Abstract
Genome-wide association studies (GWAS) have identified multiple common genetic variants associated with an increased risk of prostate cancer (PrCa), but these explain less than one-third of the heritability. To identify further susceptibility alleles, we conducted a meta-analysis of four GWAS including 5953 cases of aggressive PrCa and 11 463 controls (men without PrCa). We computed association tests for approximately 2.6 million SNPs and followed up the most significant SNPs by genotyping 49 121 samples in 29 studies through the international PRACTICAL and BPC3 consortia. We not only confirmed the association of a PrCa susceptibility locus, rs11672691 on chromosome 19, but also showed an association with aggressive PrCa [odds ratio = 1.12 (95% confidence interval 1.03–1.21), P = 1.4 × 10−8]. This report describes a genetic variant which is associated with aggressive PrCa, which is a type of PrCa associated with a poorer prognosis.
INTRODUCTION
Genome-wide association studies (GWAS) have identified more than 50 common variants associated with susceptibility to prostate cancer (PrCa). However, these variants explain less than a third of the familial risk of the disease, indicating that further susceptibility loci remain to be identified. Moreover, few variants identified by GWAS have thus far been shown to be associated with aggressive PrCa. Although the current set of SNPs contributes to overall PrCa risk prediction, overall they do not discriminate men who will develop aggressive disease, a clinically more relevant outcome. A recent GWAS of aggressive PrCa identified a novel susceptibility locus on 2q37.3, but the per-allele odds ratio (OR) did not differ between aggressive and non-aggressive cases in the replication stage (1). It has been also reported that a genetic variant in DAP2IP might be associated with the risk of aggressive PrCa (2). A recent GWAS and validation study of aggressive PrCa found an SNP on 15q13, rs6497287, to be uniquely associated with this disease trait (Preplication = 0.004); however, lack of power due to small numbers and a non-significant test for heterogeneity between less and more aggressive PrCa warrants further investigation of this finding (3). Lin et al. (4) have reported an association of PrCa mortality with five germline SNPs from a candidate gene analysis.
RESULTS
In an attempt to identify susceptibility loci for aggressive PrCa, we conducted a meta-analysis of four GWAS (Table 1 and Supplementary Material, Notes). We also included data from the second stage of the UK study, that was genotyped for 43 671 SNPs showing evidence for association in stage 1 (5). These studies included, after quality control (QC) exclusions (see Materials and Methods), a total of 11 463 controls and 11 085 cases. For the present analysis, we included data from 5953 cases with aggressive disease defined as having a Gleason score of 8 or greater (with the exception of the BPC3 study, which also includes cases with tumor stage C or greater, and the CGEMS study, which also included cases with a Gleason score of 7) and all controls (it was ensured that there was no overlap between the studies). Following imputation using HapMap Phase II CEU as a reference, approximately 2.6 million genotyped and imputed SNPs were assessed in each GWAS study using a 1 df trend tests for association. Combined association tests were generated using a fixed effects meta-analysis (see Materials and Methods).
Table 1.
Total number of cases and controls included in the analysis
| Study | Total number | Controls | Cases | Aggressive disease cases |
|---|---|---|---|---|
| GWAS meta-analysis | ||||
| Stage 1 UK | 3748 | 1894 | 1854 | 617 |
| Stage 2 UK/Melbourne | 7590 | 3940 | 3650 | 1084 |
| CGEMS | 2277 | 1101 | 1176 | 688 |
| CAPS | 2926 | 994 | 1932 | 1091 |
| BPC3 | 6007 | 3534 | 2473 | 2473 |
| Total GWAS | 22 548 | 11 463 | 11 085 | 5953 |
| Confirmation | ||||
| PRACTICAL | 34 188 | 17 324 | 16 864 | 1956 |
| BPC3 | 14 933 | 7402 | 7531 | 52 |
| Total confirmation | 49 121 | 24 726 | 24 395 | 2008 |
| Total all | 71 669 | 36 189 | 35 480 | 7961 |
In the combined analysis, two loci, rs11672691 on 19q13 (P-value = 3.8 × 10−7) and rs11704416 on 22q13 (P = 7.0 × 10−6), showed strong evidence for association. rs11672691 is in the same region as rs887391 (r2 = 0.9) that it was previously reported to be associated with PrCa by Hsu et al. (6), but it did not reach GWAS significance level in that report. These two SNPs were selected for further replication analysis in two international consortia, PRACTICAL and BPC3. The present analysis was restricted to 24 395 cases (2008 aggressive) and 24 726 controls (17 445 controls in aggressive disease analysis) from 26 studies from European populations (Table 1, Supplementary Material, Table S1 and Supplementary Material, Notes show all 29 studies, 26 of which are European).
SNP rs11672691 showed evidence of replication (P = 0.006) with a genome-wide significance of P = 1.4 × 10−8 in a combined analysis across all stages (Table 2) for aggressive PrCa. When data from non-aggressive cases were also included, the overall evidence for association was stronger (P = 2.2 × 10−12, overall). The per-allele OR for aggressive PrCa in the replication stage [1.12, 95% confidence interval (CI) 1.03–1.21; P = 0.006] was higher than that for non-aggressive cases (OR 1.08, 95% CI 1.05–1.12; P = 8.2 × 10−7); however, the difference was not statistically significant (P-value = 0.18). SNP rs11704416 showed evidence of replication for all PrCa (P = 0.002), but did not quite reach genome-wide significance overall (P = 3.7 × 10−7). The evidence for association with aggressive disease was weaker (P = 0.16 in the replication, P = 4.0 × 10−6 overall). There was no evidence that either locus was associated with serum PSA (based on 1578 control samples; Supplementary Material, Table S2). SNP rs11672691 showed stronger effect (P = 0.02) when we compared cases with a family history of PrCa (OR 1.14, 95% CI 1.06–1.22) with those with no family history (OR 1.06, 95% CI 1.02–1.10). The per-allele ORs did not differ significantly by ages (Supplementary Material, Table S3). Considering the estimated ORs in the replication stage, rs11672691 and rs11704416 together explain ∼0.16% of the familial risk of PrCa.
Table 2.
Meta-analysis and replication results
| SNP chromosome allele position | Analysis | Study | ORa (95% CI) | P-value | P-value combined | P-value combined all |
|---|---|---|---|---|---|---|
| rs11672691 19 G/A 46677427 |
Aggressive disease cases | Stage 1 UK | 1.20 (1.05–1.35) | 0.02 | 3.8 × 10−7 | 1.4 × 10−8 |
| Stage 2 UK/Melbourne | NAb | NAb | ||||
| CGEMS | 1.25 (1.09–1.41) | 0.006 | ||||
| CAPS | 1.20 (1.05–1.35) | 0.015 | ||||
| BPC3 | 1.14 (1.05–1.23) | 0.01 | ||||
| PRACTICAL replication | 1.11 (1.02–1.20) | 0.006 | 0.006 | |||
| BPC3 replication | 1.45 (.85–2.48) | 0.17 | ||||
| Replication all | 1.12 (1.03–1.21) | |||||
| All cases | Stage 1 UK | 1.11 (1.00–1.24) | 0.05 | 3.5 × 10−7 | 2.2 × 10−12 | |
| Stage 2 UK/Melbourne | NAb | NAb | ||||
| CGEMS | 1.20 (1.05–1.38) | 0.009 | ||||
| CAPS | 1.23 (1.10–1.37) | 0.002 | ||||
| BPC3 | 1.14 (1.05–1.23) | 0.006 | ||||
| PRACTICAL replication | 1.08 (1.04–1.12) | 2.6 × 10−5 | 1.7 × 10−7 | |||
| BPC3 replication | 1.10 (1.04–1.16) | 0.002 | ||||
| Replication all | 1.08 (1.05–1.12) | |||||
| rs11704416 22 G/C 38766919 |
Aggressive disease cases | Stage 1 UK | 0.85 (0.69–1.02) | 0.056 | 3.3 × 10−6 | 4.0 × 10−6 |
| Stage 2 UK/Melbourne | 0.87 (0.75–0.999) | 0.03 | ||||
| CGEMS | 0.75 (0.57–0.93) | 0.002 | ||||
| CAPS | 0.77 (0.60–0.94) | 0.003 | ||||
| BPC3 | 0.94 (0.84–1.04) | 0.21 | ||||
| PRACTICAL replication | 0.95 (0.87–1.04) | 0.28 | 0.16 | |||
| BPC3 replication | 0.62 (0.39–1.00) | 0.05 | ||||
| Replication all | 0.94 (0.86–1.02) | |||||
| All cases | Stage 1 UK | 0.90 (0.80–1.00) | 0.058 | 7.0 × 10−6 | 3.7 × 10−7 | |
| Stage 2 UK/Melbourne | 0.91 (0.84–0.99) | 0.03 | ||||
| CGEMS | 0.81 (0.69–0.94) | 0.006 | ||||
| CAPS | 0.80 (0.65–0.96) | 0.005 | ||||
| BPC3 | 0.94 (0.84–1.04) | 0.21 | ||||
| PRACTICAL replication | 0.94 (0.91–0.98) | 0.003 | 0.002 | |||
| BPC3 replication | 0.96 (0.91–1.03) | 0.26 | ||||
| Replication all | 0.95 (0.92–0.98) |
Cases not classified as aggressive were those without the features defined in the text for aggressive disease.
aPer-allele OR for the first allele.
bNA: imputation quality was poor (0.227) and this result was excluded.
DISCUSSION
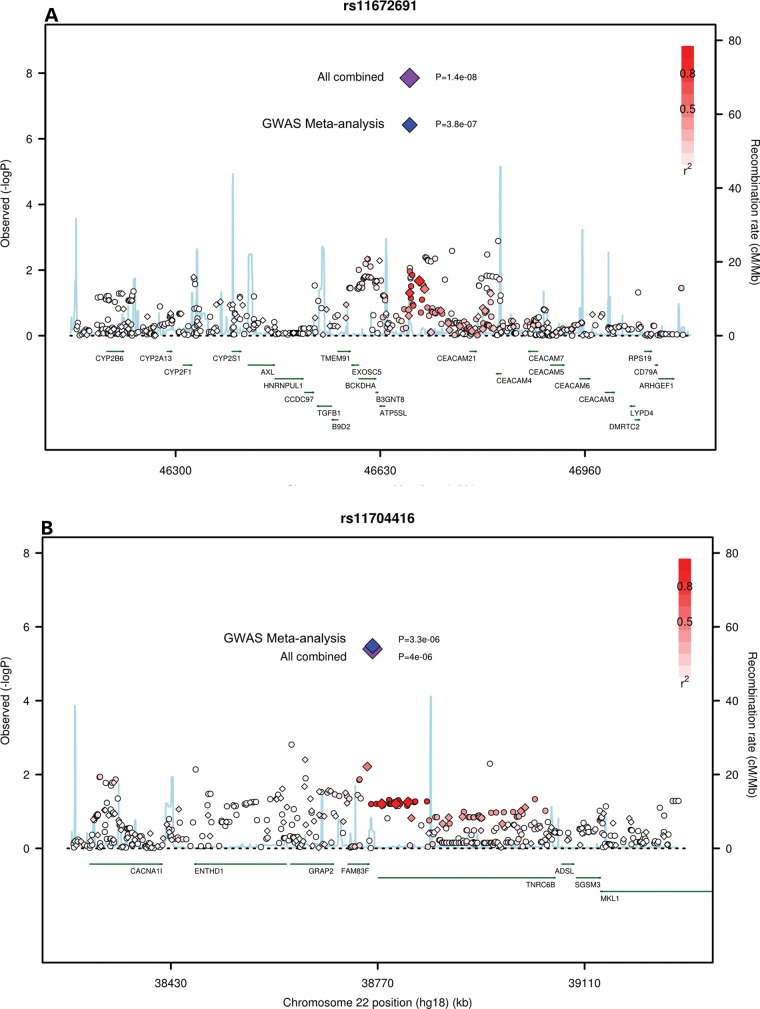
rs11672691 lies between ATP5SL and CEACAM21 (Fig. 1A) and within a hypothetical locus, LOC100505495, of a non-coding RNA. ATP5SL codes for an ATP synthase-like protein whose function is unknown; however, a variant in this gene has been associated with adult height (7). The carcinoembryonic antigen (CEA) gene family belongs to the immunoglobulin super family of genes. Several CEA subgroup members possess cell adhesion properties and some seem to function in signal transduction or regulation of signal transduction, possibly in association with other CEA sub-family members (8). Several of these proteins show a complex expression pattern in normal and cancerous tissues. Both CEACAM5 and CEACAM6 have a role in cell adhesion, invasion and metastasis (9), and are known to be overexpressed in a majority of carcinomas, including those of the gastrointestinal tract, the respiratory and genitourinary systems and breast cancer. The closest gene, CEACAM21, has been considered as a candidate gene for type 1 diabetes (10). A region on 19q13 (HPCQTL19) has been reported previously in a genetic linkage study to be a QTL for aggressive PrCa when the Gleason score was used as a quantitative measure of tumor aggressiveness (11).
Figure 1.
Regional plots of the associated SNPs at (A) 19q13 and (B) 22q13. Circles and diamonds denote imputed and genotyped SNPs, respectively. Plots show the genomic regions associated with PrCa and –log10 association P-values of SNPs. Also shown are the SNP build 36/hg18 coordinates in kilo bases, recombination rates and genes in the region. The intensity of red shading indicates the strength of LD (r2) with the index SNP. Plots were drawn with a modified R script from http://www.broadinstitute.org/mpg/snap/ldplot.php.
SNP rs11704416 lies upstream of TNRC6B on chromosome 22 (Fig. 1B). The TNRC6 (trinucleotide repeat containing 6) family of proteins have been shown to stably associate with argonaute (AGO) proteins. AGO proteins, through their association with small RNAs, perform a critical function in the effector step of RNA interference. TNRC6B protein has a role in translational inhibition through its binding to AGOs (12).
These results illustrate the value of combining GWAS to confirm candidate loci where the genome-wide significance threshold was not obtained, and improve power identifying susceptibility loci associated with sub-classifications of diseases. The original report by Hsu et al. (6) implicating the 19q13 region failed to reach genome-wide significance, whereas our findings verify a significant association. Although some samples overlap between the Hsu et al. report and our study, we expanded the discovery phase by incorporating the Stage 1 UK and Stage 2 UK/Melbourne participants and including additional samples in the replication stage. The identification of loci involved in PrCa aggressiveness has been hampered by relatively small sample sizes. The locus reported here is associated with both aggressive and non-aggressive diseases, and is therefore likely to be useful in determining those with clinically significant PrCa. Identification of such loci would aid the understanding of the biology of PrCa progression and targeted screening based on genetic risk profiling for aggressive disease.
MATERIALS AND METHODS
Samples
The four GWAS data sets have been described previously (1,5,13,14) (Table 1). Analyses were based on the data sets following standard QC procedures as previously described (5). The replication stage included 25 072 cases (2160 cases with a Gleason score of 8+) and 25 536 controls (18 255 in aggressive disease analysis) from 29 PrCa case–control studies (Supplementary Material, Table S1 and Notes). All studies were approved by the appropriate ethics committees.
Genotyping
In BPC3 and PRACTICAL, genotyping of samples from 13 studies was performed by the KASPar assay (www.kbioscience.co.uk), whereas 15 study sites performed the 5′ exonuclease assay (TaqMan™) using the ABI Prism 7900HT sequence detection system and IPO-Porto used TaqMan in a Roche LightCycler 480 Real-Time PCR System, all according to the manufacturer's instructions. Primers and probes were supplied directly by Applied Biosystems as Assays-By-Design™. Genotype counts are shown in Supplementary Material, Table S4. Assays at all sites included at least four negative controls and 2–5% duplicates on each 384-well plate. QC guidelines were followed by all the participating groups as described previously. In addition, all sites also genotyped 16 CEPH samples. We excluded individuals whose genotypes failed for at least 20% of the SNPs attempted. Data on a given SNP for a given site had to fulfill the following to be included: SNP call rate >95%, no deviation from Hardy–Weinberg equilibrium in controls at P < 0.00001, <2% discordance between genotypes in duplicate samples and in the CEPH samples. Cluster plots for SNPs that were close to failing any of the QC criteria were re-examined centrally.
Statistical methods
Imputation
Genotypes were imputed for approximately 2.6 million SNPs using the HapMap phase 2 CEU population as a reference. UK stages 1 and 2 and CGEMS were imputed using MACH 1.0 (http://www.sph.umich.edu/csg/abecasis/MACH/) to impute genotypes of autosomal markers and IMPUTE v1 (15) for chromosome X. The imputation for the BPC3 study was performed using MACH 1.0. The CAPS study used IMPUTE v1. We included imputed data from an SNP in the combined analysis if the estimated correlation between the genotype scores and the true genotypes (r2) was >0.3 (MACH) or where the quality information was >0.3 (IMPUTE).
Analyses
For UK stages 1 and 2 and CGEMS, the imputed genotype probabilities were used to derive a 1 df association score statistic for each SNP, and its corresponding variance. The test statistic for UK stage 2 was stratified by population as described previously (5). In the BPC3 study, estimated betas and standard errors were calculated for each component study, including one principal component as a covariate to adjust for population structure, using ProbABEL (16), and the results were combined to generate overall betas and standard errors, using a fixed effects meta-analysis. CAPS used SNPTEST (https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html) to estimate betas and standard errors. We converted the results from all studies into test scores and variances and derived a combined χ2 trend statistic for each SNP (equivalent to the Mantel extension test, or as in a fixed effects meta-analysis) in the R package. We repeated the same procedure to combine the results for the case/control association analysis and aggressive case/control association analysis.
We assessed associations between each SNP and PrCa in the replication stage, using a 1 df Cochran–Armitage trend test stratified by study. The combined P-values over all stages were generated similarly (using a 1 df trend test based on summing the scores and variances from each stage). SNPs were selected for validation on the basis of a significance level of P< 10−7 in a combined meta-analysis of UK stages 1 and 2, CGEMS, CAPS and BPC3, excluding SNPs that were correlated with known susceptibility SNPs (SNP rs11704416 was included since it reached P < 10−7 in an initial analysis). A total of 1921 subjects of non-European ancestry (Asian and African-American) were excluded from all analyses. Analyses were performed based on 2008 aggressive disease PrCa cases (out of 24 395 cases) and 17 445 controls (out of 24 726 controls). OR and 95% CI were estimated using unconditional logistic regression, stratified by study. In the text, we have reported the combined tests of association over all stages in European populations, but have emphasized the OR estimates from the replication stage to minimize the effect of ‘winner's curse’. ORs were computed separately for subsets of cases defined by family history, grade and age. Modification of the ORs by family history and grade was assessed using a case-only analysis, using the dichotomous variable as the endpoint (family history Yes versus No/Grade GS < 8 versus GS ≥ 8). Modification of the ORs by grade as a continuous covariate, and by age, was assessed using a case-only analysis, using polytomous regression with SNP genotype (scored 0, 1, 2) as the endpoint. The associations between SNP genotypes and PSA level were assessed using linear regression after log-transformation of PSA level to correct for skewness. We performed analyses for both all cases and only aggressive cases of PrCa. Analyses were performed in R principally using GenABEL (17), SNPTEST and ProbABEL (16) and Stata.
Publication of GWAS data
The U19, which provides funding for this work, plans to post summary data from this study onto a share point hosted by the NIH, by the end of 2012.
URLs
http://www.srl.cam.ac.uk/consortia/practical/index.html. (last accessed date 17 October, 2012)
http://www.cgems.cancer.gov/. (last accessed date 17 October, 2012)
https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html. (last accessed date 17 October, 2012)
http://www.sph.umich.edu/csg/abecasis/MACH/. (last accessed date 17 October, 2012)
http://www.broadinstitute.org/mpg/snap/ldplot.php. (last accessed date 17 October, 2012)
http://epi.grants.cancer.gov/BPC3/. (last accessed date 17 October, 2012)
http://ki.se/ki/jsp/polopoly.jsp?d=13809&a=29862&l=en. (last accessed date 17 October, 2012)
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by Cancer Research UK grants (grant numbers: C5047/A7357, C1287/A10118, C5047/A3354, C5047/A10692, C16913/A6135) and The National Institute of Health (NIH) Cancer Post-Cancer GWAS initiative grant (grant number: 1 U19 CA 148537-01 (the GAME-ON initiative)). Further support is detailed in Supplementary Material, Notes.
Supplementary Material
ACKNOWLEDGEMENTS
Acknowledgements are detailed in Supplementary Material, Notes.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Schumacher F.R., Berndt S.I., Siddiq A., Jacobs K.B., Wang Z., Lindstrom S., Stevens V.L., Chen C., Mondul A.M., Travis R.C., et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum. Mol. Genet. 2011;20:3867–3875. doi: 10.1093/hmg/ddr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duggan D., Zheng S.L., Knowlton M., Benitez D., Dimitrov L., Wiklund F., Robbins C., Isaacs S.D., Cheng Y., Li G., et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J. Natl Cancer Inst. 2007;99:1836–1844. doi: 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald L.M., Kwon E.M., Conomos M.P., Kolb S., Holt S.K., Levine D., Feng Z., Ostrander E.A., Stanford J.L. Genome-wide association study identifies a genetic variant associated with risk for more aggressive prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2011;20:1196–1203. doi: 10.1158/1055-9965.EPI-10-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin D.W., FitzGerald L.M., Fu R., Kwon E.M., Zheng S.L., Kolb S., Wiklund F., Stattin P., Isaacs W.B., Xu J., et al. Genetic variants in the LEPR, CRY1, RNASEL, IL4, and ARVCF genes are prognostic markers of prostate cancer-specific mortality. Cancer Epidemiol. Biomarkers Prev. 2011;20:1928–1936. doi: 10.1158/1055-9965.EPI-11-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eeles R.A., Kote-Jarai Z., Giles G.G., Olama A.A., Guy M., Jugurnauth S.K., Mulholland S., Leongamornlert D.A., Edwards S.M., Morrison J., et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat. Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 6.Hsu F.C., Sun J., Wiklund F., Isaacs S.D., Wiley K.E., Purcell L.D., Gao Z., Stattin P., Zhu Y., Kim S.T., et al. A novel prostate cancer susceptibility locus at 19q13. Cancer Res. 2009;69:2720–2723. doi: 10.1158/0008-5472.CAN-08-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lango Allen H., Estrada K., Lettre G., Berndt S.I., Weedon M.N., Rivadeneira F., Willer C.J., Jackson A.U., Vedantam S., Raychaudhuri S., et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal R.D., Leon E., Hansen H.J., Goldenberg D.M. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007;7:2. doi: 10.1186/1471-2407-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howson J.M., Walker N.M., Smyth D.J., Todd J.A. Analysis of 19 genes for association with type I diabetes in the Type I Diabetes Genetics Consortium families. Genes Immun. 2009;10(Suppl. 1):S74–S84. doi: 10.1038/gene.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slager S.L., Schaid D.J., Cunningham J.M., McDonnell S.K., Marks A.F., Peterson B.J., Hebbring S.J., Anderson S., French A.J., Thibodeau S.N. Confirmation of linkage of prostate cancer aggressiveness with chromosome 19q. Am. J. Hum. Genet. 2003;72:759–762. doi: 10.1086/368230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baillat D., Shiekhattar R. Functional dissection of the human TNRC6 (GW182-related) family of proteins. Mol. Cell. Biol. 2009;29:4144–4155. doi: 10.1128/MCB.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeager M., Chatterjee N., Ciampa J., Jacobs K.B., Gonzalez-Bosquet J., Hayes R.B., Kraft P., Wacholder S., Orr N., Berndt S., et al. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat. Genet. 2009;41:1055–1057. doi: 10.1038/ng.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng S.L., Liu W., Wiklund F., Dimitrov L., Balter K., Sun J., Adami H.O., Johansson J.E., Chang B., Loza M., et al. A comprehensive association study for genes in inflammation pathway provides support for their roles in prostate cancer risk in the CAPS study. Prostate. 2006;66:1556–1564. doi: 10.1002/pros.20496. [DOI] [PubMed] [Google Scholar]
- 15.Marchini J., Howie B., Myers S., McVean G., Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 16.Aulchenko Y.S., Struchalin M.V., van Duijn C.M. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics. 2010;11:134. doi: 10.1186/1471-2105-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aulchenko Y.S., Ripke S., Isaacs A., van Duijn C.M. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.