Abstract
Given the importance of glycemic control in the development of diabetes complications, the plethora of tools now available to monitor the day-to-day trends in glycemia is remarkable. In this regard, self-monitoring of blood glucose (SMBG) has been considered a key component of patient management. Arguably, there remains almost universal agreement that SMBG should be available to all diabetic patients regardless of current treatment strategy. However, recently there have been reports that have challenged the current paradigm that all patients should use SMBG and concluded that SMBG for type 2 diabetic patients not on insulin may not be beneficial on glycemic control and must be weighed against the expense and inconvenience. In this two-part point-counterpoint narrative, Malanda et al. and Polonsky and Fisher take opposing views on the utility of SMBG to be valuable for individuals with type 2 diabetes not using insulin. In the narrative below, Malanda et al. suggest that the evidence for potentially beneficial SMBG-induced effects on glycemic control, hypoglycemic periods, and potential harms in type 2 diabetic patients who are not treated with insulin does not justify the use of SMBG. Moreover, the use of SMBG is associated with huge costs, which should be better redirected to effective strategies to improve health for this category of patients.
—William T. Cefalu, md Editor in Chief, Diabetes Care
Self-monitoring of blood glucose (SMBG) is considered a key component of the treatment regimen in patients with type 2 diabetes using insulin (1). There is almost universal agreement that SMBG should be available to all diabetic patients. Even for patients not using insulin, the use of SMBG is widely taken up in clinical practice guidelines (2) and is accepted as a part of their diabetes management.
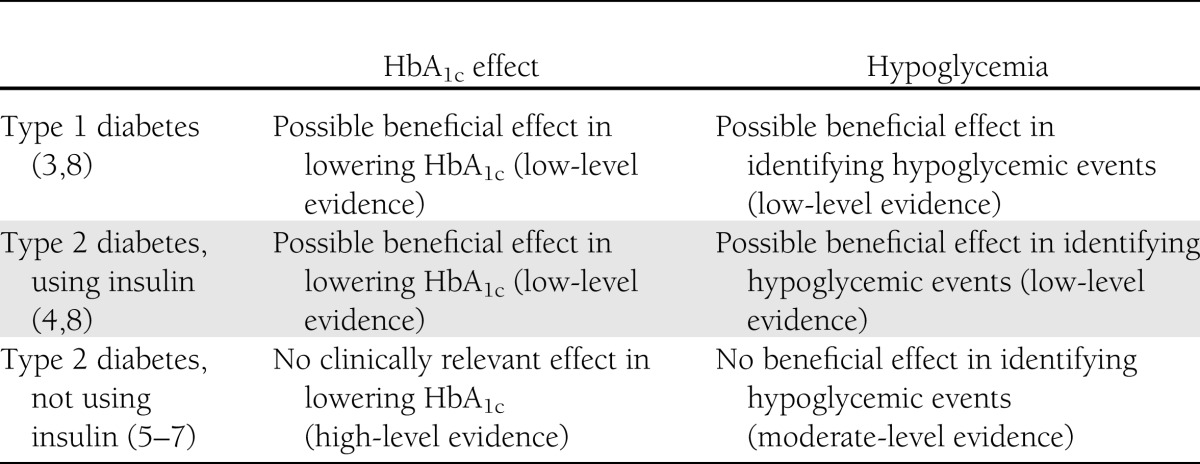
SMBG is used as an early warning to detect or confirm hypoglycemia and to improve patients’ recognition of severe hyperglycemia. Given that real-time data on blood glucose levels reflects the influence of physical activity and diet, SMBG has the potential to help patients better understand the impact of lifestyle on glycemic control (1). Furthermore, information derived from consecutive series’ of SMBG measurements could be of use for a treating physician to modify the glucose-lowering treatment. Despite these potential benefits, there is a limited evidence base for an effect of SMBG on hemoglobin A1c (HbA1c) in type 1 (3) and type 2 diabetic patients using insulin (4). We have summarized and labeled the available evidence of an effect of SMBG on HbA1c and hypoglycemia in patients using insulin (type 1 and type 2 diabetes) and patients not using insulin (type 2 diabetes) using a grading system modeled after existing methods (Table 1). At this point, we will discuss the lack of evidence of an effect of SMBG in patients with type 2 diabetes not treated with insulin.
Table 1.
Evidence of SMBG effect on HbA1c and hypoglycemia in different diabetic patient groups
SMBG, glycemic control, and noninsulin-treated type 2 diabetes
According to the available evidence, SMBG has no clinically relevant effect on glycemic control for type 2 diabetic patients not treated with insulin. Our recent systematic review and meta-analysis of all randomized controlled trials investigating effects of SMBG published since 1989 summarizes the available evidence (5). Included studies in this review varied in intervention strategies and intervention duration and included newly diagnosed or established type 2 diabetic patients. In established type 2 diabetic patients, pooled analysis resulted in a −0.26% (95% CI −0.39 to −0.13) decrease in HbA1c, in favor of SMBG after 6 months. This decrease does not meet the generally agreed clinically relevant level of 0.5% (6). The reported effects after 12 months were even smaller and lacked statistical significance (−0.13% [−0.31 to 0.04]) (5). These effects were almost similar to the effect of SMBG found in previously published reviews and meta-analyses (6,7).
In light of this evidence, the question emerges whether there are any subgroups of type 2 diabetic patients not treated with insulin that might have a glycemic benefit from SMBG. Only two studies investigated the effect of SMBG on HbA1c in newly diagnosed and noninsulin-using type 2 diabetic patients with conflicting results after 12 months’ follow-up (5). In addition, recently a meta-analysis of individual patient data of six studies investigating effects of SMBG in noninsulin-using type 2 diabetic patients reported no significant effect in predefined subgroups of age, sex, baseline HbA1c, diabetes duration, and prior experience in self-monitoring (7). So summarizing, there is no substantial evidence of a beneficial glycemic effect of SMBG in any defined subgroup of noninsulin-using type 2 diabetic patients.
SMBG and hypoglycemia
Periods of hypoglycemia are distressing events that mostly occur with tight glycemic regimens and treatments increasing insulin levels independently of blood glucose levels. It is therefore not surprising that the frequency of hypoglycemia is relatively low in diabetic patients not treated with insulin or oral antidiabetic treatments likely to cause hypoglycemia (e.g., sulfonylurea). SMBG’s potential to confirm or refute periods of hypoglycemia has generally been taken up in most management programs targeted at identifying and reducing periods of hypoglycemia, even though its effectiveness in patients using insulin has been tested in a limited number of studies (8). In noninsulin-treated diabetes, six trials investigated SMBG-related hypoglycemia (5). In only one study was the occurrence of severe hypoglycemic episodes observed. The three studies that reported hypoglycemia found only an increase in asymptomatic hypoglycemia or hypoglycemia with mild symptoms. Given the low risk patients treated with oral antidiabetic drugs have for severe hypoglycemia, especially when the drugs are used as monotherapy or in combination, SMBG would not be required for detection of hypoglycemia in these patients.
SMBG and quality of life and well-being
Underpinned by the theoretical Leventhal’s Common Sense model (9), it has been suggested that diabetes self-care activities, i.e., SMBG, may have a beneficial influence on lifestyle behavior (10). Following that suggestion, SMBG would potentially improve self-care attitudes, control beliefs, and motivational behavior, leading to positive changes in well-being and quality of life (11). To our knowledge, randomized controlled trials investigating the effect of SMBG on quality of life in insulin-using patients are not available. After the Diabetes Control and Complications Trial (12) demonstrated that an intervention including SMBG is effective in maintaining blood glucose levels near normal in diabetic patients using insulin, trials that investigated the impact of SMBG on aspects of quality of life or well-being in this patient group are hard to find. This is in sharp contrast with the interest in the contribution of SMBG to quality of life and well-being in patients who are not using insulin. Results from observational (13) and qualitative (14) studies suggest that SMBG might negatively affect aspects of quality of life. However, analyzing the studies exploring general well-being and quality of life in this subgroup of diabetic patients could not reveal any evidence of a positive or negative effect (5). In addition, the most recent studies designed to detect changes in diabetes-specific emotional distress and self-efficacy did not find an SMBG-related effect (15–17).
Costs related to SMBG
The costs of SMBG are considerable. In 2002, the Medicare B program for uncomplicated type 2 diabetic patients not using insulin in the U.S. is said to have spent more than $465 million on reagent strips, lancets, lancing devices, meters, batteries, and calibration solutions or chips, representing more than half of the medical costs declared on the International Classification of Diseases, Ninth Revision code for these patients in that year (18). In Canada, in eight provinces Can$247 million were spent on SMBG supplies (19). In the Netherlands, the estimated costs in 2006 were about EUR 70 million ($88 million) spent on SMBG supplies, ∼10% of the total costs for the treatment of type 2 diabetic patients. Although the costs seems high in respect to the total cost spent on diabetes care, the only way to make a good judgment of cost-effectiveness is to take the profits into account. The best way to evaluate the cost-effectiveness is analyses in randomized controlled trials, and this has been done in only two studies. The Diabetes Glycaemic Education and Monitoring trial in the U.K. found that the additional costs of SMBG were at least £84 ($141) combined with a negative impact on quality of life, compared with standardized usual care without SMBG. The investigators concluded that a calculation of a cost-effectiveness ratio was not meaningful while the intervention was not more effective than the control situation (20). Cameron et al. (21) evaluated the cost-effectiveness in seven randomized trials. They found an incremental cost-utility ratio of $113,643 per quality-adjusted life-year and concluded that SMBG was not cost-effective. Studies that showed that the costs of SMBG had acceptable cost-effectiveness ratios have been based on Markov/Monte Carlo models in observational data (22,23). However, these studies showed many limitations. They were based on relatively short-term observational data, the clinical effects had a low level of evidence, and not all costs were taken into account. So, reviewing the available evidence on SMBG-related costs, we have to face that the conclusion made by Davidson (18) in a previous point-counterpoint series is still applicable; SMBG in noninsulin-treated type 2 diabetic patients is a waste of money.
Conclusions
The evidence for potentially beneficial SMBG-induced effects on glycemic control, hypoglycemic periods, and potential harms in type 2 diabetic patients who are not treated with insulin does not justify the use of SMBG. Moreover, the use of SMBG is associated with huge costs, which should better be redirected toward effective strategies to improve health for this category of patients.
Acknowledgments
U.L.M., S.D.B., and G.N. were involved in a study investigating the effects of blood glucose self-monitoring in type 2 diabetic patients for which they received investigator-initiated funding from LifeScan, Inc. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Tomky D, Cypress M, Dang D, Maryniuk M, Peyrot M; AADE AADE7 self-care behaviors. Diabetes Educ 2008;34:445–449 [DOI] [PubMed] [Google Scholar]
- 2.Aakre KM, Watine J, Bunting PS, Sandberg S, Oosterhuis WP. Self-monitoring of blood glucose in patients with diabetes who do not use insulin—are guidelines evidence-based? Diabet Med 2012;29:1226–1236 [DOI] [PubMed] [Google Scholar]
- 3.Kolb H, Kempf K, Martin S, Stumvoll M, Landgraf R. On what evidence-base do we recommend self-monitoring of blood glucose? Diabetes Res Clin Pract 2010;87:150–156 [DOI] [PubMed] [Google Scholar]
- 4.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006;295:1688–1697 [DOI] [PubMed] [Google Scholar]
- 5.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012;1:CD005060. [DOI] [PubMed] [Google Scholar]
- 6.Clar C, Barnard K, Cummins E, Royle P, Waugh N; Aberdeen Health Technology Assessment Group Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess 2010;14:1–140 [DOI] [PubMed] [Google Scholar]
- 7.Farmer AJ, Perera R, Ward A, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ 2012;344:e486. [DOI] [PubMed] [Google Scholar]
- 8.Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care 2007;30:1370–1373 [DOI] [PubMed] [Google Scholar]
- 9.Leventhal L, Benyamini Y, Brownlee S, et al. Illness representations: theoretical foundations. In Perceptions of Health and Illness. Petrie KJ, Weinman J, Eds Amsterdam, Harwood Academic Publisher, 1997, p. 19–45 [Google Scholar]
- 10.Hampson SE, Glasgow RE, Toobert DJ. Personal models of diabetes and their relations to self-care activities. Health Psychol 1990;9:632–646 [DOI] [PubMed] [Google Scholar]
- 11.McAndrew LM, Musumeci-Szabó TJ, Mora PA, et al. Using the common sense model to design interventions for the prevention and management of chronic illness threats: from description to process. Br J Health Psychol 2008;13:195–204 [DOI] [PubMed] [Google Scholar]
- 12.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 13.Franciosi M, Pellegrini F, De Berardis G, et al. ; QuED Study Group The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: an urgent need for better educational strategies. Diabetes Care 2001;24:1870–1877 [DOI] [PubMed] [Google Scholar]
- 14.Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ 2007;335:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher L, Polonsky WH, Parkin CG, Jelsovsky Z, Petersen B, Wagner RS. The impact of structured blood glucose testing on attitudes toward self-management among poorly controlled, insulin-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2012;96:149–155 [DOI] [PubMed] [Google Scholar]
- 16.Fisher L, Polonsky W, Parkin CG, Jelsovsky Z, Amstutz L, Wagner RS. The impact of blood glucose monitoring on depression and distress in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin 2011;27(Suppl. 3):39–46 [DOI] [PubMed] [Google Scholar]
- 17.Malanda UL, Bot SD, Kostense PJ, Snoek FJ, Dekker JM, Nijpels G. Self-monitoring of glucose in blood or urine does not increase diabetes-specific distress in non-insulin treated patients with type 2 diabetes: results from the IN CONTROL-trial. Diabetologia 2011;54:S391 [Google Scholar]
- 18.Davidson MB. Counterpoint: Self-monitoring of blood glucose in type 2 diabetic patients not receiving insulin: a waste of money. Diabetes Care 2005;28:1531–1533 [DOI] [PubMed] [Google Scholar]
- 19.Rabi D, Johnson J, Edwards A. Self-monitoring of blood glucose for individuals with type 2 diabetes not using insulin: Leaving no cornerstone unturned. Canadian Journal of Diabetes 2010;34:24–26 [Google Scholar]
- 20.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A; Diabetes Glycaemic Education and Monitoring Trial Group Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ 2008;336:1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron C, Coyle D, Ur E, Klarenbach S. Cost-effectiveness of self-monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin. CMAJ 2010;182:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutati EI, Raptis SA. Self-monitoring of blood glucose as part of the integral care of type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S205–S210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tunis SL, Minshall ME. Self-monitoring of blood glucose (SMBG) for type 2 diabetic patients treated with oral anti-diabetes drugs and with a recent history of monitoring: cost-effectiveness in the US. Curr Med Res Opin 2010;26:151–162 [DOI] [PubMed] [Google Scholar]