Abstract
OBJECTIVE
We used fast-gradient magnetic resonance imaging (MRI) to determine the longitudinal associations between the hepatic fat content (HFF), glucose homeostasis, and a biomarker of hepatocellular apoptosis in obese youth.
RESEARCH DESIGN AND METHODS
Baseline and longitudinal liver and abdominal MRI were performed with an oral glucose tolerance test in 76 obese youth followed for an average of 1.9 years. Cytokeratin-18 (CK-18) was measured at baseline and follow-up as a biomarker of hepatic apoptosis. The relationship between baseline HFF and metabolic parameters and circulating levels of CK-18 at follow-up were assessed using a bivariate correlation.
RESULTS
At baseline, 38% had hepatic steatosis based on %HFF ≥5.5% with alterations in indices of insulin sensitivity and secretion. At follow-up, BMI increased in both groups and baseline %HFF correlated strongly with the follow-up %HFF (r = 0.81, P < 0.001). Over time, markers of insulin sensitivity and 2-h glucose improved significantly in the group without fatty liver, in contrast with the persistence of the insulin resistance and associated correlates in the fatty liver group. Baseline HFF correlated with 2-h glucose (r = 0.38, P = 0.001), whole-body insulin sensitivity (r = −0.405, P = 0.001), adiponectin (r = −0.44, P < 0.001), CK-18 levels, (r = 0.63, P < 0.001), and disposition index (r = −0.272, P = 0.021) at follow-up. In a multivariate analysis, we showed that baseline HFF is an independent predictor of 2-h glucose and whole-body insulin sensitivity.
CONCLUSIONS
In obese youth, the phenotype of MRI-measured hepatic steatosis is persistent. Baseline HFF strongly modulates longitudinally 2-h blood glucose, biomarkers of insulin resistance, and hepatocellular apoptosis.
Concurrent with the soaring rates of childhood obesity, nonalcoholic fatty liver disease (NAFLD) has emerged as the most common liver disease in children in the U.S. (1). NAFLD includes a wide spectrum of pathologies, ranging from simple steatosis (also called NAFL) to steatohepatitis (NASH) to fibrosis/cirrhosis (2,3). NAFLD is associated with hyperlipidemia, insulin resistance, and type 2 diabetes. Thirty to forty percent of obese youth have NAFLD, and ∼10% of them develop NASH, characterized by inflammation and hepatocyte ballooning on a background of hepatic steatosis (1,4–6). Although simple hepatic steatosis usually has a “benign course,” NASH, on the other hand, may progress to end-stage liver disease. Progression to more deleterious stages occurs more rapidly in children than in adults, as described by Feldstein et al. (7).
Accurate diagnosis and staging of NAFL/NASH requires liver biopsy. Due to its associated risks, high cost, and poor acceptability in pediatrics, liver biopsy is a roadblock limiting advancement in the pathogenesis and natural history of the disease in children. However, two imaging techniques (1H-nuclear magnetic resonance [1H-NMR] and fast magnetic resonance imaging [fast MRI]) have been proven to accurately quantify fatty liver content (NAFLD) in both adults and children and thus are increasingly being used in clinical research (8–10). A hepatic fat content (HFF) ≥5.5% is consistent with the diagnosis of hepatic steatosis (8–10). In our group, the fast MRI was strongly correlated with 1H-NMR measures of steatosis and with macrovesicular steatosis/NASH seen on liver biopsy in obese children (11). However, information on advanced stages, such as inflammation and fibrosis, cannot be obtained with these imaging techniques.
There is a high prevalence of hepatic steatosis in obese children and adolescents. The potential for progression to deleterious stages of liver disease and its association with type 2 diabetes creates a need to accurately identify those children and to track the putative metabolic changes associated with hepatic steatosis. Although there is vast literature on the effect of fatty liver on metabolic deterioration in adults, little is known about this process in the pediatrics. Therefore, the aim of this study was to follow a multiethnic group of obese children and adolescents with and without liver steatosis and track changes in metabolic parameters in relation to baseline HFF measured by fast MRI. We hypothesize that baseline HFF would strongly modulate the changes in glucose metabolism and insulin resistance over time in obese youth. Furthermore, circulating levels of cytokeratin-18 (CK-18), a biomarker of hepatocellullar apoptosis, known to be linked to steatohepatitis (12), was measured to follow the putative association between steatosis and hepatocellular damage longitudinally.
RESEARCH DESIGN AND METHODS
The cohort
Participants in this cohort were recruited from the Yale Pediatric Obesity Clinic and are part of a longitudinal study on the pathophysiology of type 2 diabetes. Subjects with medical conditions or using medications that may affect lipid metabolism were excluded. All subjects were nonsmokers. Information related to alcohol consumption was obtained using a questionnaire. Autoimmune hepatitis, Wilson disease, α-1-antitrypsin deficiency, hepatitis B and C, and iron overload were excluded in subjects with persistent elevation in alanine aminotransferase (ALT; >6 months). Participants were followed biannually as outpatients by the clinical staff and received standard nutrition counseling and physical activity recommendations for pediatric obesity (13). The protocol for longitudinal assessment of intra-HFF and abdominal fat distribution by MRI, glucose, and lipid metabolism was approved by the institutional review board of the Yale University School of Medicine. Written informed consent was obtained from the parents and assent from the children and adolescents.
Fast liver MRI: liver fat content
Measurement of liver fat content was performed by MRI using the 2-point Dixon (2PD) as modified by Fishbein et al. (14), based on phase-shift imaging where %HFF is calculated from the signal difference between the vectors resulting from in-phase and out-of-phase signals. Using the MRIcro software program, five regions of interest were drawn on each image, and the mean pixel signal intensity level was recorded. The %HFF was calculated in duplicate form from the mean pixel signal intensity data using the following equation: ([Sin − Sout]/[2 × Sin]) × 100. The imaging parameters were as follows: matrix size = 128 × 256, flip angle (α) = 30°, repetition time (TR) = 18 ms, echo time (TE2) = 2.38/4.76 ms, out of phase and in phase, respectively, bandwidth = 420 Hz/pixel, six averages, slice thickness = 10 mm, one slice, 2.3 s/slice. (For 2-point measurement of HFF, scan time is 14 s during a single breath hold) (15). Recent studies indicated that T1 and T2 values can introduce errors in the calculation of fat fraction (11,16,17). Without T1 correction, low-fat fractions can be overestimated (16), whereas higher-fat fractions remain relatively unaffected. In our methodology, we used a small flip angle (30°) to minimize T1 effects. A simple explanation for some of the negative fat fractions arises in low-signal-to-noise ratio regions where in-phase/out-of-phase difference is within the noise, or where the tissue is composed of either mostly all fat or mostly all water such that the in-phase/out-of-phase differences are also around zero. The T2 correction is very important in cases where iron overload may lead to T2 changes (11), but none of the patients in our study had iron overload; thus, this effect can be eliminated as a concern that might influence our results. Although some studies recently have been published using fat fractions that have been corrected for T1 and T2, this is currently an area of active research and the correction schemes are not necessarily as straightforward. Fat has multiple spectral components, and this single measurement of T2 decay curve is not adequate (such a measurement yields oscillating signal behavior) (16). For these reasons, we did not correct for T1 or T2 effects but do not feel such corrections would significantly alter the results, and could introduce additional errors (18). Subjects were grouped into the following: low HFF, where HFF% is <5.5%, or high HFF, where HFF% is ≥5.5%.
Validation of fast MRI
We validated the modified 2PD method against 1H-NMR in 34 lean and obese adolescents and found a very strong correlation between the two methods (r = 0.954, P < 0.0001) (15). To assess its repeatability, repeated assessments were performed on the same day by the same operator. The within-subject SD for %HFF was 1.9%. This degree of reproducibility is within the boundaries to make this a viable method to assess the relation between HFF and metabolic outcomes. Kim et al. (15) demonstrated that a 2PD HFF cutoff of 3.6% provided good sensitivity (80%) and specificity (87%) compared with a 1H-NMR reference (15). Comparisons between the 2PD method and histological determination of fatty liver have been made, only in adults. Fishbein et al. (17) found in 38 patients undergoing biopsy for a variety of liver diseases a highly significant correlation between liver histology and MRI determination of %HFF, particularly with macrovesicular steatosis (r = 0.92, P < 0.001). Pacifico et al. (19) validated the MRI-measured HFF with steatosis obtained from liver biopsies in 25 obese adolescents and found a strong correlation of 0.86 (P < 0.0001). Fast MRI was used to track longitudinal changes in adults during pioglitazone treatment (20) and in a case report involving obese adolescents with NAFLD undergoing gradual weight loss (9).
Abdominal MRI and total-body composition
Abdominal MRI studies were performed on a Siemens Sonata 1.5 Tesla system. Total body composition was measured by dual-energy X-ray absorptiometry with a Hologic scanner (Boston, MA).
Oral glucose tolerance test
All subjects were invited to the Yale Center for Clinical Investigation at 8:00 a.m. after an overnight fast. After the placement of an indwelling venous line, baseline samples were obtained for glucose, insulin, C-peptide, lipid profile, liver enzymes, hemoglobin A1c, and total adiponectin. A standard 3-h oral glucose tolerance test was performed as previously reported (21). Indices of insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR] and Matsuda index [whole-body insulin resistance index, WBISI]) and insulin secretion (insulinogenic index [IGI] and DI) were calculated as previously reported (21,22). The disposition index was calculated as the products of the whole body insulin sensitivity index (WBISI) and the insulinogenic index (IGI). Definition of the metabolic syndrome was based on the pediatric criteria according to Weiss et al. (23). Children and adolescents in our study were classified as having metabolic syndrome if they met three or more of the following criteria for age and sex: BMI >97th percentile (z score, 2.0 or more), triglyceride (TG) level >95th percentile, HDL cholesterol level <5th percentile, systolic or diastolic blood pressure >95th percentile, and impaired glucose tolerance. The degree of insulin resistance was determined with the use of a homeostatic model (HOMA-IR). Scores ordinarily range from 0 to 15, with higher scores indicating greater insulin resistance, and are calculated as the product of the fasting plasma insulin level (in microunits per milliliter) and the fasting plasma glucose level (in millimoles per liter), divided by 22.5.
Biochemical analysis
Plasma glucose was measured using the YSI 2700 STAT Analyzer (Yellow Springs Instruments) and lipids using an autoanalyzer (model 747-200; Roche-Hitachi). Plasma insulin and total adiponectin were measured using double-antibody radioimmunoassay from Millipore and C-peptide using double-antibody radioimmunoassay from Diagnostic Products Corp. Liver enzymes were measured using standard automated kinetic enzymatic assays. Apoptotic markers were measured using an immune-based assay (enzyme-linked immunosorbent assay), as previously reported (12,24,25). The intra-assay variations were <4% and interassay variations were <10%.
Statistical analysis
Data are expressed as mean ± SD or SE, or median (interquartile range). Differences in sex were analyzed by Fisher exact test, and dichotomous variables were analyzed by χ2 tests. Differences between subjects with low or high HFF at baseline were evaluated using independent-samples t test or Mann-Whitney U test, and baseline and follow-up variables were evaluated with paired t tests for normally distributed variables or Wilcoxon signed rank tests for variables with a skewed distribution. In order to evaluate the contribution of baseline HFF (%) to liver steatosis as well as metabolic parameters at follow-up, a simple correlation was performed with Pearson correlation coefficients. In addition, two multiple, stepwise, linear regressions were also performed in order to evaluate the effect of baseline HFF (%) on the two main glucose metabolism parameters (2-h blood glucose and WBISI). Both regressions were also adjusted for age, sex, ethnicity, BMI-Z, Δ BMI-Z, and length of follow-up. Baseline 2-h blood glucose and WBISI were also included in the two models, respectively. All analyses were performed using SPSS 15.0 for Windows, and statistical significance was assessed at the two-tailed 0.05 threshold.
RESULTS
Anthropometric and metabolic phenotypes according to liver fat content at baseline
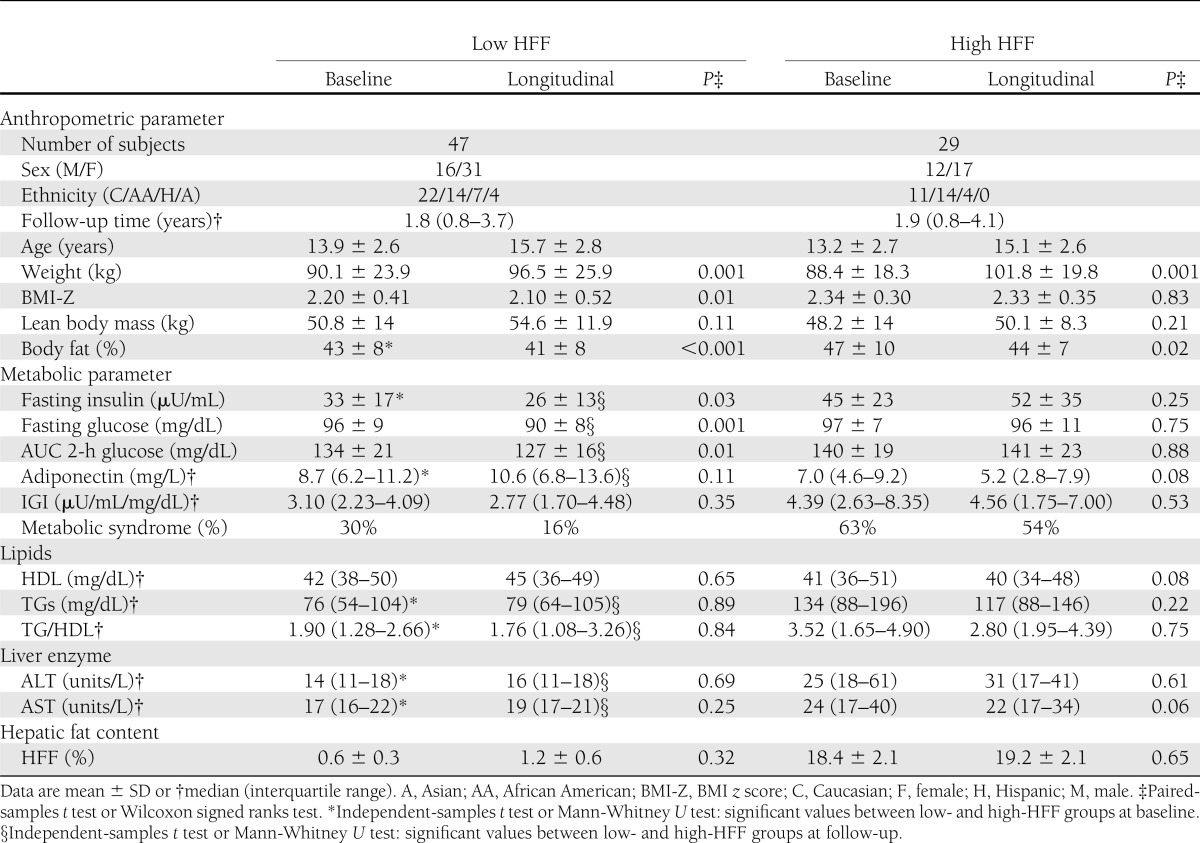
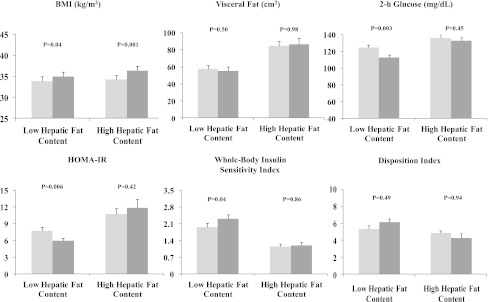
Based on the %HFF, the subjects were divided into groups with low (HFF <5.5%) and high HFF (≥HFF 5.5%). At baseline, the two groups had similar age, sex, height, weight, BMI, BMI-Z, waist circumference, and percent body fat. By definition, HFF (%) values were significantly different between the two groups. Abdominal fat distribution measurements showed only significantly higher visceral and visceral-to-subcutaneous fat ratio values in obese youth with high HFF compared with those with low HFF (Table 1 and Fig. 1).
Table 1.
Main anthropometric and metabolic parameters at baseline and follow-up in subjects with low and high HFF (HFF <5.5 and ≥5.5%, respectively)
Figure 1.
Main anthropometric and metabolic parameters at baseline (light gray) and follow-up (dark gray) in low- and high-HFF obese groups.
Fasting glucose concentrations were similar between the two groups, whereas fasting insulin and both 2-h glucose and insulin were higher in the high-HFF group, consistent with a greater degree of insulin resistance, as indicated by the HOMA-IR and lower WBISI. Baseline early insulin secretion (IGI) was lower in the group with high HFF than the group with low HFF. The high-HFF group showed a trend for a lower DI, although it did not reach a statistically significant value. Fasting adiponectin concentrations were significantly lower in the group with high HFF. ALT and aspartate aminotransferase (AST) values, TGs, and TG-to-HDL cholesterol ratio were significantly higher in the high-HFF group. Of note, the prevalence of metabolic syndrome was significantly lower in the low-HFF group (P < 0.001).
Liver fat content and glucose metabolism at follow-up
Both groups were followed for an average period of 1.8 years (P = 0.576). Those with high HFF had a significantly greater increase in BMI in contrast to the group with low HFF. Indeed BMI-Z decreased significantly in the low-HFF group, whereas no changes were observed in the high-HFF group. Of note, no significant changes were observed in HFF and visceral fat in either group. Glucose metabolism and insulin sensitivity indices significantly improved in the low-HFF group, whereas no significant changes were documented in the high-HFF group (Table 1 and Fig. 1). In particular, fasting and 2-h blood glucose and area under the curve (AUC) 2-h blood glucose significantly decreased during follow-up in the low-HFF group. In addition, fasting insulin significantly decreased whereas WBISI significantly increased at follow-up. A trend for increasing adiponectin concentrations and DI values as well as for a reduction in IGI values was documented. In particular, at follow-up, DI values were significantly higher in the group with low HFF than the group with high HFF (P = 0.01). In contrast, in the high-HFF group, both glucose and insulin sensitivity and secretion indices remained stable or showed worsening trends during the follow-up. In the low- and high-HFF groups, lipid profile as well as transaminases did not significantly change at follow-up. Circulating CK-18 levels were significantly higher in the group with fatty liver at baseline (153 ± 58 units/L) and follow-up (178 ± 83 units/L) compared with the values in the group without fatty liver (baseline 128 ± 31 units/L, follow-up 141 ± 36 units/L; P = 0.04). Of note, at follow-up, the prevalence of metabolic syndrome decreased significantly, by 50%, in the low-HFF group in contrast to no changes in prevalence in the high-HFF group.
Correlation between HFF at baseline and main metabolic parameters at follow-up
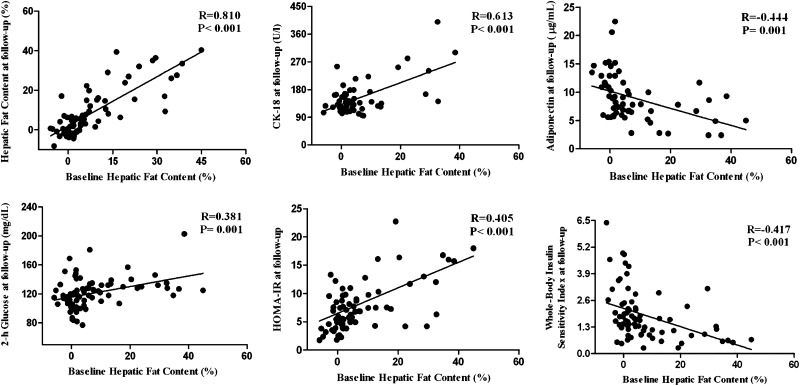
Baseline %HFF correlated strongly with the follow-up %HFF (r = 0.81, P < 0.001). Thus, obese adolescents with a high fat content at baseline remained in the same category over time. As for biomarkers of hepatocellular injury, we found a strong association between baseline HFF (%) and CK-18 levels (r = 0.613, P < 0.001) (Fig. 2). Furthermore, both ALT (r = 0.667) and AST (r = 0.606) correlated strongly with baseline %HFF (P = 0.001).
Figure 2.
Correlations between baseline HFF (%) and HFF (%), CK-18, and metabolic parameters at follow-up.
Regarding the markers of glucose and insulin metabolism, we found that a high HFF at baseline significantly correlated with 2-h glucose (r = 0.381, P = 0.001), HOMA-IR (r = 0.405, P < 0.001) (a marker of hepatic insulin sensitivity), whole-body insulin sensitivity indices (r = 0.417, P < 0.001), circulating adiponectin levels (r = 0.444, P = 0.001), and DI (r = −0.272, P = 0.021) at follow-up.
To further analyze the role of baseline HFF in determining impaired glucose regulation at follow-up, we used multiple linear regression analyses after adjusting for confounding factors. Baseline HFF was significantly associated with both 2-h glucose and WBISI at follow-up. We found that baseline HFF strongly predicts 2-h glucose (β = 0.281, P < 0.01) and insulin sensitivity (β = −0.159, P = 0.037).
CONCLUSIONS
Although hepatic steatosis is a well-recognized complication of childhood obesity, limited information regarding its future association with glucose dysregulation and potential liver damage in children is available. Our study suggests, first, that in the context of no weight loss or pharmacologic interventions, the phenotype of hepatic steatosis measured by MRI in obese youth is persistent. In fact, a strong correlation exists between the baseline and follow-up %HFF. Second, the HFF at baseline appears to be an independent modulator of post–2-h plasma glucose levels and insulin resistance. Third, baseline HFF correlates at follow-up with a circulating biomarker of liver apoptosis, CK-18.
In cross-sectional studies, our group showed that increased hepatic fat accumulation represents an independent and relevant factor related to glucose dysregulation in obese youth. As reported by Cali et al. (18), in a multiethnic group of 118 obese adolescents stratified according to tertiles of HFF, independent of obesity, the severity of fatty liver was associated with a significant decrease in insulin sensitivity and impairment in β-cell function, indicated by the decrease in the DI. Furthermore, paralleling the severity of fatty liver, there was a significant increase in the prevalence of metabolic syndrome. Hepatic steatosis may be a predictive factor of metabolic syndrome in children. D’Adamo et al. (26) reported that intrahepatic fat accumulation is more than a simple marker of insulin resistance in obese adolescents and is associated with impaired insulin sensitivity at the level of the liver, muscle, and adipose tissue. In accordance with previous reports, this study confirmed the cross-sectional association between high HFF and both impaired glucose metabolism and metabolic syndrome in obese youth. Additionally, we report here, for the first time, that the effects of HFF also strongly influence the metabolic parameters in the longitudinal setting. In particular, we showed that both glucose (fasting and 2-h blood glucose and AUC 2-h blood glucose) and insulin sensitivity (WBISI) indices at follow-up significantly improved in the low-HFF group, whereas no significant changes were documented in the high-HFF group. More importantly, during the follow-up, we documented a significant improvement of β-cell function in subjects with low compared with high HFF. In particular, at follow-up, DI values were significantly higher in obese youth with low HFF at baseline compared with the other group. These effects on relevant markers of β-cell function are of paramount importance. Indeed, the DI allows the calculation of β-cell function relative to insulin sensitivity. Our results therefore suggest that the β-cells are unable to adequately compensate for the ambient level of insulin resistance and therefore are very vulnerable, thus increasing susceptibility to type 2 diabetes. Of note, the subjects with fatty liver had elevated 2-h glucose during the oral glucose tolerance test, which indicates an imminent prediabetic state. In addition, a significant and positive association was documented between baseline HFF and longitudinal-fasting, 2-h blood glucose and AUC 2-h glucose. Insulin sensitivity and secretion indices (fasting and 2-h insulin, WBISI, and adiponectin) as well as DI at follow-up correlated with baseline HFF. These relevant correlations were further confirmed by the multiple, stepwise, linear regression analysis showing an independent relationship between baseline HFF and longitudinal metabolic parameter (2-h blood glucose and WBISI) even after adjusting for confounding factors (age, sex, ethnicity, BMI-Z, Δ BMI-Z, and duration of follow-up).
NAFLD encompasses a wide spectrum of conditions associated with overaccumulation of fat in the liver, ranging from NAFL or simple steatosis to NASH and cirrhosis. Although NAFL typically follows a benign, nonprogressive clinical course, NASH is a potentially serious condition; as many as 25% of patients may progress to cirrhosis and experience complications of portal hypertension, liver failure, and hepatocellular carcinoma (8,9,27). At present, the available noninvasive tests to distinguish NASH from NAFL include clinical signs and symptoms, routine laboratory and radiological imaging tests, and combinations of clinical and blood test results. Unfortunately, these tests are of limited use, and liver biopsy remains the only reliable way of diagnosing NASH and grading the severity of liver damage. However, an invasive liver biopsy is poorly suited as a diagnostic test, especially in children. Thus, in the current study, we elected to measure a biomarker of hepatocyte apoptosis as an indicator of putative NASH. Hepatocyte apoptosis is a prominent morphologic and pathogenic feature of NASH (28). During apoptosis, caspase-cleaved CK-18 is released into the cytoplasm and in the serum after cell death. Therefore, circulating soluble forms of CK-18 are being used to quantify apoptotic activity. Wieckowska et al. (29) quantified CK-18 fragments in adults with biopsy-proven NASH, finding that a cutoff value of 380 units/L gave specificity for NASH diagnosis of 94% and a sensitivity of 90.5%. The predictive value of CK-18 was further confirmed in the NASH-Clinical Research Network study (12). Together with A.E. Feldstein, we measured CK-18 levels in the obese adolescents with varying degrees of MRI-measured hepatic steatosis at baseline and follow-up. Thus, the strong relationship between baseline hepatic steatosis and increasing CK-18, a biomarker of NASH, indeed suggests that the mere presence of MRI-measured hepatic steatosis is not only a modulator of diabetes and cardiovascular risk but may also be a harbinger of NASH in obese kids. It is important to note that the all participants received standard lifestyle recommendations (i.e., nutrition counseling and physical activity recommendations). Of note, only in those subjects with low HFF did we document an improvement of the main outcomes of interest during the follow-up period, whereas no similar changes were documented in those with high HFF.
The main strength of our study is the use of fast MRI as a noninvasive imaging technique for monitoring fatty liver disease. This technique reliably measures hepatic fat and is in strong agreement with magnetic resonance spectroscopy, the gold standard for the imaging of liver steatosis. Fast-gradient MRI is closely correlated with biopsy-proven macrovesicular steatosis obtained in obese children (19). Moreover, for the first time, we report about a measure of hepatocellular apoptosis and its relation to baseline hepatic steatosis. Weaknesses are the relatively small sample size and short period of follow-up.
In conclusion, in obese children and adolescents, the phenotype of MRI-measured hepatic steatosis is persistent. In obese youth, the degree of HFF is a strong modulator both cross-sectionally and longitudinally of insulin and glucose metabolism and of hepatocellular apoptosis, as determined by CK-18.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) (grants R01-HD-40787, R01-HD- 28016, and K24-HD-01464, to S.C., and DK-076852 and DK-082451 to A.E.F.), the National Center for Research Resources (NIH Clinical and Translational Science Award UL1-RR-0249139), and the Yale Diabetes Endocrinology Research Center (P30 DK-045735).
No potential conflicts of interest relevant to this article were reported.
G.K. analyzed the data and wrote the manuscript. C.G. researched and analyzed the data. B.P., A.E.F., N.S., R.K., M.S., E.D., and R.G. researched data. J.D. analyzed the data. S.C. wrote, reviewed, and edited the manuscript. S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–1393 [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–1231 [DOI] [PubMed] [Google Scholar]
- 3.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011;332:1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis 2004;8:549–558, viii–ix [DOI] [PubMed] [Google Scholar]
- 5.Roberts EA. Nonalcoholic steatohepatitis in children. Curr Gastroenterol Rep 2003;5:253–259 [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005;115:e561–e565 [DOI] [PubMed] [Google Scholar]
- 7.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 2009;58:1538–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab 2006;91:4287–4294 [DOI] [PubMed] [Google Scholar]
- 9.Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non-invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr Radiol 2001;31:806–809 [DOI] [PubMed] [Google Scholar]
- 10.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westphalen AC, Qayyum A, Yeh BM, et al. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology 2007;242:450–455 [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009;50:1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007;120(Suppl. 4):S254–S288 [DOI] [PubMed] [Google Scholar]
- 14.Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging 1997;15:287–293 [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Taksali SE, Dufour S, et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med 2008;59:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging 2008;26:347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishbein M, Castro F, Cheruku S, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol 2005;39:619–625 [DOI] [PubMed] [Google Scholar]
- 18.Cali AM, Zern TL, Taksali SE, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care 2007;30:3093–3098 [DOI] [PubMed] [Google Scholar]
- 19.Pacifico L, Martino MD, Catalano C, et al. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol 2011;17:3012–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004;39:188–196 [DOI] [PubMed] [Google Scholar]
- 21.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab 2004;89:1096–1101 [DOI] [PubMed] [Google Scholar]
- 22.Yeckel CW, Taksali SE, Dziura J, et al. The normal glucose tolerance continuum in obese youth: evidence for impairment in beta-cell function independent of insulin resistance. J Clin Endocrinol Metab 2005;90:747–754 [DOI] [PubMed] [Google Scholar]
- 23.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–2374 [DOI] [PubMed] [Google Scholar]
- 24.Tamimi TI, Elgouhari HM, Alkhouri N, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol 2011;54:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr 2010;51:500–506 [DOI] [PubMed] [Google Scholar]
- 26.D’Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 2010;33:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes 2011;60:2011–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003;125:437–443 [DOI] [PubMed] [Google Scholar]
- 29.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006;44:27–33 [DOI] [PubMed] [Google Scholar]