Abstract
OBJECTIVE
To examine, for the first time, the association between a novel inflammatory cytokine, angiopoietin-like protein (ANGPTL) 2, and the development of type 2 diabetes (T2DM).
RESEARCH DESIGN AND METHODS
A total of 2,164 community-dwelling Japanese individuals aged 40 to 79 years without diabetes were followed up for 7 years. Serum ANGPTL2 levels were divided into quartile categories at baseline: <2.15, 2.16–2.71, 2.72–3.40, and ≥3.41 ng/mL. During follow-up, 221 participants developed T2DM.
RESULTS
In multivariate analyses, after adjusting for comprehensive risk factors and high-sensitivity C-reactive protein (hs-CRP) levels, the risk of developing T2DM was significantly higher in the highest ANGPTL2 quartile than in the lowest quartile (hazard ratio, 1.80; 95% CI, 1.14–2.85; P = 0.01).
CONCLUSIONS
Elevated serum ANGPTL2 levels were positively associated with the development of T2DM in a general population, independent of other risk factors including hs-CRP levels.
Angiopoietin-like proteins (ANGPTLs), which are structurally similar to angiopoietins, are characterized by a coiled-coil domain in the N-terminus and a fibrinogen-like domain in the C-terminus. Seven ANGPTLs have been identified to date (1–3); one of them, ANGPTL2, has been shown to be expressed abundantly in adipose tissues and to be a key mediator linking obesity to adipose tissue inflammation and systemic insulin resistance in mice (4,5). In humans, ANGPTL2 is also closely related to adiposity and inflammation (4). However, the association of serum ANGPTL2 levels with the risk of developing type 2 diabetes (T2DM) has not been investigated to date. The objective of this study was to examine this issue in a cohort of the general Japanese population, taking into account a comprehensive range of confounders.
RESEARCH DESIGN AND METHODS
Study population and follow-up survey
In 2002, a baseline survey for this study was performed in the town of Hisayama, Japan. A detailed description of this survey was published previously (6). Briefly, of the total of 3,896 residents aged 40 to 79 years, 3,000 consented to participate in the survey (participation rate, 77.0%). Among them, 178 participants were not administered a 75-g oral glucose tolerance test (OGTT): 100 refused the test, 46 had already eaten breakfast, and the other 32 were receiving insulin therapy for diabetes. Consequently, 2,822 participants completed the OGTT. After further excluding 485 participants who had newly diagnosed or known diabetes and 8 for whom there was no measurement of ANGPTL2, the remaining 2,329 (953 men and 1,376 women) were enrolled in the baseline examination.
The baseline participants were followed up prospectively, from 2002 to 2009, by yearly health examinations during which an OGTT was administered. Of the baseline participants, 2,164 (865 men and 1,299 women) who underwent reexaminations during the follow-up period were finally selected for this study (follow-up rate, 92.9%; mean follow-up period, 6.0 years). These participants completed the follow-up examinations an average of 4.9 times, and among them, 861 (39.8% of the follow-up population) underwent all 7 annual OGTTs. During the follow-up, T2DM occurred in 221 participants (115 men and 106 women).
Clinical evaluation and laboratory measurements
In the baseline and follow-up examinations, the study participants underwent the OGTT after an overnight fast of at least 12 h. Diabetes was defined by the 2003 American Diabetes Association criteria (7). Serum ANGPTL2 concentrations were measured with the human ANGPTL2 sandwich enzyme-linked immunosorbent assay using two mouse monoclonal antibodies that were confirmed to recognize only ANGPTL2 and not to react with other ANGPTLs or angiopoietins (4).
Statistical analysis
ANGPTL2 levels were divided into quartile categories: ≤2.15, 2.16–2.71, 2.72–3.40, and ≥3.41 ng/mL. The incidence of T2DM was calculated by the person-year method and adjusted for age and sex by the direct method using 10-year age groupings. The adjusted hazard ratios (HRs) and their 95% CIs were calculated using the Cox proportional hazards model.
RESULTS
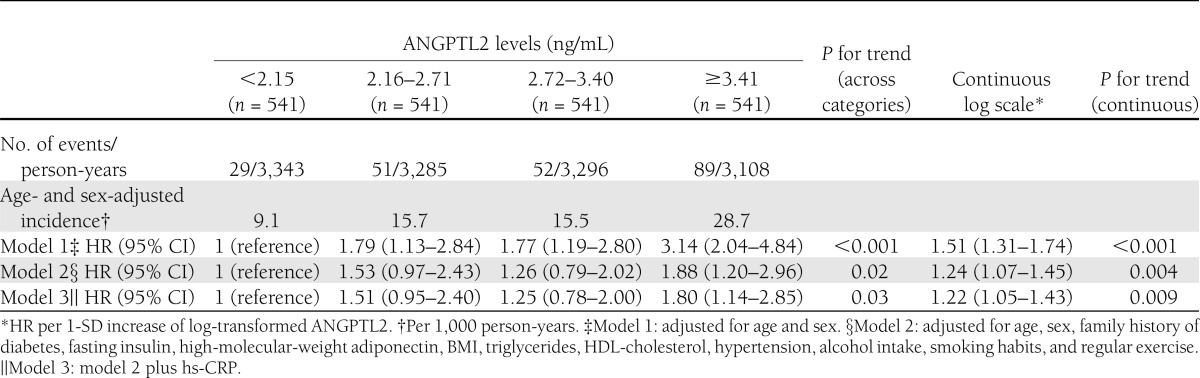
At baseline, the mean age of participants was 58.6 years, and the proportion of men was 40.9%. The age- and sex-adjusted incidences of T2DM increased significantly with elevating quartiles of ANGPTL2 concentrations, and the risk was significantly higher in the second, third, and fourth quartiles than in the first quartile (Table 1, model 1). In the multivariate analysis, this association remained substantially unchanged even after adjustment for age, sex, family history of diabetes, fasting insulin, high-molecular-weight adiponectin, BMI, triglycerides, HDL cholesterol, hypertension, alcohol intake, smoking habits, and regular exercise (model 2). As shown in model 3, after further adjustment for high-sensitivity C-reactive protein (hs-CRP) values, the risk of developing T2DM was significantly higher in the highest ANGPTL2 quartile than in the lowest quartile (HR, 1.80; 95% CI, 1.14–2.85; P = 0.01). These findings remained substantially unchanged when waist circumference was used instead of BMI in the adjusted models.
Table 1.
Adjusted incidences and HRs of type 2 diabetes according to ANGPTL2 levels, 2002–2009
CONCLUSIONS
In a prospective study of a cohort of the general Japanese population, we clearly demonstrated that the risk for the development of T2DM increased with increasing serum ANGPTL2 levels. This association remained robust even after controlling for other confounding factors, including hs-CRP levels.
To our knowledge, this is the first report to indicate that serum ANGPTL2 levels are an independent risk factor for developing T2DM in a general population. The concept that heightened inflammation is important in the pathogenesis of T2DM (8) is supported by the evidence that inflammation in islets, adipose tissue, liver, and muscle may provoke insulin resistance and β-cell dysfunction (9,10) and may therefore antedate the diagnosis of T2DM. Prospective observational studies have demonstrated that several nonspecific indicators of inflammation were found to be predictive of incident T2DM (11–14). Among them, C-reactive protein is a nonspecific inflammatory marker and the most commonly measured circulating marker for subclinical inflammation (13,15). The standardized assays for its measurement are widely available (13,15). In this study, the association between serum baseline ANGPTL2 levels and incident T2DM was found to be independent of the hs-CRP levels. Nevertheless, further studies would be required to reveal whether the association is truly independent of other established inflammatory markers.
This analysis clearly showed that elevated serum ANGPTL2 levels were independently associated with incident T2DM. Further studies are needed to reveal the role of ANGPTL2 in inflammation in human adipose tissue and the development of T2DM.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research on Innovative Areas (22116010) and for Scientific Research (A) (22240073) and (C) (24590797, 22590892, 23590797, 23590798, 23500842, and 24590796) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (Comprehensive Research on Aging and Health: H20-Chouju-004; Comprehensive Research on Life-Style Related Diseases including cardiovascular diseases and diabetes mellitus: H22-Junkankitou [Seishuu]-Ippan-005, H22-Junkankitou [Seishuu]-Ippan-017, H23-Junkankitou [Seishuu]-Ippan-002, and H23-Junkankitou [Seishuu]-Ippan-005; and Comprehensive Research on Dementia: H23-Ninchishou-Ippan-004). This study also was supported by the Funding Program for Next Generation World-Leading Researchers (LS098) from the Japan Society for the Promotion of Science.
No potential conflicts of interest relevant to this article were reported.
Y.D., Y.O., and Y.K. planned the study and directed its implementation, including quality assurance and control. T.N. and Y.H. designed the study’s analytic strategy. O.T. measured the samples. N.M. and J.H. helped conduct the literature review. M.I. and T.K. helped supervise the field activities.
T.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff of the Division of Health and Welfare of Hisayama for their cooperation in this study.
References
- 1.Kim I, Moon SO, Koh KN, et al. Molecular cloning, expression, and characterization of angiopoietin-related protein. angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem 1999;274:26523–26528 [DOI] [PubMed] [Google Scholar]
- 2.Kim I, Kim HG, Kim H, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J 2000;346:603–610 [PMC free article] [PubMed] [Google Scholar]
- 3.Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med 2005;11:473–479 [DOI] [PubMed] [Google Scholar]
- 4.Tabata M, Kadomatsu T, Fukuhara S, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab 2009;10:178–188 [DOI] [PubMed] [Google Scholar]
- 5.Okada T, Tsukano H, Endo M, et al. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol 2010;176:2309–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi Y, Kubo M, Yonemoto K, et al. Fasting plasma glucose cutoff for diagnosis of diabetes in a Japanese population. J Clin Endocrinol Metab 2008;93:3425–3429 [DOI] [PubMed] [Google Scholar]
- 7.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 8.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286–1292 [DOI] [PubMed] [Google Scholar]
- 9.Varma V, Yao-Borengasser A, Rasouli N, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 2009;296:E1300–E1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999;353:1649–1652 [DOI] [PubMed] [Google Scholar]
- 12.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM, Insulin Resistance Atherosclerosis Study Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2002;51:1131–1137 [DOI] [PubMed] [Google Scholar]
- 13.Doi Y, Kiyohara Y, Kubo M, et al. Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: the Hisayama Study. Diabetes Care 2005;28:2497–2500 [DOI] [PubMed] [Google Scholar]
- 14.Duncan BB, Schmidt MI. The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther 2006;8:7–17 [DOI] [PubMed] [Google Scholar]
- 15.Pearson TA, Mensah GA, Alexander RW, et al; Centers for Disease Control and Prevention. American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511 [DOI] [PubMed] [Google Scholar]