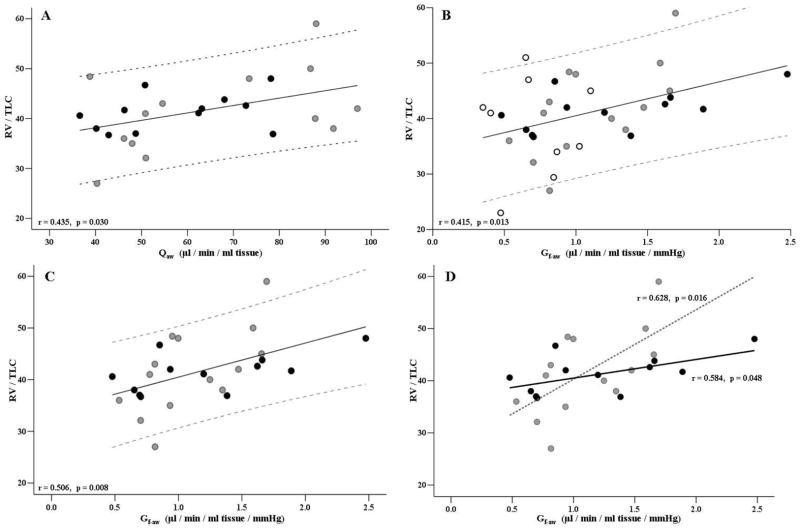
Figure 3.
Airway blood flow and bronchovascular conductance versus RV/TLC ratio. Q̇aw=Airway blood flow, Gf-aw=bronchovascular conductance flow, RV=residual volume (L), TLC=total lung capacity, HF = heart failure. White dots represent control group (CTRL), grey dots represent patients with HF-A (HF-A group, NYHA class I-II), and black dots represent (HF-B group, NYHA class III–IV). (A) Plot and regression for Q̇aw versus RV/TLC for all HF subjects. (B) Plot and regression for Gf-aw versus RV/TLC for all subjects. (C) Plot and regression for Gf-aw versus RV/TLC for all HF patients. (D) Plot and regression for HF-A group (dotted line) and for HF-B group (solid line)
Relationships between Q̇aw and Gf-aw to the RV/TLC ratio are represented in Figure 3. When considering all the HF patients, Q̇aw was correlated to RV/TLC (Figure 3A). Furthermore, Gf-aw was correlated to RV/TLC in all subjects (i.e. CTRL and HF subjects, Figure 3B), in all HF patients (Figure 3C), and individually in each of the separate HF groups (Figure 3D). Hence, individuals with higher Q̇aw and higher Gf-aw tended to also have higher RV/TLC ratios.
There were no significant relationships between Q̇aw and diffusing capacity or hemodynamics for either the CTRL group or HF groups.
4. Discussion
Our data demonstrate firstly that while there is no measurable change in Q̇aw with HF, Gf-aw is increased in stable, ambulatory HF patients compared to healthy, control participants. Secondly our data demonstrate that Q̇aw and Gf-aw are negatively associated with measures of pulmonary function (FVC, FEV1, and RV/TLC) and as such, higher Q̇aw and Gf-aw are associated with poorer pulmonary function. These data suggest a possible contribution of Q̇aw and Gf-aw to lung function abnormalities in the HF patient population.
The mucosa of non-gas exchanging airways is a relatively small tissue volume that is highly vascularized. Blood vessels within the airway walls are predominantly dispersed within the subepithelial tissue space and it is widely hypothesized that conductance and/or resistance characteristics of this vascular bed could impact on global pulmonary function (Wagner et al. 1996; McIlveen 2000). In humans, blood inflow to the airway vasculature arises from the systemic circulation and the majority of blood outflow from the airway mucosa is into the pulmonary venous system via post-capillary bronchopulmonary anastomotic structures (Agostoni et al. 1987; Wagner et al. 1990). Given this anatomical configuration, both systemic and pulmonary hemodynamics directly affect flow and conductance in the airway vascular bed. In HF patients, elevated pulmonary pressures, cardiac stretch, and increases in several neurohumoral factors have been hypothesized to alter Q̇aw and Gf-aw and subsequently contribute to observed restrictive/obstructive spirometry patterns (McIlveen 2000). Determination of Q̇aw to estimate Gf-aw by the soluble gas technique is reflective of the airway mucosal blood flow beginning at the level of the main stem bronchi down to the level of the terminal bronchioles and thus provides some insight on the role of this vascular bed in the observed pulmonary function abnormalities of HF (Scuri et al. 1995).
4.1
Airway Blood Flow, Conductance and In the present study, no differences in the direct measures of Q̇aw were observed between CTRL and HF groups. However, when examining these values alone, it is not possible to separate the relative contributions of the perfusion pressure of the vascular bed and that of the resistance/conductance values of the conduit vessels. Subjects with HF presented with decreased systemic pressures and increased PCWP, serving to decrease the perfusion pressure (ΔP) across the bronchovascular bed. Taking intoaccount ΔP, it was demonstrated that Gf-aw was elevated in HF which is consistent with distension of the airway vasculature in these subjects.
While we found PCWP was increased in HF, surprisingly there was no significant relationship between Q̇aw and Gf-aw and PCWP for any group. An overview of Q̇aw studies in the literature conducted primarily in animals consistently demonstrates a reduction in Q̇aw with increasing downstream vascular pressures(Auld et al. 1960; Goetz et al. 1965; Modell et al. 1981; Baile et al. 1984; Agostoni et al. 1987; Wagner et al. 1990). In dogs, Agostoni et al. (1987) showed that anastomotic Q̇aw was reduced under the following conditions: increasing downstream pulmonary vascular pressure, decreasing upstream systemic pressure, or an overall decrease in the pressure gradient across the vascular bed. Wagner et al. (1990) demonstrated in sheep that increasing left atrial pressure, which in turn increases pulmonary vascular pressure, produced a reduction in total bronchial artery flow by means of an increase in bronchovascular resistance.
However it is important to recall that HF patients present with a constellation of secondary physiologic adaptations resulting in marked interference between normal bronchovascular regulation and cardiopulmonary alterations occurring with the disease. These adaptations in early phases of HF may differ from those seen in the latter stages of the disease. The tendency for an d Q̇aw in mild HF-A (mild HF) and the tendency for a diminished Q̇aw in moderate/severe HF-B relative to CTRL may reflect the progressive nature of any physiologic adaptation occurring in early vs more advanced stages of HF.
4.2 Pulmonary Function
Patients with HF frequently exhibit combined mild obstructive and restrictive pulmonary function abnormalities (Puri et al. 1995). The HF patients that participated in the present study displayed characteristics of restrictive pulmonary function as demonstrated by tendency for decreased values for FVC and FEV1. However these subjects also displayed obstructive elements in the collected spirometry tracings. Both HF groups had a significantly lower FEF25-75 and FEV1 compared to CTRL, suggesting some marked small airway obstruction. These flow data are characteristic of a “non-specific ventilatory limitation” or non-specific pattern of pulmonary dysfunction (Hyatt et al. 2009). Although these pulmonary function findings are consistent with those of other investigators for HF patients, the specific mechanisms that contribute to non-specific spirometry tracings are presently not clear. One theory is that obstruction of the small airways contributes to mixed restrictive/obstructive pulmonary function abnormalities and that closer attention should be also be given to measured lung volumes, specifically RV, TLC, and the RV/TLC ratio (Stanescu et al. 2004; Hyatt et al. 2009).
4.3 Relationships of Q̇aw and Gf-aw to Pulmonary Function and Hemodynamics
In analyses inclusive of all subjects (Figure 2), modest associations were observed between Gf-aw and %predicted FVC and with %predicted FEV1, suggesting that there may be a link between blood flow in the airways and global pulmonary function. Moreover we found strong relationship between Q̇aw and Gf-aw to measures of the RV/TLC ratio. In HF patients, higher Q̇aw are related to higher RV/TLC ratios (Figure 3A). In all subjects and in the individual HF patient groups, higher mucosal blood flow conductance are also related to higher RV/TLC ratios that is likely associated with increased air trapping (Figure 3B–D).
Taken together, we believe the results of the current study support the hypothesis that an increase in bronchial conductance may contribute to an increase in airway resistance in HF. Firstly we are confident that measurements obtained by the soluble gas method used in this study are sensitive to and inclusive of mucosal blood flow in the walls of airways susceptible to changes in resistance. This method allows for penetration of the test gas deep into the lung but excludes mixing and/or uptake in the alveolar regions of the lung. Secondly non-specific pattern of spirometry tracings in combination with elevated RV/TLC values in HF may be an indication of gas trapping and airway obstruction (Stanescu et al. 2004; Hyatt et al. 2009). The strong positive relationship we found between RV/TLC and Gf-aw. in both HF groups (and not CTRL) suggests that a worsening of airway obstruction is related to greater bronchial conductance which may be translated into greater bronchial engorgement. Thirdly, cartilage content is reduced in the walls of smaller airways making small bronchi and bronchioles structurally more compliant and more susceptible to narrowing (Noble et al. 2002). The inverse relationship between airflow resistance and lumen radius (raised to the 4th power) dictates that small decreases in absolute lumen dimension will translate into exponential increases flow resistance in these conduit vessels.
4.4 Limitations
As highlighted, measurement of bronchial blood flow is difficult and the soluble gas technique represents an indirect measure of a complex system. For the purpose of this study, the measures of Q̇aw refer to mucosal blood flow rather than total blood flow into the bronchial circulation. Clearly a more direct measure of Q̇aw would be ideal, however this is difficult in vivo. However we would argue that changes mucosal Q̇aw, i.e. those anatomically closest to the airway lumen, would provide the best insight into any related changes in pulmonary function. Our results showed a weak, but significant relationship between PF and Q̇aw and Gf-aw. We would have expected more pronounced changes in pulmonary function with greater disease severity, however this was not always the case. This lack of a clear differentiation between the HF groups may have been due to the fact that these were all stable, relatively well-compensated group of HF patients. Moreover differentiating between HF groups is complex and we may not always see distinct differences between groups. While we chose to categorize subjects based on NYHA class, others factors such as B-type natriuretic peptide may better at predicting disease severity (Doust et al. 2005) and hence categorize the HF group.
In calculating the bronchovascular perfusion pressure we used PCWP an estimate left atrial pressure. Venous drainage of the bronchvascular tree is complex, with best estimates suggesting two-thirds of the blood flow returns to the pulmonary circulation, with the remaining blood flow returning to the low pressure right side of the heart. Clearly we are unable to accurately determine the downstream pressure for all bronchial blood flow, however even if we were able to do so, one would argue that this would not change the overall results of the current study. Finally, the non-invasive method used to estimate PCWP may represent another potential limitation. In the current study we estimated PCWP using the regression equation described by Nagueh et al (1997). However there is some debate as to the applicability of this regression equation in healthy subjects with others questioning the accuracy of this approach (Firstenberg et al. 2000). However, given that the method we have outlined is widely cited in the literature (Nagueh et al. 1997) we feel confident that our results for healthy subjects are consistent with published data.
5. Conclusions
Numerous physiologic systems are affected by a failing heart, with particular interest given here as to how HF impacts the bronchial circulation and subsequently pulmonary function. The present investigation found that Gf-aw was elevated in stable HF patients relative to healthy control subjects. Increases in Gf-aw were associated with decreased FVC and FEV1. Increases in Q̇aw and Gf-aw are also associated with increased RV/TLC ratios suggestive of a relationship between the bronchial circulation and air-trapping in the lung.
Acknowledgments
The authors would like to thank the subjects for their participation in our study and the important contributions of Jennifer Fitz-Gibbon and Dr. Lyle J. Olson in the recruitment and daily testing. We would also like to thank Dr. Kenneth C. Beck for his input regarding this study as well as for his technical expertise and Dr Adam Wanner for his input and guidance in setting up the airway blood flow technique in our laboratory. This study was supported by the National Institute of Health Grant HL71478 and Center for Translational Science Activities Grant Number 1 UL1 RR024150.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostoni PG, Deffebach ME, Kirk W, Lakshminarayan S, Butler J. Upstream pressure for systemic to pulmonary flow from bronchial circulation in dogs. J Appl Physiol. 1987;63(2):485–491. doi: 10.1152/jappl.1987.63.2.485. [DOI] [PubMed] [Google Scholar]
- Agostoni PG, Doria E, Bortone F, Antona C, Moruzzi P. Systemic to pulmonary bronchial blood flow in heart failure. Chest. 1995;107(5):1247–1252. doi: 10.1378/chest.107.5.1247. [DOI] [PubMed] [Google Scholar]
- Auld PA, Rudolph AM, Golinko RJ. Factors affecting bronchial collateral flow in the dog. Am J Physiol. 1960;198:1166–1170. doi: 10.1152/ajplegacy.1960.198.6.1166. [DOI] [PubMed] [Google Scholar]
- Baile EM, Albert RK, Kirk W, Lakshaminarayan S, Wiggs BJ, Pare PD. Positive end-expiratory pressure decreases bronchial blood flow in the dog. J Appl Physiol. 1984;56(5):1289–1293. doi: 10.1152/jappl.1984.56.5.1289. [DOI] [PubMed] [Google Scholar]
- Cabanes L, Costes F, Weber S, Regnard J, Benvenuti C, Castaigne A, Guerin F, Lockhart A. Improvement in exercise performance by inhalation of methoxamine in patients with impaired left ventricular function. N Engl J Med. 1992;326(25):1661–1665. doi: 10.1056/NEJM199206183262503. [DOI] [PubMed] [Google Scholar]
- Cabanes LR, Weber SN, Matran R, Regnard J, Richard MO, Degeorges ME, Lockhart A. Bronchial hyperresponsiveness to methacholine in patients with impaired left ventricular function. N Engl J Med. 1989;320(20):1317–1322. doi: 10.1056/NEJM198905183202005. [DOI] [PubMed] [Google Scholar]
- Ceridon M, Wanner A, Johnson BD. Does the bronchial circulation contribute to congestion in heart failure? Med Hypotheses. 2009 doi: 10.1016/j.mehy.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330(7492):625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, Zuckerman J, Thomas JD. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36(5):1664–1669. doi: 10.1016/s0735-1097(00)00909-8. [DOI] [PubMed] [Google Scholar]
- Goetz RH, Rohman M, State D. The Hemodynamics of Bronchopulmonary Anastomoses. Surg Gynecol Obstet. 1965;120:517–529. [PubMed] [Google Scholar]
- Hennessy E, White S, van der Touw T, Quail A, Porges W, Glenfield P. Control of resting bronchial hemodynamics in the awake dog. Am J Physiol. 1993;265(2 Pt 2):H649–660. doi: 10.1152/ajpheart.1993.265.2.H649. [DOI] [PubMed] [Google Scholar]
- Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135(2):419–424. doi: 10.1378/chest.08-1235. [DOI] [PubMed] [Google Scholar]
- Hyatt RE, Scanlon PD, Nakamura M. Interpretation of pulmonary function tests: a practical guide. Philadelphia: Wolter Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117(2):321–332. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Pulmonary function in patients with reduced left ventricular function: influence of smoking and cardiac surgery. Chest. 2001;120(6):1869–1876. doi: 10.1378/chest.120.6.1869. [DOI] [PubMed] [Google Scholar]
- Lockhart A, Dinh-Xuan AT, Regnard J, Cabanes L, Matran R. Effect of airway blood flow on airflow. Am Rev Respir Dis. 1992;146(5 Pt 2):S19–23. doi: 10.1164/ajrccm/146.5_Pt_2.S19. [DOI] [PubMed] [Google Scholar]
- Long WM, Yerger LD, Abraham WM, Lobel C. Late-phase bronchial vascular responses in allergic sheep. J Appl Physiol. 1990;69(2):584–590. doi: 10.1152/jappl.1990.69.2.584. [DOI] [PubMed] [Google Scholar]
- McIlveen S, White S, Parsons G. Autonomic control of bronchial circulation in awake sheep during rest and behaviour. Clin Exp Pharmacol Physiol. 1997;24(12):940–947. doi: 10.1111/j.1440-1681.1997.tb02723.x. [DOI] [PubMed] [Google Scholar]
- McIlveen SA. Bronchovascular role in pulmonary congestion. Clin Exp Pharmacol Physiol. 2000;27(12):1045–1048. doi: 10.1046/j.1440-1681.2000.03374.x. [DOI] [PubMed] [Google Scholar]
- Meyer M, Schuster KD, Schulz H, Mohr M, Piiper J. Pulmonary diffusing capacities for nitric oxide and carbon monoxide determined by rebreathing in dogs. J Appl Physiol. 1990;68(6):2344–2357. doi: 10.1152/jappl.1990.68.6.2344. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Modell HI, Beck K, Butler J. Functional aspects of canine bronchial-pulmonary vascular communications. J Appl Physiol. 1981;50(5):1045–1051. doi: 10.1152/jappl.1981.50.5.1045. [DOI] [PubMed] [Google Scholar]
- Morris NR, Ceridon ML, Beck KC, Strom NA, Schneider DA, Mendes ES, Wanner A, Johnson BD. Exercise-related change in airway blood flow in humans: relationship to changes in cardiac output and ventilation. Respir Physiol Neurobiol. 2008;162(3):204–209. doi: 10.1016/j.resp.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NR, Snyder EM, Beck KC, Haseler LJ, Olson LJ, Johnson BD. The relationship between resting lung-to-lung circulation time and peak exercise capacity in chronic heart failure patients. J Card Fail. 2007;13(5):389–394. doi: 10.1016/j.cardfail.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30(6):1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- Noble PB, Turner DJ, Mitchell HW. Relationship of airway narrowing, compliance, and cartilage in isolated bronchial segments. J Appl Physiol. 2002;92(3):1119–1124. doi: 10.1152/japplphysiol.00662.2001. [DOI] [PubMed] [Google Scholar]
- Oh JK, Seward JB, Tajik AJ. The echo manual. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Puri S, Baker BL, Dutka DP, Oakley CM, Hughes JM, Cleland JG. Reduced alveolar-capillary membrane diffusing capacity in chronic heart failure. Its pathophysiological relevance and relationship to exercise performance. Circulation. 1995;91(11):2769–2774. doi: 10.1161/01.cir.91.11.2769. [DOI] [PubMed] [Google Scholar]
- Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- Scuri M, McCaskill V, Chediak AD, Abraham WM, Wanner A. Measurement of airway mucosal blood flow with dimethylether: validation with microspheres. J Appl Physiol. 1995;79(4):1386–1390. doi: 10.1152/jappl.1995.79.4.1386. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol. 2006;101(6):1623–1632. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Turner ST, Hoffman EA, Joyner MJ, Johnson BD. Genetic variation of the beta2-adrenergic receptor is associated with differences in lung fluid accumulation in humans. J Appl Physiol. 2007;102(6):2172–2178. doi: 10.1152/japplphysiol.01300.2006. [DOI] [PubMed] [Google Scholar]
- Stanescu D, Veriter C. A normal FEV1/VC ratio does not exclude airway obstruction. Respiration. 2004;71(4):348–352. doi: 10.1159/000079638. [DOI] [PubMed] [Google Scholar]
- Wagner EM, Mitzner W. Effects of bronchial vascular engorgement on airway dimensions. J Appl Physiol. 1996;81(1):293–301. doi: 10.1152/jappl.1996.81.1.293. [DOI] [PubMed] [Google Scholar]
- Wagner EM, Mitzner WA. Effect of left atrial pressure on bronchial vascular hemodynamics. J Appl Physiol. 1990;69(3):837–842. doi: 10.1152/jappl.1990.69.3.837. [DOI] [PubMed] [Google Scholar]
- Wanner A, Mendes ES, Atkins ND. A simplified noninvasive method to measure airway blood flow in humans. J Appl Physiol. 2006;100(5):1674–1678. doi: 10.1152/japplphysiol.01349.2005. [DOI] [PubMed] [Google Scholar]
- Wetzel RC, Herold CJ, Zerhouni EA, Robotham JL. Intravascular volume loading reversibly decreases airway cross-sectional area. Chest. 1993;103(3):865–870. doi: 10.1378/chest.103.3.865. [DOI] [PubMed] [Google Scholar]
- White S, McIlveen S, Parsons G, Quail A, Cottee D, Gunther R, Bishop R, McLeod D, Blake R. Neural control of the bronchial circulation. Arch Physiol Biochem. 2003;111(4):305–308. doi: 10.3109/13813450312331337450. [DOI] [PubMed] [Google Scholar]