Abstract
Sub-maximal exercise gas analysis may be a useful method to assess and track pulmonary arterial hypertension (PAH) severity. The aim of the present study was to develop an algorithm, using exercise gas exchange data, to assess and monitor PAH severity. Forty PAH patients participated in the study completing a range of clinical tests and a novel sub-maximal exercise step test, which lasted 6-minutes and incorporated resting (2-min), exercise (3-min) and recovery (1-min) ventilatory gas analysis. Using gas exchange data, including breathing efficiency (VE/VCO2 slope), end-tidal CO2 (PETCO2), oxygen saturation (SaO2) and oxygen pulse (O2 pulse), a Pulmonary Hypertension Gas Exchange Severity (PH-GXS) score was developed. Patients were re-tested after ~6-months. There was significant separation between healthy controls and moderate PAH (WHO I/II) and more severe PAH (WHO III/IV) patients for VE/VCO2 slope, PETCO2, SaO2 and O2 pulse. The PH-GXS score developed significantly correlated with WHO class (r=0.51), six-minute walk distance (r=−0.59), right ventricle systolic pressure [RVSP] (r=0.49), N-terminal pro-B-type natriuretic peptide [log NT-Pro BNP] (r=0.54) and pulmonary vascular resistance [PVR] (r=0.71). The PH-GXS score remained unchanged in 22 patients re-tested (1.50±0.92 vs. 1.48±0.94), as did WHO class (2.3±0.8 vs. 2.3±0.8) and six-minute walk distance (455±120 vs. 456±103). Small individual changes were observed in the PH-GXS score, with 8 patients improving and 8 deteriorating. In conclusion, the PH-GXS score differentiated between PAH patients and correlated with traditional clinical measures. The PH-GXS score was unchanged in our cohort after 6-months, consistent with traditional clinical metrics, but individual differences were evident. A PH-GXS score may be a useful way to track patient responses to therapy.
Keywords: Pulmonary arterial hypertension, exercise, gas analysis
Introduction
We propose that cardiopulmonary gas exchange obtained with sub-maximal exercise may provide an important way to track disease severity in pulmonary arterial hypertension (PAH)1 and that building a scoring system based on the most important variables may provide an index that is more robust than one single gas exchange variable on its own and yet still sensitive to changes in disease severity. Thus, the purpose of this study was to develop a scoring system using non-invasive gas exchange measures. The goal was to take the gas exchange metrics that most clearly separated PAH from healthy controls (i.e. breathing efficiency [VE/VCO2 slope], and end-tidal CO2 [PETCO2], oxygen saturation [SaO2] and oxygen pulse [O2 pulse] at end exercise) and build a table grading each variable according to severity. While one would expect this preliminary score to somewhat correlate with other metrics currently used in a clinical setting to determine disease severity in PAH, it was designed to be primarily a gas exchange severity score and thus under certain conditions (i.e. surgical right to left shunt), may not reflect these traditional measures.
Methods
Forty consecutive patients with PAH being treated in the Mayo Clinic Pulmonary Hypertension Unit participated in this study. Thirty-two patients presented with idiopathic PAH, hereditary PAH, PAH associated with the use of diet drugs or portopulmonary hypertension while the remaining 8 patients had PAH associated with connective tissue disease. There was very little difference between these two groups of patients as demonstrated in our recent manuscript.1 Twenty-five age- and sex- matched controls, free of any cardio-respiratory disease, also participated in the study. All participants gave written informed consent after being provided a description of the study requirements. The study was approved by the Mayo Clinic institutional review board and was performed in accordance with the ethical standards of the Declaration of Helsinki.
Patients completed a range of standard tests, including right heart catheterization, echocardiography, six-minute walk and pulmonary function testing; in accordance with current clinical practice. In addition, each participant performed a novel sub-maximal exercise test lasting 6-minutes and consisting of 2-min resting baseline, 3-min progressive step exercise and 1-min recovery as described in our recent publication.1 Throughout the test, breathing pattern, gas exchange and heart rate were monitored using a simplified gas analysis system (SHAPE Medical Systems, Inc). This new miniaturized and portable device is self calibrating, containing built-in automated temperature, barometric pressure and humidity corrections, and has been previously validated against a standardized metabolic gas analysis system.2 Following two minutes of baseline measurements, subjects performed a 3-min step exercise test. For the first minute of exercise step rate was set at 1 stride per second (60 strides per minute [spm]) and was maintained or increased (typically to 90 or 120spm, depending on patient performance), depending on respiratory exchange ratio (RER) and perceived exertion (RPE); the primary aim being an RER equal to 0.9 and RPE < 13, as described previously.1 On completion of 3-minutes step exercise recovery data was collected for a further minute. Clinical and sub-maximal exercise testing was repeated at three and / or six months in patients returning for clinical appointments, according to normal clinical follow up procedures.
Minute ventilation (VE), breathing frequency (fR), tidal volume (VT), oxygen consumption (VO2), carbon dioxide production (VCO2), RER and end tidal CO2 (PETCO2) were obtained breath-by-breath and averaged over a 30-second period at rest and the last 30-seconds of each minute during exercise. In addition, heart rate (HR) and oxygen saturation (SaO2) were obtained continuously using pulse oximetry. From this data, derived variables such as VE/VCO2 ratio and oxygen pulse (VO2/HR) were calculated. Ventilatory efficiency slopes (VE/VCO2 slope) [VE L/min = m (VCO2, L/min) + b, where m = VE/VCO2 slope] were calculated using all exercise data points via least squares linear regression.
From our previous publication four key exercise gas exchange variables were determined that differentiated PAH patients and healthy controls and also between differing disease severities. This included VE/VCO2 slope, and end exercise PETCO2, SaO2 and O2 pulse. Using these variables a pulmonary hypertension gas exchange severity (PH-GXS) score was developed.
To create the PH-GXS score each individual gas exchange variable was given the same weighting in the model. Normal values for each variable were determined as the mean ± standard deviation of the control group (i.e. the control group mean and standard deviation VE/VCO2 slope was 30 and 3, respectively, hence the normal cutoff value was considered to be 27–33 or <33). Equal cutoff points, that reflected the distribution of abnormal values, were then chosen for each individual parameter in an effort to represent mild PAH, moderate PAH, severe PAH and very severe PAH (see scoring matrix in Table 1). Patient data were then categorized accordingly into one of the 5 groups (normal – 0, mild – 1, moderate – 2, severe – 3 and very severe – 4) and this was done for each of the four gas exchange variables. The total score was then tallied and divided by the number of variables in the model as described in Equation 1.
Table 1.
Scoring matrix.
| Breathing Efficiency | End-tidal CO2 (mmHg) | Oxygen saturation (%) | Oxygen pulse | |
|---|---|---|---|---|
| 0 (Normal) | <33 | >37 | >92 | >9 |
| 1 (Mild) | 33 – 38.9 | 32 – 36.9 | 88 – 91.9 | 7.5 – 8.9 |
| 2 (Moderate) | 39 – 44.9 | 27 – 31.9 | 84 – 87.9 | 6.0 – 7.4 |
| 3 (Severe) | 45 – 50.9 | 22 – 26.9 | 80 – 83.9 | 4.5 – 5.9 |
| 4 (Very Severe) | >51 | <22 | <80 | <4.5 |
| Equation 1 |
This allowed us to create a 0–4 gas exchange severity score, where normal = 0 – 0.49, mild PAH = 0.5 – 1.49, moderate PAH = 1.5 – 2.49, severe PAH = 2.5 – 3.49 and very severe = 3.5 – 4.
To compare differences in key exercise gas exchange variables between the healthy controls and PAH patients (WHO class I/II and III/IV) a one-way ANOVA was performed. The Levenes test was used to determine equal variance and subsequently a Bonferroni (equal variance assumed) or Games-Howell (equal variance not assumed) post hoc correction, was used to compare between different groups. Following the development of the PH-GXS score, correlation analysis was performed to determine how well, or not, this novel classification system compared with traditional clinical measures such as right heart catheterization derived pressure data, echocardiography data, six-minute walk data and WHO class. Finally, to compare PAH patient re-test data (i.e. test 1 vs. test 2) and the ability of the PH-GXS score to track diseases severity a paired t test was performed. The acceptable type I error was set at p < 0.05. Results are expressed as means ± SD and as frequency (%). Statistical analyses were performed using SPSS software, version 12.0 for Windows (Chicago, Illinois, US).
Results
PAH patient and healthy controls were well matched for age, weight and height as demonstrated in Table 2. Approximately two-thirds of the patients had a diagnosis of idiopathic PAH (63%) and 20% of patients had PAH associated with connective tissue disease (Table 3). Patient severity ranged from WHO class 1 to 4. Patients were taking a variety of medications with 65% on a combined therapy, which consisted of an endothelin antagonist, a PDE-5 inhibitor or a prostacyclin.
Table 2.
Pulmonary arterial hypertension patient and control demographics.
| Controls (n = 25 ) | PAH (n = 40) | |
|---|---|---|
| Female (%) | 80 | 80 |
| Age (years) | 51 ± 15 | 50 ± 13 |
| Height (cm) | 167.8 ± 8.2 | 167.7 ± 7.0 |
| Weight (kg) | 70.1 ± 12.7 | 75.8 ± 16.5 |
Data are presented as frequency and mean ± standard deviation.
Table 3.
Pulmonary arterial hypertension characteristics and clinical data
| Pulmonary arterial hypertension etiology | |
| Idiopathic | 25 (63%) |
| Hereditary | 4 (10%) |
| Associated with diet drug use | 2 (5%) |
| Portopulmonary Hypertension | 1 (2%) |
| Associated with Connective Tissue Disease | 8 (20%) |
| WHO Class | |
| I/II | 27 (67.5%) |
| III/IV | 13 (32.5%) |
| Six-minute walk distance (m) [% pred.] | 453 ± 105 [80 ± 19] |
| Log N-terminal pro-B-type nautriuretic peptide (pg/mL) | 752 ± 1229 [2.28 ± 0.68] |
| Echocardiography Data | |
| Right Ventricle Systolic Pressure (mmHg) | 76 ± 23 |
| Tricuspid Annular Plane Systolic Excursion by M-mode (mm) | 21 ± 5 |
| Lateral Annulus Systolic Velocity (msec) | 0.13 ± 0.03 |
| Pulmonary Function Data | (n = 32) |
| Forced Ventilatory Capacity (L) [% pred.] | 3.2 ± 0.8 [86 ± 16] |
| Forced Expiratory Volume in 1 second (L) [% pred.] | 2.5 ± 0.6 [80 ± 14] |
| Diffusion Capacity for Carbon Monoxide (ml/min/mmHg) [% pred.] | 17.6 ± 4.8 [71 ± 14] |
| Medications | |
| Endothelin Antagonist | 58% |
| Phosphodiesterase type-5 Inhibitor | 73% |
| Prostacyclin | 48% |
| Digitalis | 33% |
| Diuretic | 60% |
Data presented as mean ± standard deviation, and frequency (%).
Patient clinical data are also shown in Table 3. Group mean echocardiography and six-minute walk data demonstrated a right ventricle systolic pressure (RVSP) and six-minute walk distance of 76 ± 23 mmHg and 453 ± 105 metres, respectively. A subgroup (n = 15) of PAH patients who had a right heart catheterization procedure had a mean pulmonary artery pressure (mPAP), mean right atrial pressure (mRAP), cardiac index (CI) and pulmonary vascular resistance (PVR) of 55 ± 10 mmHg, 10.5 ± 5.3 mmHg, 2.35 ± 0.59 L/min/m2 and 10.9 ± 4.4 Wood Unit, respectively.
Significant differences were observed in VE/VCO2 slope, PETCO2 and O2 pulse between healthy controls, moderate PAH (WHO class I/II) and more severe PAH (WHO class III/IV) patients (Table 4), as described in our previous manuscript.1 The pulmonary hypertension gas exchange severity (PH-GXS) score was developed using these key gas exchange variables, the results of which are demonstrated in Table 5. The majority of PAH patients had a mild (43%) to moderate (20%) classification, while 26% of patients had severe/very severe PAH according to gas exchange. The remaining patients (13%) had a relatively normal gas exchange response to exercise, which is likely due to the therapeutic treatments being taken.
Table 4.
Differences in key sub-maximal exercise gas exchange data between the controls and pulmonary arterial hypertension groups.
| Variable | Control (n = 25) | PAH - WHO I/II (n = 27) | PAH - WHO III/IV (n = 13) |
|---|---|---|---|
| Breathing Efficiency | 29 ± 4 | 35 ± 8* | 48 ± 8*† |
| End-tidal CO2 @ end exercise (mmHg) | 40 ± 3 | 34 ± 6* | 26 ± 5*† |
| Oxygen Saturation @ end exercise (%) | 94 ± 2 | 90 ± 4* | 88 ± 6* |
| Oxygen Pulse @ end exercise | 11.8 ± 3.1 | 9.5 ± 3.0* | 6.9 ± 2.4*† |
Data are presented as mean ± standard deviation.
Significant differences between control and pulmonary arterial hypertension groups (p < 0.05).
Significant differences between the pulmonary arterial hypertension groups (p < 0.05).
Table 5.
Pulmonary Hypertension Gas Exchange Severity [PH-GXS] score classification (n = 40).
| Classification | Patients |
|---|---|
| Normal (0 – 0.49) | 5 (13%) |
| Mild (0.5 – 1.49) | 17 (43%) |
| Moderate (1.5 – 2.49) | 8 (20) |
| Severe (2.5 – 3.49) | 9 (23%) |
| Very Severe (3.5 – 4) | 1 (3%) |
To compare the PH-GXS score with current clinical data simple linear regression analysis was performed. Our PH-GXS score correlated well with WHO class, six-minute walk distance, RVSP and log NT Pro BNP (Figure 1). In patients who had a right heart catheterization procedure (n = 15) there was a significant correlation between the PH-GXS score and PVR, but no correlation between mPAP or mRAP and PH-GXS score (Figure 2); whilst there was a trend that suggested a relationship between CI and PH-GXS score (Figure 2).
Figure 1.
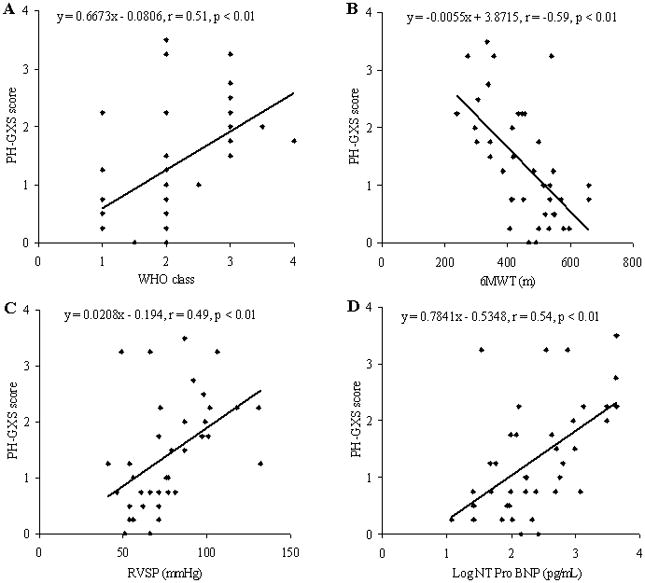
Correlation of Pulmonary Hypertension Gas Exchange Severity (PH-GXS) score and key clinical measures, including WHO class (A), six-minute walk distance (B), Right Ventricle Systolic Pressure (C) and log NT Pro BNP (D).
Pearson’s (r) value and the significance achieved are demonstrated on each individual graph.
Figure 2.
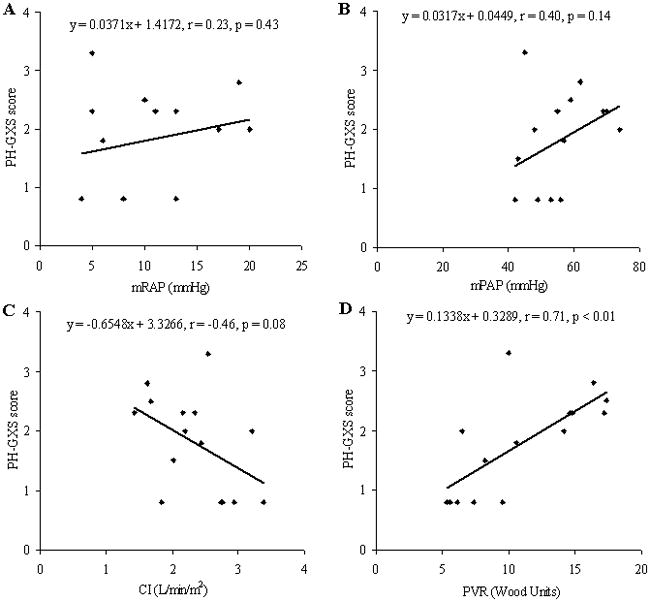
Correlation of Pulmonary Hypertension Gas Exchange Severity (PH-GXS) score and right heart catheterization data, including mean right atrial pressure (A), mean pulmonary artery pressure (B), cardiac index (C) and pulmonary vascular resistance (D).
Pearson’s (r) value and the significance achieved are demonstrated on each individual graph.
Twenty two patients underwent follow up tests on average 6 (range 3–8) months after their first test. These patients had been tracked by the Mayo Clinic Pulmonary Hypertension Unit for a number of years and they were taking a range of medications. Following our first exercise step test 8 patients had a change in medication, 3 were enrolled in blinded drug trials and 11 patients remained on the same therapy. Our aim was to track these patients over a 6-month period to see if it was possible to identify changes in the severity of PAH using traditional markers and the PH-GXS score, regardless of the treatment received. There was no change in six-minute walk distance, WHO class or RVSP within the PAH patients, as demonstrated in Table 6. While PAH patient gas exchange data, including PETCO2, SaO2 and O2 pulse at end exercise, was largely unchanged from Test 1 to Test 2, VE/VCO2 slope was significantly lower on re-testing. Despite this, and similar to traditional clinical metrics, the PH-GXS score remained relatively stable and unchanged within the PAH patient group. Taken as a whole this data suggests that PAH severity remained unaltered over this short time period. However, when looking at each individual patient subtle changes in the PH-GXS score were evident, with 8 patients showing an improved exercise gas exchange while 8 patients had a slight deterioration, as demonstrated in Figure 3.
Table 6.
Tracking pulmonary arterial hypertension patient (n = 22) responses over time (6-months on average) using clinical metrics and exercise gas exchange
| Variable | PAH Test 1 | PAH Test 2 |
|---|---|---|
| WHO class | 2.3 ± 0.8 | 2.3 ± 0.8 |
| Six-minute walk distance (m) | 455 ± 120 | 456 ± 103 |
| Right Ventricle Systolic Pressure (mmHg) | 73 ± 24 | 67 ± 24 |
|
| ||
| Breathing Efficiency | 41 ± 9 | 38 ± 9* |
| End-tidal CO2 @ end exercise (mmHg) | 30 ± 6 | 31 ± 5 |
| Oxygen Saturation @ end exercise (%) | 89 ± 5 | 89 ± 6 |
| Oxygen Pulse @ end exercise | 8.2 ± 2.4 | 7.4 ± 2.8 |
|
| ||
| Pulmonary Hypertension Gas Exchange Severity Score | 1.50 ± 0.92 | 1.48 ± 0.94 |
Data are presented as mean ± standard deviation.
Significant differences between test 1 and test 2 within the pulmonary arterial hypertension group (p < 0.05).
Figure 3.
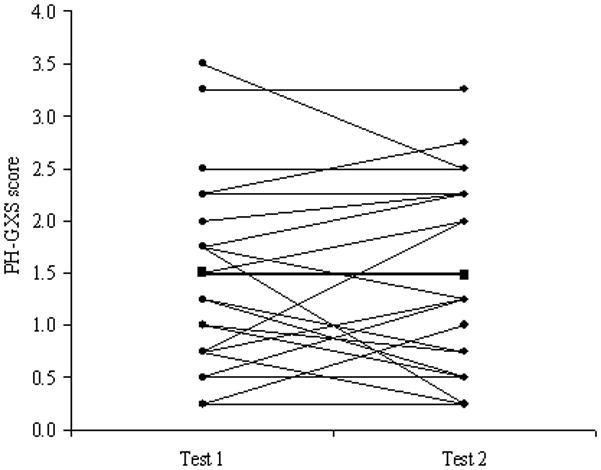
Individual (circle) and group mean (square) changes in the PH-GXS score from test 1 to test 2, which on average were separated by a 6-month period.
The individual changes observed in PH-GXS score over this time period were related to the delta change in log NT-Pro BNP (r = 0.45, p < 0.05; Figure 4). No relationships were observed between the delta PH-GXS score and delta WHO class, delta six-minute walk distance and delta RVSP.
Figure 4.
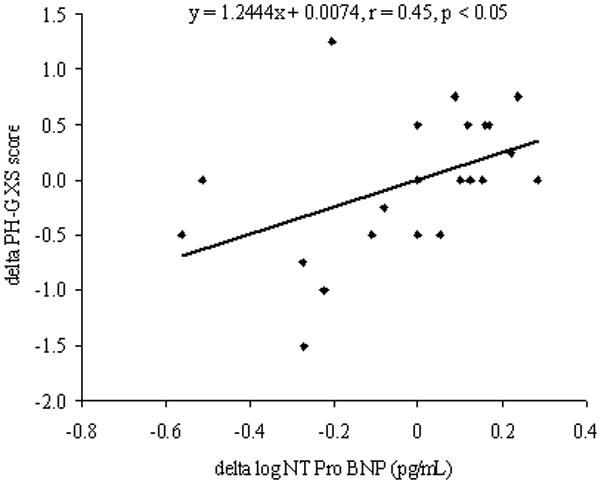
The relationship of the change in PH-GXS score and log NT Pro BNP between tests 1 and 2. Pearson’s (r) value and the significance achieved are highlighted on the graph.
Discussion
Gas exchange during a light sub-maximal exercise step test separates healthy controls and PAH patients. In particular, breathing efficiency (VE/VCO2), end-tidal CO2 (PETCO2), oxygen saturation (SaO2) and oxygen pulse (O2 pulse) were significantly different between the controls and PAH patients, while VE/VCO2 slope, PETCO2 and O2 pulse also differed between moderate PAH (WHO class I/II) and more severe PAH (WHO class III/IV) patients. Using these key gas exchange variables an equally weighted pulmonary hypertension gas exchange severity (PH-GXS) score was developed to help assess PAH severity. This scoring system, which was purposely based solely on exercise gas analysis, correlated well with traditional clinical metrics used to determine disease severity in PAH populations, including right heart catheterization derived PVR, echocardiographic data (RVSP and pulmonary artery capacitance), six-minute walk distance and WHO class. The PH-GXS score remained unchanged between PAH patient first and second test, which were separate by ~ 6 months. This was consistent with traditional clinical metrics, such as six-minute walk distance and WHO class, which were also unaltered over this relatively short time period suggesting that disease severity remained stable in this cohort of PAH patients who were well managed and relatively well compensated.
A number of recent studies have demonstrated an altered ventilatory response to exercise in PAH patients,3–5 which is unsurprising given that the main symptom of PAH is dyspnea on exertion. Indeed we recently demonstrated that there were differences between healthy controls and PAH patients in the ventilatory response to a low level exercise step test, with patients having a poorer breathing efficiency (increased VE/VCO2 slope), and a reduced PETCO2, SaO2 and O2 pulse at end exercise.1 A reduced blood flow to the pulmonary vessels, resulting in a V/Q mismatch and altered physiological dead space are the most likely explanation for the changes observed in VE/VCO2 slope and PETCO2.4–6 In addition, a reduced blood supply to skeletal muscles and associated early lactic acidosis, arterial hypoxemia and right to left shunting, all of which alter the concentrations of CO2, O2 and H+ ions in blood stimulating peripheral chemoreceptors, likely contribute to the hyper-ventilatory response and altered VE/VCO2 and PETCO2 seen in PAH.4,5,7,8 The decreased SaO2 during exercise in PAH can also be explained by a V/Q mismatch, as well as a reduced blood cell transit time in a restricted pulmonary vascular bed and a reduced diffusing capacity of the lungs.4,5,7,9 Finally the decreased O2 pulse, which is an indirect measure of stroke volume, detected in the PAH group at end exercise may be explained by the hearts inability to increase stroke volume in the face of high pulmonary artery pressures with a reliance placed on increasing heart rate during exercise to provide a sufficient blood supply.3,10
It is clear that each of the alterations evident in VE/VCO2 slope, and PETCO2, SaO2 and O2 pulse at end exercise in PAH patients are related to and representative of the pathophysiological changes associated with PAH. For that reason we decided to included these four variables together, in an equally weight algorithm, to formulate a Pulmonary Hypertension Gas Exchange Severity (PH-GXS) score that could be used to assess and track PAH disease severity. Considering the pathophysiology described above it was our belief that a PAH patient who had a very high VE/VCO2 slope (>51) and very low PETCO2 (<22), SaO2 (<80) and O2 pulse (<4.5) at end exercise would signify a very severe condition where the pathophysiological changes (i.e. high pulmonary pressures or right to left shunting) responsible for the altered ventilatory response during exercise would be extreme. Conversely, a PAH patient with a relatively normal VE/VCO2 slope (33–39), and a normal to low PETCO2 (32–37 mmHg), SaO2 (88–92%) and O2 pulse (7.5–9) at end exercise would signify a mild condition where the pathophysiological changes would be minimal.
The PH-GXS score was used to assess PAH severity, independently of all traditional clinical measures, and it was found to correlate well with other clinical metrics, including six-minute walk distance, WHO class, RVSP and PVR. Conversely to this no relationship was observed between PH-GXS score and mPAP or mRAP, which is interesting given both of these invasive measures have been linked with prognosis.11 These invasive measures were obtained under resting conditions, whereas the PH-GXS score implemented exercise gas exchange metrics and this may explain the lack of a relationship. The PH-GXS score developed may well be a better predictor of patient prognosis, in comparison to traditional clinical metrics, and this will need to be assessed in future studies.
While linear regression analysis produced some interesting findings it remains difficult to fully validate a PH-GXS score to assess and track PAH severity as there is no real gold standard measure for comparison. For many years the six-minute walk test has been used as a primary end-point measure to determine the effectiveness of new therapies and to track disease severity, however this test is quite rudimentary and questions remain regarding its sensitivity and accuracy to detect positive or negative changes, particularly in milder forms of PAH.12–15 The WHO classification is reliant on receiving accurate information from patients and can therefore be subjective.15 Echocardiography derived right heart pressures are estimated and semi-subjective, and require a high expertise to obtain.12 Finally, while right heart catheterization pressure data would be considered the gold standard measure to assess PAH severity, it is an invasive procedure usually performed under resting conditions which cannot be easily used to serially track disease severity.12,15 As a result of these limitations our PH-GXS score may not always track or relate to typical measures of disease severity. Nevertheless the PH-GXS score is meant to be an independent gas exchange severity score, related to pathophysiological alterations evident in PAH, providing additional useful information beyond that of traditional metrics to assist with the assessment and monitoring of PAH patients. More work is undoubtedly required to fully elucidate the usefulness of an exercise gas exchange score in this population.
In the present study the PH-GXS score remained unchanged between patient first and second tests, which were separated by ~6 months. Upon examining other clinical metrics (i.e. six-minute walk distance, WHO class and RVSP) it was apparent that these measures were also unchanged. This data suggests that this cohort of PAH patients remained relatively stable over this short time frame, with little differences observed in exercise gas exchange, functional class, walking distance or estimated right heart pressures. The patients examined in this study were well managed and had been seen periodically in the PH clinic for a number of years. Hence, these patients were well compensated and it is likely that following them for only 6 months was not long enough to see discernable alterations in disease severity. The PH-GXS score did slightly improve or deteriorate in some patients over this time and these individual changes in PH-GXS score were significantly related to alterations in log NT Pro BNP. The PH-GXS score, and also log NT Pro BNP, may be more sensitive to changes in PAH severity compared to traditional clinical metrics which would allow for the early detection of an improving or worsening condition.
In summary, we created a gas exchange severity score that was related to the exercise limitations experienced by PAH patients. This gas exchange score correlates reasonably well with traditional metrics, which supports its validity, but also provides added information not traditionally obtained during PAH management. It is possible that the PH-GXS score could be a new independent measure used to track disease severity. This could be used alone or in conjunction with other clinical data, providing more specific information and allowing better clinical decisions to be made. Additional longitudinal follow up is needed in order to correlate results of the PH-GXS score with patient outcome.
Most patients involved in this study had been followed in the PH clinic for a number of years and had been taking a variety of prescribed drug treatments. These patients were therefore relatively stable and it was not surprising that few differences, in clinical metrics and exercise gas exchange data, were observed between Test 1 and Test 2. The average time period between the two tests was 6 months (ranging from 3–8 months) and this was likely too short to see any clear changes in disease severity. Future studies should monitor patients over a longer time period so that the effectiveness of exercise gas exchange (PH-GXS score) as a tracking tool can be fully elucidated.
Acknowledgments
We would like to thank the subjects for their participation, as well as Kathy O’Malley and Joshua O’Malley for their technical assistance. This study was supported by Gilead Sciences, Inc and NIH Grant HL71478.
Footnotes
Conflict of Interest
Dr B Johnson has previously received funding from Shape Medical Systems, Inc. for the testing and validation of their medical equipment prior to participation in the present study, while Dr R Frantz has received a research grant form United Therapeutics. The remaining authors have no conflicts of interest to declare.
References
- 1.Woods PR, Frantz RP, Taylor BJ, Olson TP, Johnson BD. The usefulness of submaximal exercise gas exchange to define pulmonary arterial hypertension. J Heart Lung Transplant. 2011 doi: 10.1016/j.healun.2011.03.021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AD, Woods PR, Olson TP, Hulsebus ML, O’Malley KA, MacCarter D, Johnson BD. Validation of a simplified, portable cardiopulmonary gas exchange system for submaximal exercise testing. Open Sports Med J. 2010;4:34–40. [Google Scholar]
- 3.Riley MS, Porszasz J, Engelen MPKJ, Brundage BH, Wasserman K. Gas exchange responses to continuous incremental cycle ergometry exercise in primary pulmonary hypertension in humans. Eur J Appl Physiol. 2000;83:63–70. doi: 10.1007/s004210000240. [DOI] [PubMed] [Google Scholar]
- 4.Sun XG, Hansen JE, Oudix RJ, Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104:429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 5.Yasunobu Y, Oudix RJ, Sun XG, Hansen JE, Wasserman K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127:1637–1646. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 6.Reybrouck T, Mertens L, Schulze-Neick I, Austenat I, Eyskens B, Dumoulin M, Gewillig M. Ventilatory inefficiency for carbon dioxide during exercise in patients with pulmonary hypertension. Clinical Physiology. 1998;18:337–344. doi: 10.1046/j.1365-2281.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JE, Ulubay G, Chow BF, Sun XG, Wasserman K. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest. 2007;132:977–983. doi: 10.1378/chest.07-0619. [DOI] [PubMed] [Google Scholar]
- 8.Oudiz RJ, Midde R, Hovanesyan A, Sun XG, Roveran G, Hansen JE, Wasserman K. Usefulness of right-to-left shunting and poor exercise gas exchange for predicting prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2010;105:1186–1191. doi: 10.1016/j.amjcard.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steenhuis LH, Groen HJM, Koeter GH, van der Mark TW. Diffusion capacity and hemodynamics in primary and chronic thromboembolic pulmonary hypertension. Eur Respir J. 2000;16:276–281. doi: 10.1034/j.1399-3003.2000.16b15.x. [DOI] [PubMed] [Google Scholar]
- 10.Holverda S, Gan CT, Marcus JT, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Impaired stroke volume response to exercise in pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:1732–1733. doi: 10.1016/j.jacc.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology foundation task force on expert consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc. ; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Peacock AJ, Naeije R, Galie N, Rubin L. End-points and clinical trial design in pulmonary arterial hypertension: have we made progress? Eur Respir J. 2009;34:231–242. doi: 10.1183/09031936.00107108. [DOI] [PubMed] [Google Scholar]
- 13.Badesch DB, Champion HC, Sanchez MAG, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Rubin L, Simonneau G. Perspective on the optimal endpoints for pulmonary arterial hypertension trials. Current Opinion in Pulmonary Medicine. 2010;16:S43–S46. doi: 10.1097/01.mcp.0000370210.53379.b3. [DOI] [PubMed] [Google Scholar]
- 15.Peacock A, Keogh A, Humbert M. Endpoints in pulmonary arterial hypertension: the role of clinical worsening. Current Opinion in Pulmonary Medicine. 2010;16:S1–S9. doi: 10.1097/01.mcp.0000370205.22885.98. [DOI] [PubMed] [Google Scholar]


