Abstract
OBJECTIVE
To identify the most important pretreatment characteristics and changes in psychological and behavioral factors that predict weight outcomes in the Diabetes Prevention Program (DPP).
RESEARCH DESIGN AND METHODS
Approximately 25% of DPP lifestyle intervention participants (n = 274) completed questionnaires to assess weight history and psychological and behavioral factors at baseline and 6 months after completion of the 16-session core curriculum. The change in variables from baseline to 6 months was assessed with t tests. Multivariate models using hierarchical logistic regression assessed the association of weight outcomes at end of study with each demographic, weight loss history, psychological, and behavioral factor.
RESULTS
At end of study, 40.5% had achieved the DPP 7% weight loss goal. Several baseline measures (older age, race, older age when first overweight, fewer self-implemented weight loss attempts, greater exercise self-efficacy, greater dietary restraint, fewer fat-related dietary behaviors, more sedentary activity level) were independent predictors of successful end-of-study weight loss with the DPP lifestyle program. The DPP core curriculum resulted in significant improvements in many psychological and behavioral targets. Changes in low-fat diet self-efficacy and dietary restraint skills predicted better long-term weight loss, and the association of low-fat diet self-efficacy with weight outcomes was explained by dietary behaviors.
CONCLUSIONS
Health care providers who translate the DPP lifestyle intervention should be aware of pretreatment characteristics that may hamper or enhance weight loss, consider prioritizing strategies to improve low-fat diet self-efficacy and dietary restraint skills, and examine whether taking these actions improves weight loss outcomes.
The Diabetes Prevention Program (DPP) demonstrated that a lifestyle intervention aimed at 7% weight loss and 150 min of physical activity per week reduced the risk of diabetes by 58% over 2.8 years in 1,079 ethnically diverse participants at high risk to develop diabetes (1). Weight loss was the predominant predictor of diabetes prevention, and for every kilogram of weight loss, there was a 16% risk reduction in development of diabetes for lifestyle intervention participants (2). Of the DPP lifestyle intervention participants, 49% achieved the 7% weight loss goal at 6 months after completion of a core curriculum, and 37% maintained the 7% weight loss at end of study (3). Weight loss in DPP was greater for those who were older, engaged in more frequent self-monitoring of fat intake, reported a lower percentage of calories from fat, and increased physical activity (2,3). However, many participants did not achieve study weight loss targets.
Little has been published about the potential role of other modifiable psychological and behavioral variables on the ability to achieve and maintain weight loss. Understanding participant characteristics that independently predict weight loss and maintenance is critical for DPP clinical translation efforts and will help identify those who are most likely to succeed with this evidence-based lifestyle intervention to prevent or delay type 2 diabetes. Moreover, additional insights on the most important modifiable predictors of weight outcomes should help health care providers focus on enhancing abilities and skills of participants in relevant, change-worthy, cognitive-behavioral domains.
On the basis of previous research (4–11), we hypothesized that achievement and maintenance of weight loss in DPP lifestyle group participants at 6 months and end of study would be greater for those with the following pretreatment characteristics: fewer previous self-implemented weight loss attempts and weight loss programs; higher self-efficacy for diet, exercise, and weight loss; lower perceived stress; less frequent emotional eating; less binge eating; and more dietary restraint. In addition, we hypothesized that changes in several cognitive and behavioral factors targeted during the initial active weight loss intervention period of the trial, including improvements in self-efficacy and dietary restraint and reductions in emotional eating and binge eating, would predict greater weight loss at end of study.
RESEARCH DESIGN AND METHODS
The DPP design and methods have been described (1). In brief, eligible participants had impaired glucose tolerance and elevated fasting glucose levels, BMI ≥24 kg/m2 (>22 kg/m2 for Asian Americans), and were at least age 25 years (1). The lifestyle intervention (12) had two specific goals: to lose at least 7% of body weight and increase moderate intensity activity to at least 150 min/week. Lifestyle coaches met with participants individually over the first 24 weeks to review a 16-session core curriculum that focused on diet, physical activity, and behavioral modification strategies. After the first 6 months, lifestyle coaches tailored individual sessions to the needs of each participant and also offered group classes/campaigns three times per year to help improve activity levels and weight loss.
Study participants
DPP participants in the lifestyle intervention arm were recruited for this substudy between 1998 and 2001; 18 of 27 DPP centers agreed to participate and obtained approval from their institutional review boards. Of the nine centers that did not participate, two were not invited because they had already randomized almost all of their center cohorts; the other seven (four of which were American Indian centers) declined to participate to minimize participant burden because they had elected to participate in other ancillary studies. Of the final 293 lifestyle participants who were randomized, written informed consent was obtained from 274 (94%), representing almost 25% of the entire lifestyle intervention cohort.
At baseline, the entire lifestyle intervention cohort was aged 50.6 ± 11.3 years, 32% male, and weighed 94.1 ± 20.8 kg. By race ethnicity, the cohort was 54% white, 18.9% black, 16.5% Hispanic, 5.6% American Indian, and 5.3% Asian. As reported previously (4), the subgroup in the current study was representative of the total lifestyle group with regard to age, sex, initial weight, and race-ethnicity, except that American Indian centers did not participate (Table 1). The mean weight loss and percentage of participants achieving the 7% weight loss goal at 6 months and end of study were also similar in this subgroup as in the entire DPP lifestyle cohort, who lost 6.9 ± 4.5% at 6 months and 4.9 ± 7.4% at study-end (1,3).
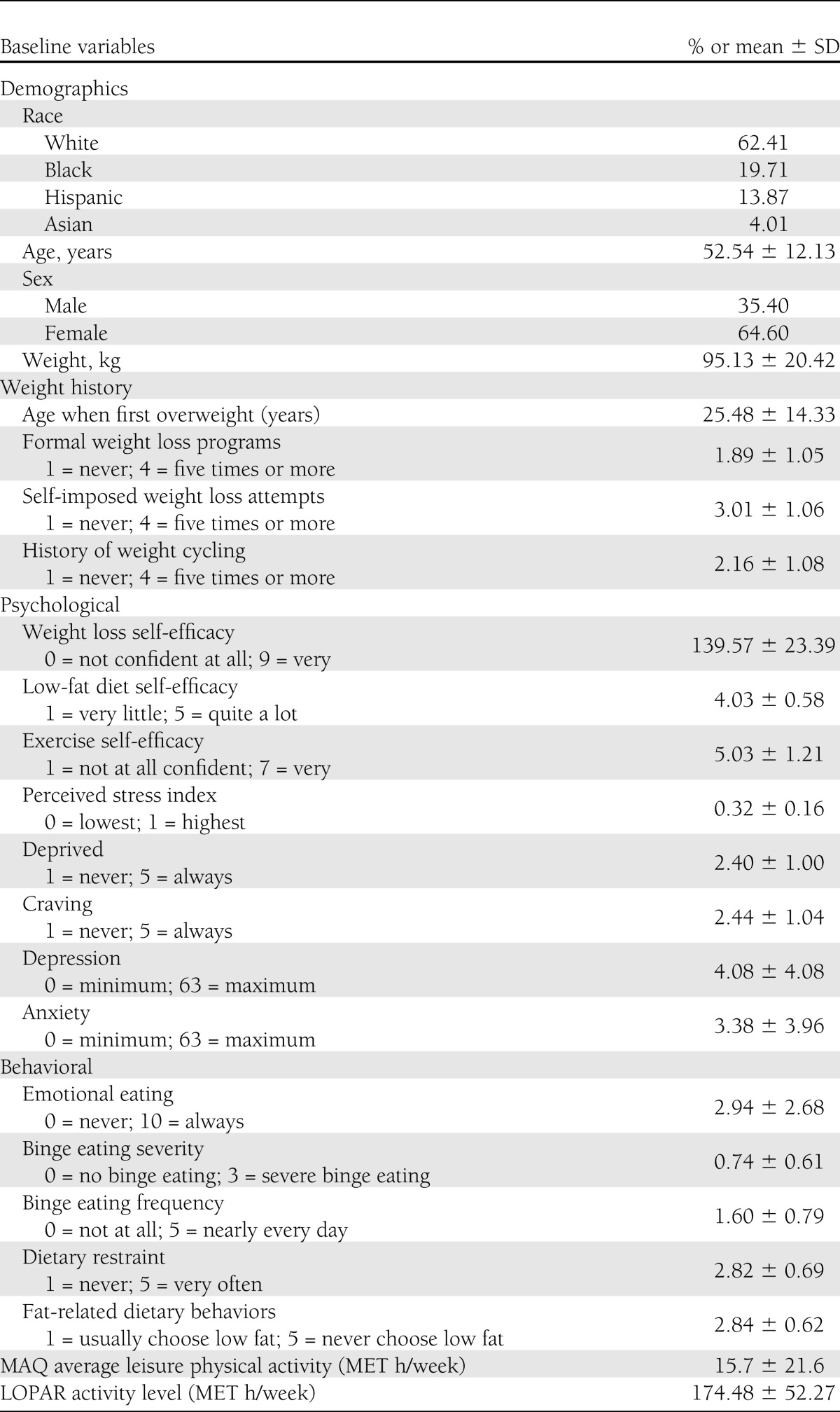
Table 1.
Pretreatment demographic, weight history, psychological and behavioral characteristics
Measures
Participants were asked to complete self-administered questionnaires assessing weight loss history and psychological and behavioral variables at baseline after randomization to the lifestyle intervention group and again at 6 months. Ninety-eight percent of the study subjects completed the questionnaires at 6 months, which was the data used in the current analyses. Participants reported their weight history, including age of onset of obesity, frequency of weight cycling (change of 20 pounds or more), and number of previous attempts with self-implemented and formal weight loss programs.
The DPP lifestyle intervention was based on Social Cognitive Theory, which was the theoretical framework for selection of the psychological and behavioral measures included in this study (12). The psychological and behavioral variables selected have been frequently cited as important predictors of weight loss and have the potential to be modified (4–11). The validated psychological and behavioral measures used have been previously described (4) and are briefly summarized.
Psychological measures.
The Weight Efficacy Lifestyle Questionnaire assesses confidence in resisting overeating in 20 tempting situations (5). The 16-item Low-Fat Diet Self-Efficacy Scale measures confidence in performing healthy dietary behaviors (13). The 5-item Exercise Self-Efficacy Scale (6) measures self-confidence to persist with exercise in various situations (14). The 30-item Perceived Stress Questionnaire (15) measures frequency of feeling pressured by life events. Participants also answered one question regarding how often they felt deprived while dieting and one question regarding how often they “fantasized a lot about favorite foods while dieting” (craving) (4). All DPP participants completed Beck Depression and Anxiety Inventories (16,17) at baseline and annually.
Behavioral measures.
The 5-item Emotional Eating Questionnaire measures frequency of eating due to negative emotions (6). A 5-item version of the Binge Eating Scale measures binge eating severity (18,19). Binge eating frequency was assessed using the first three questions from the Questionnaire on Eating and Weight Patterns (20). The Restraint subscale of Dutch Eating Behavior Questionnaire (21) rates frequency of using 10 different restraint behaviors. The 22-item Fat-Related Diet Questionnaire (22) measures dietary patterns related to selecting low-fat diets. All DPP participants completed the Modifiable Activity Questionnaire (MAQ) at baseline and annually and Low Level Physical Activity Recall (LOPAR) at baseline, 6 months, and annually. The MAQ assesses occupational and leisure activity during the past year, whereas LOPAR provides a general measure of physical activity (occupational, leisure, and home) during the preceding week (23).
Statistical analysis
To minimize the issue of multiple comparisons, weight outcome at end of study was defined as the primary outcome. Short-term weight outcomes at 6 months are presented separately to permit comparison of predictors at the end of the initial intervention period with predictors of long-term weight outcomes at end of study. This comparison distinguishes between predictors of the intervention effect across time to observe which effects are persistent.
Change in variables from baseline was tested with t tests. Multivariate models were constructed using hierarchical logistic regression to assess the association of weight outcomes (the dichotomous measure of whether the 7% weight loss goal was met) with each variable block (demographics, weight loss history, cognitive/psychological factors, and behaviors). In the first block of variables, race-ethnicity, age, sex, and baseline weight were forced into the models. Stepwise regression was performed using variables from remaining blocks, one block at a time. A value of P = 0.05 was required to enter the model, although the P value could become nonsignificant after adding subsequent variables or after adjusting for cluster effect. Stepwise regression was used to select variables from each successive block for entry into the model, retaining any variables from previous blocks. This process was repeated until all blocks were used. Once this final model was constructed, we used generalized estimating equations to adjust models for study site and center cluster effect; the results of this analysis represent the key findings of the study.
For cognitive/psychological and behavioral factors, variables were assessed as pairs—baseline levels and change scores—because baseline levels and change scores are confounded with each other; the worse the baseline score, the greater the improvement in that factor. Each pair of variables was entered into the model, and the significance level of each variable was assessed. If one or both variables of a pair were significantly related to the outcome, those pairs of variables were evaluated in terms of their contribution to the model r2; the pair of variables that made the largest contribution to r2 was entered into the model. Then, remaining variable pairs in that block were evaluated for entry into the model, following the same procedure.
Results for multivariate analyses of weight outcomes are presented for two models: 1) after all demographic and statistically significant weight history and cognitive/psychological variables had entered the model; and 2) after all statistically significant behavioral variables had been added to the first model. This analytic strategy allows us to determine whether behavioral variables explained the association of other factors with weight outcomes. We also present the r2 at the end of each block to assess the contribution of each set of factors to weight outcomes. Because results other than those for the multivariate analysis of the end-of-study outcome are supplementary analyses designed to provide a context for interpreting the primary results, we do not adjust for multiple comparisons. All analyses were performed using SAS 9.1 software (SAS Institute, Inc., Cary, NC).
RESULTS
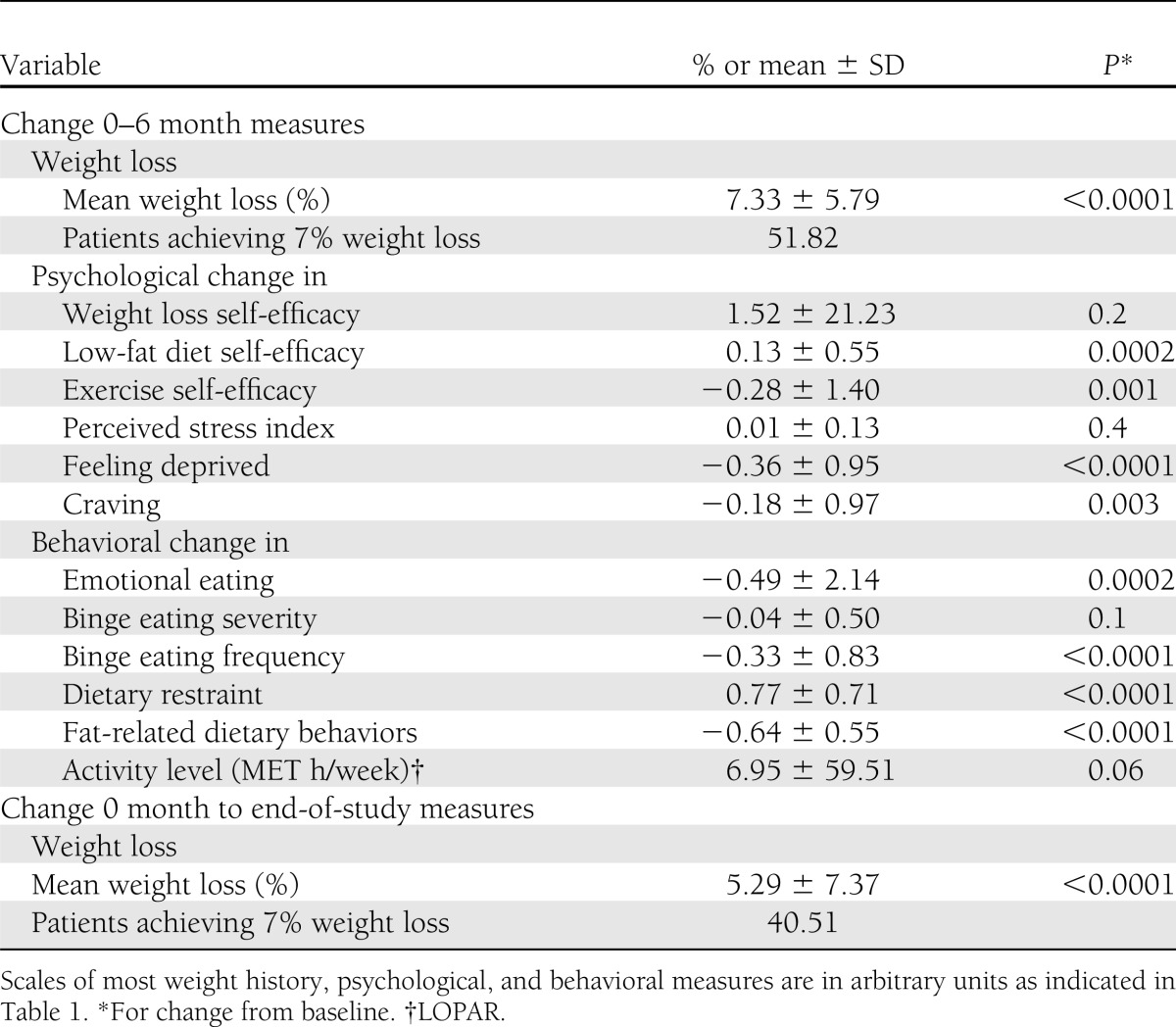
Baseline characteristics related to weight history, previous weight loss experiences, and scores on psychological and behavioral variables that were potential predictors of weight outcomes are reported in Table 1. The 6-month changes in psychological and behavioral variables selected to represent putative predictors for end-of-study long-term weight outcomes are reported in Table 2. Statistically significant improvements were documented in all measured psychological and behavioral factors, except for perceived stress, after completion of the 6-month core curriculum.
Table 2.
Changes in psychological and behavioral factors and weight outcomes
Multivariate analyses of the effects of pretreatment characteristics and changes in behavioral and psychological variables on achieving 7% weight loss
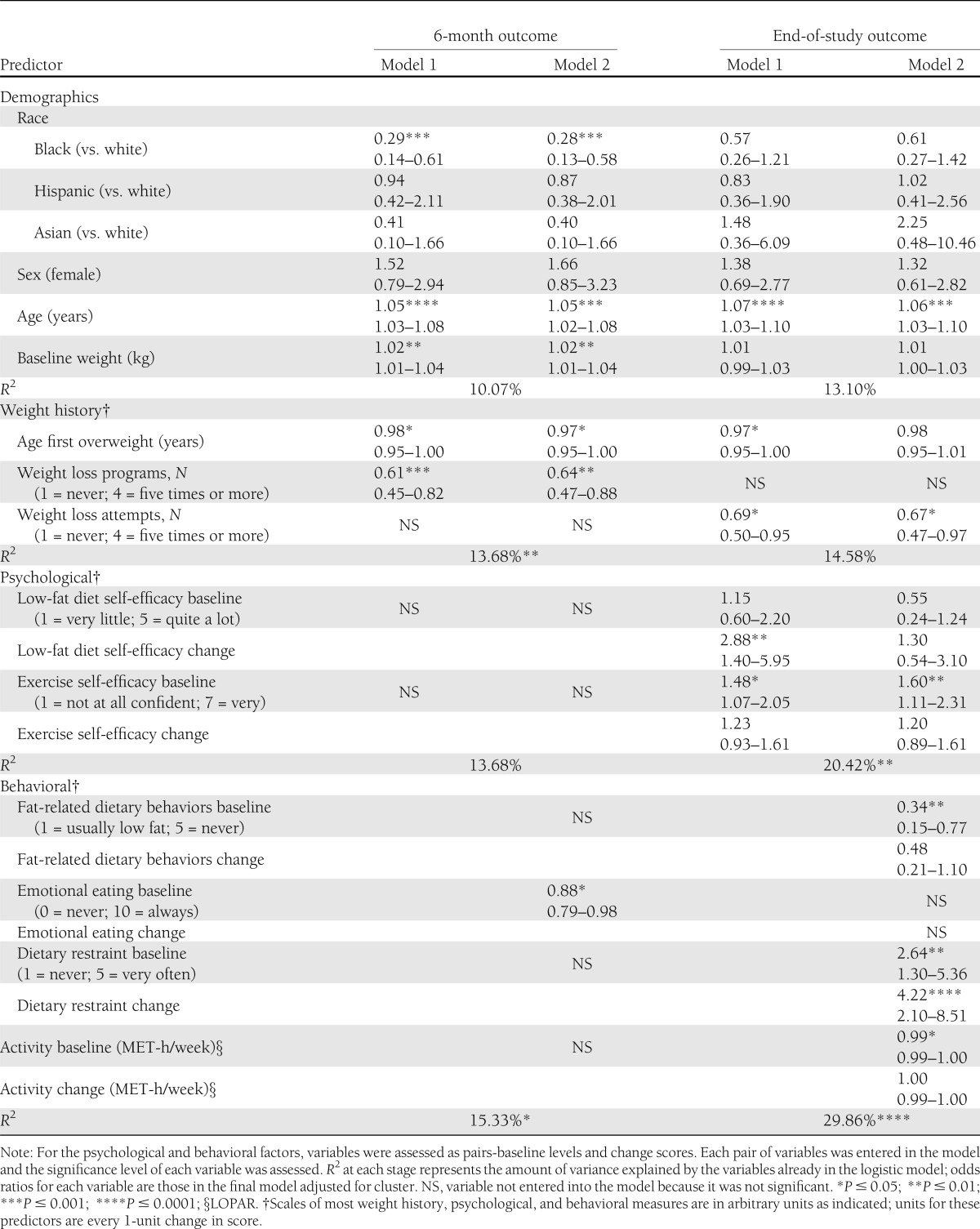
Race (being white compared with black), older age, older age when first overweight, and fewer past self-implemented weight loss attempts were the independent, nonmodifiable, pretreatment predictors of achieving 7% weight loss at end of study. Greater exercise self-efficacy, greater dietary restraint, fewer high-fat dietary behaviors, and a more sedentary activity level were independent, modifiable, pretreatment predictors of achieving the 7% weight loss goal at end of study (Table 3). At baseline, lower activity levels were correlated with larger increases in activity (Pearson correlation coefficient: r = −0.49; P < 0.0001). Fewer previous formal weight loss programs (nonmodifiable) and less frequent emotional eating (modifiable) were positive predictors of achieving 7% weight loss at 6 months but not at end of study. At baseline, more previous weight loss attempts were associated with younger age when first overweight (r = −0.35, P < 0.0001), more frequent weight cycling (r = 0.52, P < 0.0001), more cravings (r = 0.36, P < 0.0001) and feeling deprived while dieting (r = 0.43, P < 0.0001), and less self-efficacy for weight loss (r = −0.22, P < 0.0001). Once behavioral variables were entered into the regression (model 2), they explained the effect of race and age when first overweight on end-of-study weight outcomes.
Table 3.
Multivariate predictors (odds ratios and CI) of categorical weight loss outcomes (adjusted for cluster)
Only 6-month improvements in low-fat diet self-efficacy and dietary restraint skills remained important as predictors of achieving 7% weight loss at end of study. Once behavioral variables were entered into the regression (model 2), they explained the effect of changes in low-fat diet self-efficacy on weight outcomes.
Although baseline and early changes in perceived stress, feelings of being deprived while dieting, binge eating severity, weight loss self-efficacy, and exercise self-efficacy were significantly (P < 0.05) associated with ability to achieve weight loss outcomes at 6 months and end of study (data not shown), these variables were not independent predictors of weight outcomes when confounders were eliminated. Depression, anxiety, binge eating frequency, and craving were not significantly associated with weight outcomes (data not shown).
CONCLUSIONS
This study expands on previous research by examining the relative importance of pretreatment demographic, weight history, psychological and behavioral characteristics, and early changes in cognitive and behavioral factors targeted by the intervention in predicting short- and long-term weight outcomes in an ethnically diverse group of men and women. Moreover, the multivariate models identify the nonmodifiable demographic and weight history factors and the modifiable psychological and behavioral factors that independently influence success in achieving weight loss. Understanding mediators of success (and failure) in lifestyle intervention programs is critical to maximize success.
Moving beyond previous analyses showing that race, older age, and higher initial body weight are highly significant predictors of weight loss (24), we have now found that several additional baseline patient characteristics were independent and durable predictors of successful weight loss with the DPP lifestyle program at end of study in multivariate models: older age when first overweight, fewer previous self-implemented weight loss attempts, greater exercise self-efficacy, fewer fat-related dietary behaviors, greater dietary restraint, and a more sedentary activity level. In addition, improvements in low-fat diet self-efficacy and dietary restraint skills were independent and durable predictors of end-of-study long-term weight outcomes.
Consistent with previous findings (9,11), more previous weight loss attempts, whether formal or self-implemented programs, was an important independent predictor of inability to achieve 7% weight loss. Those with previous weight loss attempts also reported a profile of being younger when first overweight, more frequent weight cycling, more cravings and feeling deprived while dieting, and less self-efficacy for weight loss. These patterns of repeated negative experiences with previous programs and associated weight cycling may indicate the degree of experience with “repeated failure” and potentially affect long-term motivation and self-efficacy.
It is noteworthy that lower overall activity level at baseline was an independent predictor of greater likelihood of achieving 7% weight loss at end of study. Those with lower activity levels at baseline had larger increases in activity, which has been previously reported to predict greater weight loss (2,3).
Our findings that less frequent emotional eating at baseline was an independent predictor of achieving a 7% weight loss at 6 months and that greater dietary restraint and exercise self-efficacy at baseline are important factors in predicting 2- to 3-year weight outcomes are consistent with those of Teixeira et al. (25) in overweight and obese women. The persistent interference of emotional eating in achieving weight loss goals (6) at the 6- and 12-month follow-up is important to consider, as others have reported that emotional eaters lose less weight with standard behavioral treatment programs and seem to lose more weight with acceptance-based behavioral treatment (26–28). This approach uses acceptance and mindfulness processes, along with commitment and behavior change processes, to produce psychological flexibility; the ability to defuse from difficult thoughts and accept difficult feelings while persisting in value-based action (29). Notably, emotional eating at baseline was not an independent predictor of weight loss at end of study, implying that other factors became more important in determining longer-term weight outcomes. The significant improvements in low-fat diet self-efficacy, fat-related dietary behaviors, and dietary restraint after participation in the DPP core curriculum may have helped participants to manage the effect of any emotional eating better by end of study.
We expand on previous research by confirming that low-fat diet self-efficacy and dietary restraint skills are important modifiable, persistent predictors of long-term weight outcomes durably after 2–3 years of follow-up. Improvement in low-fat diet self-efficacy during the core curriculum was the most important independent psychological predictor of long-term weight outcomes. For each unit improvement in low-fat diet self-efficacy score, there was almost a threefold greater likelihood of achieving 7% weight loss at end of study. Behavioral variables explained the relationship between low-fat diet self-efficacy and end-of-study long-term weight outcomes, which may explain why there have been conflicting reports on the relationship of diet self-efficacy with weight loss outcomes (9,11).
Improvement in dietary restraint skills was the most important independent behavioral predictor of achieving long-term weight outcomes. For each unit increase in dietary restraint change score, there was a 4.3-fold greater likelihood of achieving 7% weight loss at end of study.
The DPP core curriculum resulted in significant improvements in low-fat diet self-efficacy and dietary restraint skills, each of which predicted better weight loss outcomes. The core curriculum focused on learning skills, such as setting small achievable goals, options for reducing fat intake, self-monitoring and balancing of fat gram intake, stimulus control, managing stress/high-risk situations, and problem solving, which could explain these improvements. Improvements in dietary restraint skills appear to be key to sustainable lifestyle change and weight loss. When participants learn to respond to lapses in meeting fat gram or activity goals by compensating with improved behaviors at the next opportunity, they are likely to feel more self-efficacious and less likely to engage in dichotomous thinking that is related to weight regain (10).
Clinically, these results point to the importance of a flexible approach to modifying fat- related dietary behaviors and to flexible dietary restraint as potential determinants of sustainable lifestyle change and weight loss (25). In the DPP, participants learned that there were three ways that they could choose to eat less fat in a given situation: eat smaller portions of the high-fat food, eat the high-fat food less often, or substitute a lower-fat alternative. This flexible approach to self-managing fat intake goals allowed each participant to tailor his or her own means to long-term success with lifestyle change and did not rely on adhering to a specific or rigid diet plan. Moreover, participants learned flexible dietary restraint behaviors. They practiced the skill of responding to an increase in weight or an overeating episode by eating less than usual on the following day(s) to compensate for and balance out fat and calorie intake so that on average they still met their goals over a given week. The clinical implications of these results may begin to address the issues raised by the Study to Help Improve Early Evaluation and Management of Risk Factors Leading to Diabetes (SHIELD) study, which underscored the need for programs that facilitate the development of lifestyle change skills to fill the critical gap that exists between knowing what to do and knowing how to do it for people with type 2 diabetes or at risk to develop it (30).
Strengths of this study are inclusion of an ethnically diverse group of men and women, very high retention rate, weight loss success levels that were defined a priori, sufficient follow-up time to study weight loss, use of a large number and broad scope of baseline and follow-up assessments, and examination of associations between psychological and behavioral factors targeted by the 6-month core curriculum and subsequent end-of-study weight outcomes.
The findings here must be interpreted with several limitations in mind. Although we conducted longitudinal analyses, the design is observational because we did not have a control group. We studied only one-quarter of the DPP lifestyle cohort; however, they resembled the entire cohort with regard to baseline characteristics and weight outcomes achieved, including univariate associations among age, race, and weight loss (3,24). Another limitation is that the clinical trial participants we studied may not be representative of all individuals with prediabetes seeking to lose weight. We acknowledge that nonsignificant associations with weight outcomes may be due to the narrow range of some of the factors (depression and anxiety scores were low, with little variance in this subgroup likely due to screening procedures for study entry).
We have expanded on previous research by identifying which pretreatment factors and psychological and behavioral factors targeted by the intervention are independent predictors of weight loss, by examining how long these associations persist, and by determining which factors explain the associations of other factors with weight loss. Our findings offer important insights into which modifiable and nonmodifiable pretreatment characteristics and which cognitive-behavioral factors targeted by the intervention were associated with successful long-term weight loss in DPP. Health care providers who are translating the DPP lifestyle intervention should be aware of participant characteristics that may hamper or enhance success with weight loss and consider prioritizing strategies that improve self-efficacy and dietary restraint skills to maximize sustainable weight outcomes. The findings presented here on the most important independent predictors of short-term and long-term weight outcomes may also help researchers in community settings to streamline and prioritize the number and type of survey measures used as they translate these results to real world practice settings. Future research is needed to examine whether taking action on the basis of these results is effective in improving weight loss outcomes, a key component of diabetes prevention and treatment.
Acknowledgments
This work was supported by a research grant from the American Diabetes Association to L.M.D. J.B.M. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K24 DK-080140.
No potential conflicts of interest relevant to this article were reported.
L.M.D. and M.P. researched data; contributed to discussion; and wrote, reviewed, and edited the manuscript. P.J.S. researched data and wrote, reviewed, and edited the manuscript. D.A.W., J.B.M., and D.M.N. contributed to discussion and reviewed and edited the manuscript. L.M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 62nd Scientific Sessions of the American Diabetes Association, San Francisco, California, 14–18 June 2002 and at the 63rd Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 13–17 June 2003.
The authors are grateful to the principal investigators and program coordinators and especially to the DPP volunteers who agreed to support and participate in this study. The authors are particularly indebted to the lifestyle coaches and program coordinators who assisted in the data collection process.
APPENDIX
The following 18 DPP centers participated: Pennington Biomedical Research Center, Baton Rouge, Louisiana; Jefferson Medical College, Philadelphia, Pennsylvania; University of Miami, Miami, Florida; The University of Texas Health Science Center, San Antonio, Texas; Joslin Diabetes Center, Boston, Massachusetts; University of Washington and Veterans Affairs Puget Sound Health Care System, Seattle, Washington; University of Tennessee, Memphis, Tennessee; Northwestern University Medical School, Chicago, Illinois; Massachusetts General Hospital, Boston, Massachusetts; University of California, San Diego, San Diego, California; St. Luke’s Roosevelt Hospital, New York, New York; Indiana University, Indianapolis, Indiana; MedStar Research Institute, Washington, DC; Washington University, St. Louis, Missouri; John Hopkins School of Medicine, Baltimore, Maryland; University of New Mexico School of Medicine, Albuquerque, New Mexico; Albert Einstein College of Medicine, Bronx, New York; and University of Hawaii, Honolulu, Hawaii.
Footnotes
Clinical trial reg. no. NCT00004992, clinicaltrials.gov.
A list of the groups participating in this study can be found in the appendix.
References
- 1.The Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamman RF, Wing RR, Edelstein SL, et al. Diabetes Prevention Program Research Group Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group Achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res 2004;12:1426–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM; DPP Research Group Psychological and behavioral correlates of baseline BMI in the Diabetes Prevention Program (DPP) Diabetes Care 2002;25:1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol 1991;59:739–744 [DOI] [PubMed] [Google Scholar]
- 6.Blair AJ, Lewis VJ, Booth DA. Does emotional eating interfere with success in attempts at weight control? Appetite 1990;15:151–157 [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Bursac Z, Dillo V, White DB, West DS. Stress, race and body weight. Health Psychol 2009;28:131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalle Grave R, Calugi S, Corica F, Di Domizio S, Marchesini G, QUOVADIS Study Group Psychological variables associated with weight loss in obese patients seeking treatment at medical centers. J Am Diet Assoc 2009;109:2010–2016 [DOI] [PubMed] [Google Scholar]
- 9.Teixeira PJ, Going SB, Houtkooper LB, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes 2004;28:1124–1133 [DOI] [PubMed] [Google Scholar]
- 10.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6:67–85 [DOI] [PubMed] [Google Scholar]
- 11.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obes Rev 2005;6:43–65 [DOI] [PubMed] [Google Scholar]
- 12.The Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program: a description of the lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey ML, Owen SV, Froman RD. Instrument development: cardiac diet and exercise self-efficacy. Nurs Res 1992;41:347–351 [PubMed] [Google Scholar]
- 14.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992;63:60–66 [DOI] [PubMed] [Google Scholar]
- 15.Levenstein S, Prantera C, Varvo V, et al. Development of the perceived stress questionnaire: a new tool for psychosomatic research. J Psychosom Res 1993;37:19–32 [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571 [DOI] [PubMed] [Google Scholar]
- 17.Beck AT. Beck Anxiety Inventory. San Antonio, Psychological Corporation, 1993 [Google Scholar]
- 18.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47–55 [DOI] [PubMed] [Google Scholar]
- 19.Greeno CG, Marcus MD, Wing RR. Diagnosis of binge eating disorder: discrepancies between a questionnaire and clinical interview. Int J Eat Disord 1995;17:153–160 [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Yanovski S, Wadden T, et al. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord 1993;13:137–153 [PubMed] [Google Scholar]
- 21.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord 1986;5:295–315 [Google Scholar]
- 22.Kristal AR, Shattuck AL, Henry J. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc 1990;90:214–220 [PubMed] [Google Scholar]
- 23.Kriska AM, Edelstein SL, Hamman RF, et al. Physical activity in individuals at risk for diabetes: Diabetes Prevention Program. Med Sci Sports Exerc 2006;38:826–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West DS, Prewitt TE, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program Obesity (Silver Spring) 2008;16:1413–1420 [DOI] [PubMed] [Google Scholar]
- 25.Teixeira PJ, Silva MN, Coutinho SR, et al. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity (Silver Spring) 2010;18:725–735 [DOI] [PubMed] [Google Scholar]
- 26.Forman EM, Butryn ML, Hoffman KL, Herbert JD. An open trial of an acceptance-based behavioral intervention for weight loss. Cognit Behav Pract 2009;16:223–235 [Google Scholar]
- 27.Forman EM, Butryn ML, Shaw JA, et al. Preliminary outcomes from the Mind Your Health project: a randomized controlled trial comparing standard behavioral and acceptance-based behavioral interventions for obesity. Paper presented at the 44th Annual Convention of the Association for Behavioral and Cognitive Therapies, 18–21 November 2010, San Francisco, California [Google Scholar]
- 28.Niemeier HM, Leahey T, Palm Reed K, Brown RA, Wing RR. An acceptance-based behavioral intervention for weight loss: a pilot study. Behav Ther 2012;43:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillis J, Hayes SC, Bunting K, Akihiko M. Teaching acceptance and mindfulness to improve the lives of the obese: a preliminary test of a theoretical model. Ann Behav Med 2009;37:58–69 [DOI] [PubMed] [Google Scholar]
- 30.Green AJ, Bazata DD, Fox KM, Grandy S; the SHIELD Study Group. Health-related behaviours of people with diabetes and those with cardiometabolic risk factors: results from SHIELD Int J Clin Pract 2007;61:1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]