Abstract
Bacterial community composition in blood-sucking arthropods can shift dramatically across time and space. We used 16S rRNA gene amplification and pyrosequencing to investigate the relative impact of vertebrate host-related, arthropod-related and environmental factors on bacterial community composition in fleas and ticks collected from rodents in southern Indiana (USA). Bacterial community composition was largely affected by arthropod identity, but not by the rodent host or environmental conditions. Specifically, the arthropod group (fleas vs ticks) determined the community composition of bacteria, where bacterial communities of ticks were less diverse and more dependent on arthropod traits—especially tick species and life stage—than bacterial communities of fleas. Our data suggest that both arthropod life histories and the presence of arthropod-specific endosymbionts may mask the effects of the vertebrate host and its environment.
Keywords: bacterial diversity, microbiome, pyrosequencing, rodents, fleas, ticks
The composition of bacterial communities in blood-sucking arthropods can shift dramatically across time and space (Jones et al., 2010). These shifts are linked in part to variable arthropod characteristics (for example, arthropod species, life stage and engorgement level; Pidiyar et al., 2004; Moreno et al., 2006; Heise et al., 2010). However, blood-sucking arthropods acquire a portion of their microbial communities from vertebrate animals during feeding and additional microbes from the environment during free-living stages (fleas and mosquitos) or host-questing periods (ticks). Therefore, it is likely that both vertebrate host-related (for example, age, gender and reproductive status) and environmental (for example, temperature and habitat type) factors will also influence the bacterial communities of blood-sucking arthropods. However, there is a paucity of studies on the potential role of the vertebrate host and environment on bacterial composition within blood-sucking arthropods, and thus the relative impacts of vertebrate host-related, arthropod-related and environmental factors on bacterial community composition in blood-sucking arthropods are not well understood.
We used 16S rRNA pyrosequencing to characterize the bacterial community composition in 66 fleas (Orchopeas leucopus and Ctenophthalmus pseudagyrtes) and 132 ticks (Dermacentor variabilis and Ixodes scapularis) collected from 193 rodents (Peromyscus leucopus and Microtus ochrogaster) in southern Indiana, USA (Supplementary Material and Methods).
Most sequences (92%) were assigned to 103 phylotypes, representing six presumptive phyla (Actinobacteria, Bacteroidetes, Cyanobacteria, Firmicutes, Proteobacteria and Tenericutes) and 30 genera (Supplementary Figure S2). Similar to other studies on blood-sucking arthropods, we found diverse bacterial communities where most phylotypes were sparsely represented or occurred in only a few individual arthropods (Reed and Hafner, 2002; Pidiyar et al., 2004; Jones et al., 2010; Andreotti et al., 2011). For D. variabilis and I. scapularis, bacteria similar to Francisella spp. and Rickettsia spp., respectively, were most abundant. The high prevalence of Arsenophonus 1 and Francisella 1 and 2 in D. variabilis and of Rickettsia 1 in I. scapularis, and their similarity to previously characterized endosymbionts (Supplementary Table S2), suggests that they are stable residents of these tick species. Bartonella spp. were the most frequently identified bacteria in the two flea species. In particular, Bartonella 1, a phylotype with high resemblance to the vertebrate (including human) pathogen, B. grahamii (Kerkhoff et al., 1999; Breitschwerdt and Kordick, 2000), infected the two flea species at high frequencies. Brevibacillus spp., the second most common sequences in all four arthropod species, may also have an important role in bacterial communities of arthropods, and thus deserve additional study.
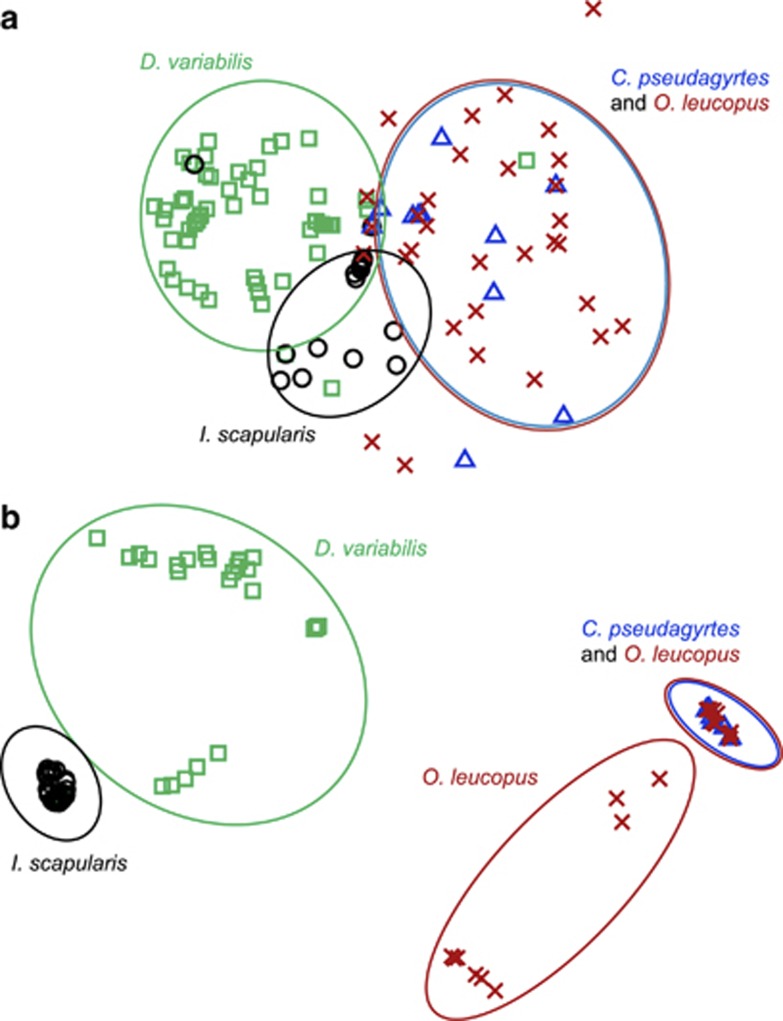
To the best of our knowledge, this is the first study that simultaneously quantified the effects of vertebrate host-related, arthropod-related and environmental factors on the bacterial community composition of blood-sucking arthropods. Only the effect of arthropod-related variables was significant and accounted for 18.6% of the variation in the bacterial composition (Supplementary Table S3). In particular, the arthropod group (fleas vs ticks) had a major effect on bacterial composition (F=31.9, P<0.0005). The major clusters of bacterial communities in our ordinations corresponded to (i) O. leucopus and C. pseudagyrtes fleas, (ii) I. scapularis and (iii) D. variabilis ticks (Figure 1). The unexplained variance in bacterial community composition could be a result of other factors not quantified in this study, such as interspecific interactions between bacteria, or a result of stochastic events.
Figure 1.
Non-metric multidimensional scaling ordination of (a) all 198 arthropods based on Bray–Curtis similarities in abundances of the 103 bacterial phylotypes and (b) a subset of 146 arthropods based on Bray–Curtis similarities in abundances of five bacterial phylotypes belonging to the commonest arthropod-specific genera (Rickettsia 1, Francisella 1, Francisella 2, Bartonella 1 and Bartonella 2; Supplementary Table S2 and Supplementary Figure S2). Closer points represent higher similarity in bacterial community composition than points that are further apart. Each point represents an individual arthropod. The division of arthropod to species (green squares for D. variabilis, black circles for I. scapularis, blue triangles for C. pseudagyrtes, and red X's for O. leucopus) was the best explanatory variable of the bacterial community composition within ticks but not fleas. Oval shapes surround bacterial communities in individual arthropods of the same species or two species that were not distinguished based on their bacterial communities.
Independent analyses for fleas and ticks significantly improved the explanatory power of the tested variables for ticks (39.7%) but not for fleas (20.4%), for which none of the tested variables were significant. Arthropod species had a major effect on bacterial composition in ticks (F=55.8, P<0.0005). The Rickettsia 1 and Francisella 1 phylotypes were primarily responsible for differences across tick species, as the former was found mainly in I. scapularis and the latter was found only in D. variabilis. Arthropod life stage also significantly affected the bacterial community composition of ticks (F=9.8, P<0.0005). The Francisella 1 phylotype was 37 times more abundant in nymphs than in larvae and was the primary determinant of differences across life stages. Life-stage differences in blood engorgement (for example, nymphs consume larger and more blood meals than larvae) may explain those differences, as blood ingestion often induces bacteria multiplication in the arthropod gut (Heise et al., 2010). Alternatively, Francisella bacteria may accumulate with transmission from one developmental stage of the tick to the next.
The lower diversity of bacterial species in ticks (Supplementary Table S2) and their higher sensitivity to arthropod-related variables compared to fleas may be a result of the dominance of endosymbionts in ticks, which are likely to be highly dependent on the arthropod and may potentially exclude competing bacteria (Lively et al., 2005). In addition, free-living flea larvae (unlike ticks) may acquire bacteria including Comamonas, Pelomonas, Ralstonia (Supplementary Figure S2) from soil and transtadially transmit these to adult fleas (in addition to acquisition of bacteria from blood meals).
The most intriguing results of this study are that the vertebrate host and environmental characteristics do not seem to affect bacterial community composition in fleas and ticks. Fleas and ticks were collected over a range of environmental conditions and sites, but none of those conditions significantly affected the bacterial communities of the arthropods. Geographic differences have been detected in other tick-associated bacterial communities (Wielinga et al., 2006; Clay et al., 2008; van Overbeek et al., 2008). While we collected ticks from rodents, in these other studies ticks were sampled from the environment. Rodents could have transported ectoparasites and their associated bacteria among sites or may have provided more stable conditions and buffered potential environmental variability. However, none of the seven host-related factors tested had a significant impact on bacterial community composition. The minor effects of host traits on the microbial community composition may reflect the fact that vertically-transmitted endosymbionts are the dominant members of these communities, at least in ticks. The nature of association between fleas and their dominant bacterial association with Bartonella deserve further study.
Taken together, our study suggests that bacterial communities in blood-sucking arthropods are composed of both pathogens and endosymbionts, where the exact species composition is determined largely by arthropod traits.
Acknowledgments
We thank Dr John Whitaker, Dr Bob Pinger, Dr Hans Klompen, Jonathan Kagemann, Arian Avalos, Will Snydermann, Vidhya Silvanose and Kashi Revanna for their assistance in data collection and analysis. Funding was provided through NIH/NSF joint grant DEB-03268742 to KC and CF, by NIH grant 1RC2HG005806-01 to QD and DN, by the Indiana Metabolomics and Cytomics Initiative of Indiana University (IU) to KC and CF. HH was supported by a grant from the IU Center for Research in Environmental Sciences to KC. DM was supported by the UNT-HHMI undergraduate student research grant.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Andreotti R, de Leon AAP, Dowd SE, Guerrero FD, Bendele KG, Scoles GA.2011. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol11 (article number: 6).doi: 10.1186/1471-2180-1111-1186.http://www.biomedcentral.com/1471-2180/11/6 . [DOI] [PMC free article] [PubMed]
- Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–438. doi: 10.1128/cmr.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K, Klyachko O, Grindle N, Civitello D, Oleske D, Fuqua C. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol Ecol. 2008;17:4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- Heise SR, Elshahed MS, Little SE. Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. J Med Entomol. 2010;47:258–268. doi: 10.1603/me09197. [DOI] [PubMed] [Google Scholar]
- Jones RT, Knight R, Martin AP. Bacterial communities of disease vectors sampled across time, space, and species. ISME J. 2010;4:223–231. doi: 10.1038/ismej.2009.111. [DOI] [PubMed] [Google Scholar]
- Kerkhoff FT, Bergmans AMC, van der Zee A, Rothova A. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol. 1999;37:4034–4038. doi: 10.1128/jcm.37.12.4034-4038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively CM, Clay K, Wade MJ, Fuqua C. Competitive co-existence of vertically and horizontally transmitted parasites. Evol Ecol Res. 2005;7:1183–1190. [Google Scholar]
- Moreno CX, Moy F, Daniels TJ, Godfrey HP, Cabello FC. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environ Microbiol. 2006;8:761–772. doi: 10.1111/j.1462-2920.2005.00955.x. [DOI] [PubMed] [Google Scholar]
- Pidiyar VJ, Jangid K, Patole MS, Shouche YS. Studies on cultured and uncultured microbiota of wild Culex quinquefasciatus mosquito midgut based on 16s ribosomal RNA gene analysis. Am J Trop Med Hyg. 2004;70:597–603. [PubMed] [Google Scholar]
- Reed DL, Hafner MS. Phylogenetic analysis of bacterial communities associated with ectoparasitic chewing lice of pocket gophers: a culture-independent approach. Microb Ecol. 2002;44:78–93. doi: 10.1007/s00248-002-0009-4. [DOI] [PubMed] [Google Scholar]
- van Overbeek L, Gassner F, van der Plas CL, Kastelein P, Rocha UND, Takken W. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. FEMS Microbiol Ecol. 2008;66:72–84. doi: 10.1111/j.1574-6941.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Gaasenbeek C, Fonville M, de Boer A, de Vries A, Dimmers W, et al. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in the Netherlands. Appl Environ Microbiol. 2006;72:7594–7601. doi: 10.1128/AEM.01851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.