Abstract
OBJECTIVE
To examine the cost-effectiveness of a hepatitis B vaccination program for unvaccinated adults with diagnosed diabetes in the U.S.
RESEARCH DESIGN AND METHODS
We used a cost-effectiveness simulation model to estimate the cost-effectiveness of vaccinating adults 20–59 years of age with diagnosed diabetes not previously vaccinated for or infected by hepatitis B virus (HBV). The model estimated acute and chronic HBV infections, complications, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios. Data sources included surveillance data, epidemiological studies, and vaccine prices.
RESULTS
With a 10% uptake rate, the intervention will vaccinate 528,047 people and prevent 4,271 acute and 256 chronic hepatitis B infections. Net health care costs will increase by $91.4 million, and 1,218 QALYs will be gained, producing a cost-effectiveness ratio of $75,094 per QALY gained. Results are most sensitive to age, the discount rate, the hepatitis B incidence ratio for people with diabetes, and hepatitis B infection rates. Cost-effectiveness ratios rise with age at vaccination; an alternative intervention that vaccinates adults with diabetes 60 years of age or older had a cost-effectiveness ratio of $2.7 million per QALY.
CONCLUSIONS
Hepatitis B vaccination for adults with diabetes 20–59 years of age is modestly cost-effective. Vaccinating older adults with diabetes is not cost-effective. The study did not consider hepatitis outbreak investigation costs, and limited information exists on hepatitis progression among older adults with diabetes. Partly based on these results, the Advisory Committee on Immunization Practices recently recommended hepatitis B vaccination for people 20–59 years of age with diagnosed diabetes.
The hepatitis B vaccine was first recommended in the U.S. in 1982 for groups known to be at high risk of hepatitis B virus (HBV) infection. Selective vaccination of adults and infants at increased risk for HBV infection was followed by adoption of universal hepatitis B vaccination for infants (1991) and catch-up vaccination for adolescents up to 18 years of age (1995 and 1999) (1). This incremental and selective vaccination strategy for eliminating HBV transmission was associated with an 84% decrease in reported cases of acute hepatitis B from 1990 to 2009 (from 8.5 to 1.1 incident cases per 100,000). When asymptomatic infection, underdiagnosis, and underreporting are taken into account, the estimated number of new HBV infections is >10 times higher than the number of confirmed acute cases (2,3).
Despite these impressive improvements, in recent years ∼60% of acute hepatitis B cases with risk factor information had none of the previously recognized risks for HBV infection (e.g., employment in a health care field involving contact with human blood; dialysis; injection drug use; multiple sexual partners; men who have sex with men; or household or sexual contact with a confirmed or suspected individual with an HBV infection), suggesting that other risks for HBV infection have not been identified (2). From 1996 to 2011, 29 outbreaks of hepatitis B infection in long-term care institutional facilities were reported to the Centers for Disease Control and Prevention (CDC). Twenty five of the outbreaks involved adults with diabetes receiving assisted blood glucose monitoring (4). From 2008 to 2011, news media reported instances in which >5,700 people were placed at risk for bloodborne infection from misuse of diabetes equipment (infection control lapses related to assisted blood glucose monitoring; use of diabetes equipment [e.g., insulin pens and lancing devices] designed for single-person use on multiple people) (5). These events raised the possibility that diabetes is a marker for increased risk of HBV transmission through exposure to contaminated blood during diabetes care and monitoring.
The diabetes status of people with acute hepatitis infection is not routinely collected for national hepatitis surveillance purposes. To examine the risk of acute hepatitis B infection among adults with diabetes, diabetes status was obtained for 865 confirmed cases of acute hepatitis B identified during 2009–2010 at eight Emerging Infections Program sites (6). After controlling for demographic characteristics and stratifying by traditional risk factors for HBV infection (injection drug use, multiple sexual partners, and men who have sex with men), adults with diagnosed diabetes 23–59 years of age who lacked traditional risk factors for HBV infection had twice the odds of acute hepatitis B compared with adults without diabetes. Moreover, adults with diagnosed diabetes 60 years of age or older who lacked traditional risk factors had a 50% higher odds of acute hepatitis B compared with adults without diabetes, although the difference was not statistically significant (6). These results were consistent with seroprevalence data from the 1999–2010 National Health and Nutrition Examination Survey, which demonstrated a 60% increase in the seroprevalence of current or past HBV infection among adults with diagnosed diabetes compared with adults without diagnosed diabetes, and a 30% increase for adults with diagnosed diabetes 60 years of age or older, which was statistically significant (CDC, unpublished data). Together, these data support the hypothesis that people with diagnosed diabetes are at increased risk of HBV infection.
Options for preventing HBV infection among adults with diagnosed diabetes include increased emphasis on infection control practice for diabetes care procedures (4), modifications in the design of diabetes care equipment to reduce the potential for exposure to blood (7), and consideration of pre-exposure hepatitis B vaccination for adults with diabetes. In this article, we examine the cost-effectiveness of a hypothetical hepatitis B vaccination program for unvaccinated adults with diagnosed diabetes.
RESEARCH DESIGN AND METHODS
We modified an existing decision-analytic Markov model of vaccination for hepatitis B and outcomes of HBV infection (8,9) to reflect the impact of hepatitis B in adults with diagnosed diabetes. The modifications accounted for higher incidence of HBV infection among adults with diagnosed diabetes, higher mortality among people with diabetes, and older age at peak diabetes prevalence. Other model parameters were updated to reflect current data.
The analysis begins with the choice of vaccination strategy, which may include vaccination or no vaccination. Outcomes were assessed for the entire population of U.S. adults 20–59 years of age who currently have diagnosed diabetes. The study population was stratified into 5-year age-groups. The model tracks hepatitis-related events from both acute and chronic HBV infections.
Acute infection
More than half of patients who are acutely infected with HBV are asymptomatic (Supplementary Fig. 1A). For those who develop symptoms, some recover without hospitalization, whereas others are hospitalized. Some hospitalized patients develop fulminant hepatic failure, which can lead to death, liver transplant, or recovery. Most patients who receive a liver transplant survive, but some die.
Chronic infection
Acute infection is a necessary precursor to chronic infection. Approximately 6% of people who experience acute hepatitis B develop chronic hepatitis B (Supplementary Fig. 1B). People with uncomplicated chronic hepatitis can become inactive carriers, develop cirrhosis or hepatocellular carcinoma (HCC), or remain with uncomplicated chronic hepatitis. Inactive carriers can return to the chronic hepatitis state, and they can transmit HBV to others. People with cirrhosis can remain in that state, develop compensated cirrhosis or HCC, or die of hepatitis-related causes. People with decompensated cirrhosis can remain in that state, develop HCC, receive a liver transplant, or die of hepatitis-related causes. People with HCC can remain in that state, receive a liver transplant, or die of hepatitis-related causes. People in the liver transplant state can survive or die of hepatitis-related causes. At any time, people can die of other causes. Other-cause mortality rates are age specific and represent all-cause mortality for people with diabetes.
Incidence rates for susceptible people with diagnosed diabetes and other transition probabilities
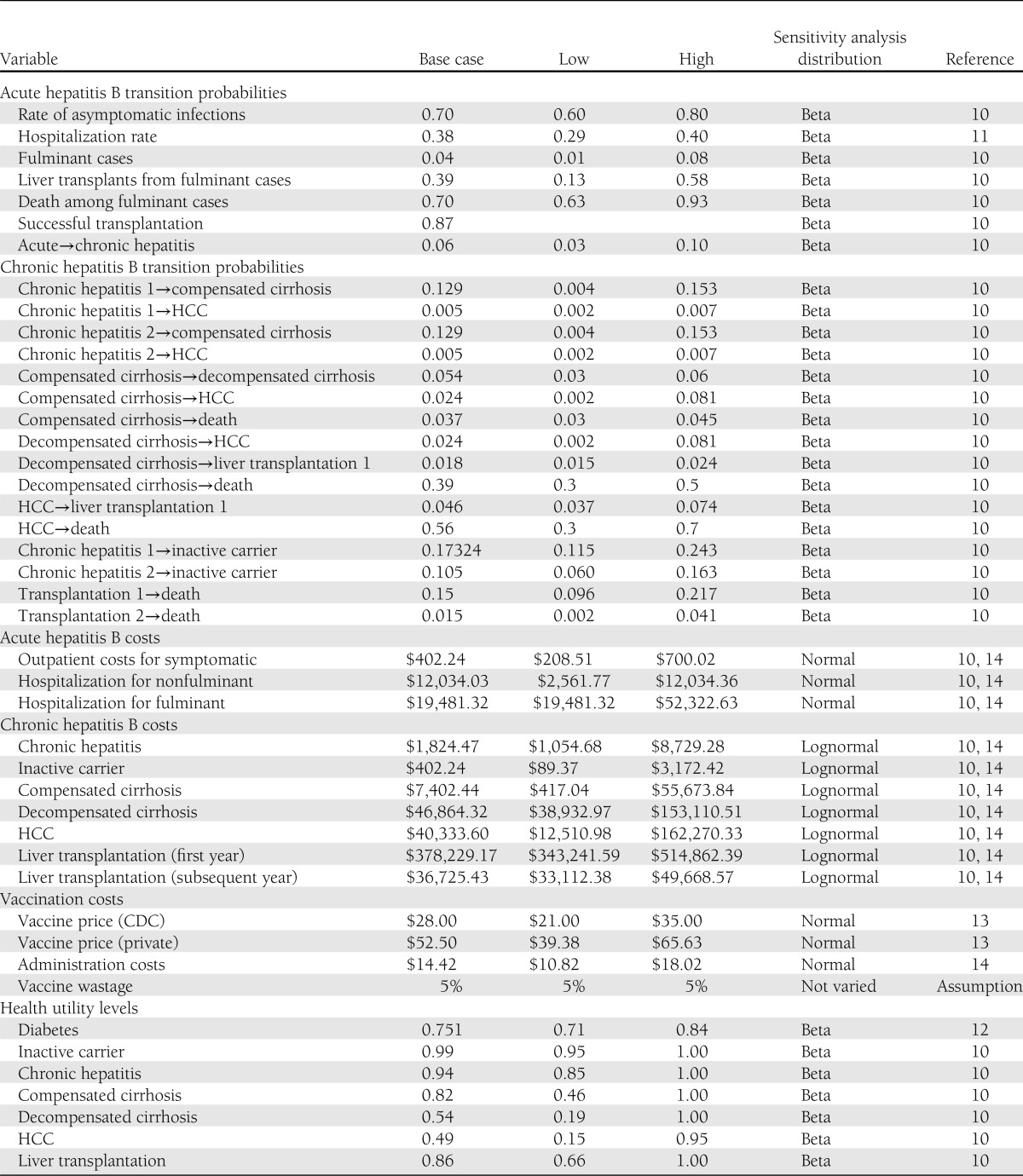
The HBV infection rates for people with diagnosed diabetes who are susceptible to infection depend on the incidence of HBV infection among all people with diagnosed diabetes, the prevalence of previous HBV infection, existing vaccine coverage, and the efficacy of prior vaccination. The Supplementary Data describes components of the calculation. Incidence rates for susceptible people with diagnosed diabetes increase from 91 per 100,000 people with diabetes 20–24 years of age to a peak of 101 per 100,000 people 35–39 years of age, and then decline to 13 per 100,000 people 70 years of age or older (Supplementary Table 2). Other disease progression probabilities and epidemiologic parameters are listed in Table 1 (10–14).
Table 1.
Hepatitis B disease progression, cost, and health utilities parameters
Efficacy of vaccination
Efficacy of vaccination is based on a CDC review (S.S., T.M., B. Baack, unpublished data) (the Supplementary Data lists source studies) of vaccine studies in adults (19 studies) and people with diagnosed diabetes (10 studies). Efficacy declines with age, from 95% for 15–29 years of age to 40% for 70 years of age or older (Supplementary Data).
Costs, health utilities, and mortality rates
Table 1 contains all cost values and utility values for health states. Direct program costs and direct medical costs are included. All final cost figures were converted to 2010 U.S. dollars using the U.S. Bureau of Labor Statistics’ Consumer Price Index (All Urban Consumers, Medical Care, U.S. City Average). The Supplementary Data describes the calculation of other-cause mortality rates for people with diabetes.
Intervention strategy
The primary vaccination strategy considered is vaccination with adult hepatitis B vaccine (recombinant). Analyses of routine vaccination of adults 20–59 years of age with diabetes are based on assumptions that vaccination will be offered to all adults 20–59 years of age with diagnosed diabetes; 10% of adults with diagnosed diabetes who are susceptible to hepatitis B and who report no previous vaccination will accept the vaccination (the assumption is based on hepatitis B vaccine uptake for adults with previously recognized risk factors, uptake for other adult vaccines, and manufacturer projections) (Supplementary Data); all people who accept the vaccine will complete a primary series of three doses (1 mL each) given on a 0-, 1-, and 6-month schedule or other approved schedule; immunity will not wane over time for people achieving seroprotection; and vaccination will occur during regularly scheduled patient visits (76% of primary care offices stock hepatitis B vaccine for adults) (15).
The age-group 20–59 years was chosen based on evidence of declining vaccination efficacy in older age-groups and preliminary analyses indicating that vaccination had very high cost-effectiveness ratios among older age-groups. For completeness, the vaccination of adults 60 years of age or older was analyzed as an alternative intervention strategy. The intervention may be viewed as a catch-up strategy because vaccination will be offered to all people who currently have diagnosed diabetes. For purposes of the model, it is assumed that people who refuse vaccination in the first year will not accept vaccination in subsequent years; thus, all vaccination associated with the catch-up program will occur during the first year.
Baseline strategy
The vaccination of adults with diagnosed diabetes was compared with the status quo of no additional vaccination. Under the status quo strategy, some adults with diabetes have previously been vaccinated but no additional vaccination is assumed to occur.
Analyses
For each vaccination strategy, incremental cost-effectiveness ratios were calculated as net costs per quality-adjusted life-year (QALY) gained. Net costs of vaccination were estimated as vaccine costs and administration costs minus averted medical costs. The net costs were then divided by the estimated improvement in QALYs resulting from vaccination.
The perspective used is the health care system perspective. The costs included the direct costs to the health care system of providing hepatitis B vaccination and the direct medical costs of hepatitis B–related illness and complications that are averted by preventing HBV infection. The summary measure of effectiveness is QALYs saved by the program. Productivity losses associated with hepatitis-related morbidity or mortality are not included in the analysis because they are implicitly included in the QALY measures (16).
QALYs saved by preventing acute and chronic hepatitis B are estimated for the remaining life expectancy of the target population. The analytical horizon was selected because available data suggest that vaccine-induced immunity against hepatitis B does not wane over time and because chronic hepatitis B can lead to serious health consequences years after an acute infection. All future costs and benefits were discounted at a 3% annual rate.
Sensitivity analyses
In one-way sensitivity analyses, model input parameters were individually varied from their low to high values while keeping all other variables fixed. Parameter ranges are based on 95% CIs. When 95% CIs were not available, parameters were varied between the highest and lowest values identified in the literature or by 25% if only one value was available.
In probabilistic sensitivity analyses, parameters were varied simultaneously, and the model was run 1,000 times. For each run, key input parameters were drawn from appropriate distributions. Probability and utility decrements were drawn from beta distributions to ensure values between 0 and 1. Other disease parameters and disease state costs were drawn from lognormal distributions to account for skewness. Costs for small, distinct medical events were varied based on normal distributions. For groupings of clearly associated variables (vaccine efficacy, hepatitis incidence, and utility decrements), correlation between variables was allowed when parameters were drawn for individual runs. We estimated 95% credible intervals of the cost-effectiveness ratio by bootstrapping the results of the probabilistic sensitivity analysis (17).
The private cost of vaccine (which is known) and the cost of administering the vaccine were separately varied by a multiplicative factor between 0.75 and 1.25 for one-way sensitivity analyses and with a corresponding normal distribution in probabilistic sensitivity analyses. The discount factor was varied from 0 to 5% in the one-way sensitivity analysis and was not varied in the probabilistic sensitivity analysis.
In addition to the one-way and probabilistic sensitivity analyses, we considered several alternative scenarios. Separately, the age at vaccination was varied in 10-year increments, the vaccine uptake rate was varied from 5 to 40%, and the vaccine was available at the CDC price.
RESULTS
Health outcomes
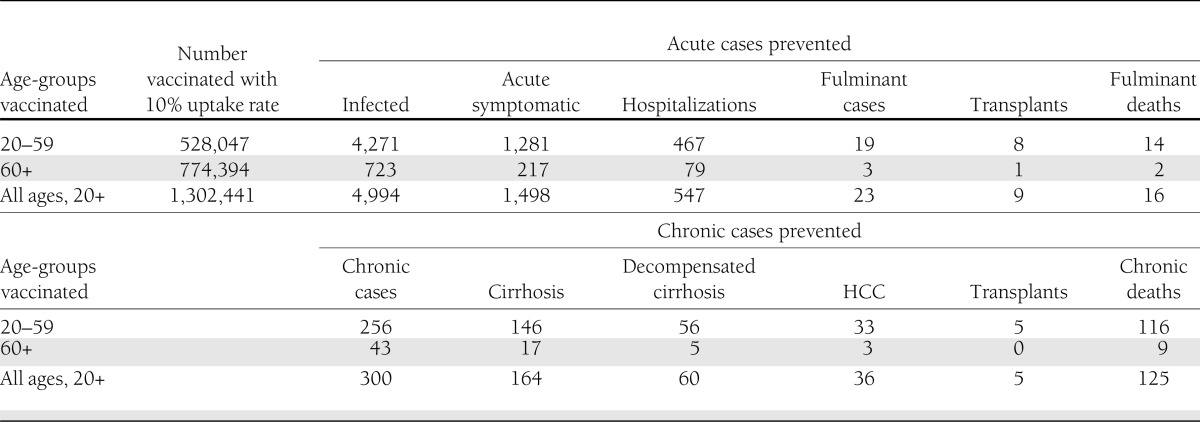
Projected reductions in acute and chronic health outcomes resulting from vaccination are shown in Table 2. Vaccinating susceptible people with diagnosed diabetes 20–59 years of age with an assumed 10% uptake rate (main analysis) results in vaccination of 528,047 people. This program will prevent 4,271 acute HBV infections over the course of the vaccinated individuals’ lifetimes, 467 hospitalizations for acute infections, 19 fulminant cases, 8 transplants, and 14 deaths from fulminant hepatitis. The program will also prevent 256 cases of chronic HBV infection, thereby preventing 146 cirrhosis cases, 56 decompensated cirrhosis cases, 33 HCC cases, 5 transplants, and 116 deaths from chronic hepatitis.
Table 2.
Acute and chronic health outcomes prevented by hepatitis B vaccination
Vaccinating people with diagnosed diabetes 60 years of age or older and people with diagnosed diabetes 20 years of age or older (the alternative strategies analyzed) would prevent 723 and 4,994 acute HBV infections, respectively. Although a large number of people 60 years of age or older would be vaccinated, few acute and chronic cases would be prevented.
Cost-effectiveness
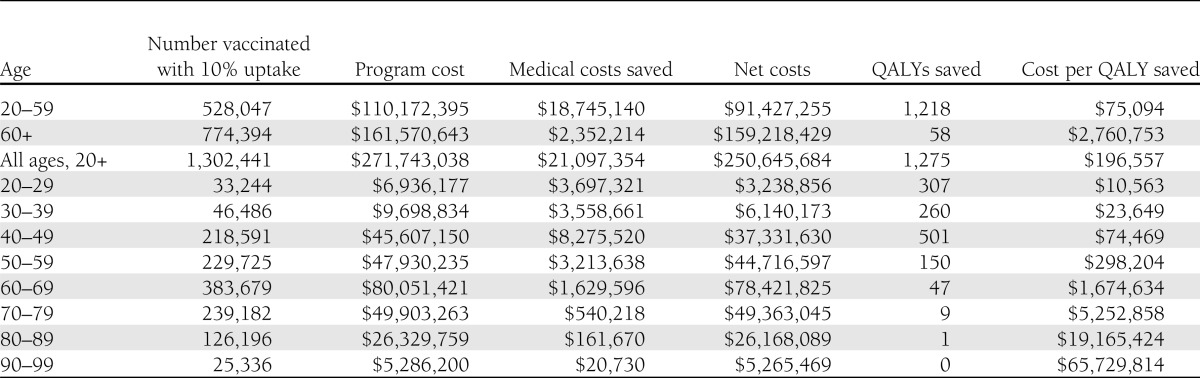
The vaccination program increases net costs and increases QALYs (Table 3). The point estimate of the incremental cost-effectiveness ratio of hepatitis B vaccination for people 20–59 years of age with diagnosed diabetes is $75,094 per QALY. The cost-effectiveness ratio for the alternative strategy of vaccinating adults with diagnosed diabetes 20 years of age or older is $196,557 per QALY; vaccinating only adults with diagnosed diabetes 60 years of age or older produces a cost-effectiveness ratio of $2,760,753 per QALY.
Table 3.
Estimated outcomes and impact of age at hepatitis B vaccination on results
The cost-effectiveness ratios increase with age at vaccination. The factors underlying this increase are discussed below.
Sensitivity analyses
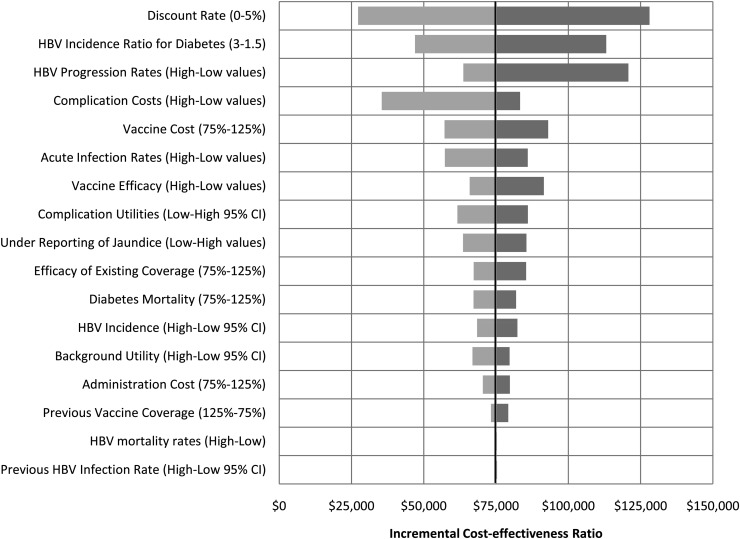
Figure 1 shows a tornado diagram depicting the cost-effectiveness results of the one-way sensitivity analyses. The center line of the tornado is the baseline cost-effectiveness ratio of $75,094 per QALY gained. For presentation purposes for certain related groups of parameters (complication costs, chronic hepatitis transition probabilities, acute infection transition probabilities, and complication utilities), we set all parameters in the group to their low and high values. The effects of varying individual parameters within these groups were smaller than the aggregated effects for the group. The parameter labels in Fig. 1 indicate which values were associated with improved cost-effectiveness and which resulted in worse cost-effectiveness. Results were most sensitive to the discount rate, with no discounting improving cost-effectiveness to $27,000 per QALY, and a 5% discount rate resulting in $128,000 per QALY. Results were also sensitive to the hepatitis B incidence ratio for people with diagnosed diabetes relative to people without diabetes, hepatitis B progression rates, and complication costs.
Figure 1.
One-way sensitivity analyses of cost-effectiveness of hepatitis B vaccination for people with diagnosed diabetes, 20–59 years of age.
The cost-effectiveness ratio based on the probabilistic sensitivity analysis results is $74,478 per QALY gained, slightly lower than the results using baseline values. The bootstrapped 95% credible interval for the cost-effectiveness ratio ranges from $69,000 to $80,400 per QALY gained.
Alternative uptake rates
As uptake rates increase, net costs and QALYs saved increase as more people are vaccinated. The cost-effectiveness ratio does not change because its numerator and denominator increase proportionately (Supplementary Data).
CDC vaccine prices
If the hepatitis B vaccine were available at the lower CDC price, the program cost would decline and the cost-effectiveness ratio would decrease to $41,622 per QALY gained (Supplementary Data).
CONCLUSIONS
No previous study has analyzed the cost-effectiveness of hepatitis B vaccination among adults with diabetes. Hepatitis B vaccination for adults with diagnosed diabetes <60 years of age costs ∼$75,000 per QALY saved, a value substantiated over a probabilistic range of input parameters. For all adults with diagnosed diabetes 20 years of age or older, hepatitis B vaccination is less cost-effective at a cost per QALY saved of ∼$197,000.
Three factors contribute to the varying cost-effectiveness of hepatitis B vaccination for people with diagnosed diabetes by age-group at vaccination: hepatitis B incidence decreases after 40 years of age (2), vaccine immunogenicity declines with age (Supplementary Data), and older people spend fewer years at risk for hepatitis complications because their remaining life expectancy is shorter. These considerations suggest both clinical and economic rationales for vaccination soon after diabetes diagnosis.
The cost-effectiveness of hepatitis B vaccination for adults with diagnosed diabetes is within the range of cost-effectiveness of selected diabetes management interventions and adult immunizations. Li et al. (18) reviewed the cost-effectiveness of 44 interventions for diabetes and classified most as cost-saving, very cost-effective (cost-effectiveness ratio between $0 and $25,000 per QALY), or cost-effective (cost-effectiveness ratio between $25,001 and $50,000 per QALY). Hepatitis B vaccination would be classified as marginally cost-effective (cost-effectiveness ratio between $50,001 and $100,000 per QALY) under the Li et al. (18) approach, along with four of the interventions reviewed by Li et al. Cost-effectiveness ratios for adult zoster and pneumococcal vaccination range from $16,229 to >$100,000 (19,20) and from cost saving to $66,818 (21) per QALY saved, respectively.
Assuming a 10% vaccine uptake rate, a hepatitis B vaccination program for adults with diagnosed diabetes 20–59 years of age would cost ∼$110 million in its first year. If uptake rates increase, program costs will increase but the cost per QALYs saved will remain unchanged because net costs and QALYs saved increase proportionately. Unlike other adult vaccination programs, costs would be expected to decline over time as the vaccinated pediatric cohort ages into adulthood.
This analysis has several limitations. The private vaccine price ($52.50) was used as an input parameter because the proportion of people who would receive vaccine at the CDC vaccine price ($28.00, the price for vaccine obtained through CDC contracts for immunization programs that receive CDC immunization grant funds; private providers and private citizens cannot directly purchase vaccines through CDC contracts) could not be estimated. Some studies used to derive figures for vaccine efficacy had small sample sizes or included people with diabetes in a secondary analysis. We did not include adverse events associated with vaccination. However, the hepatitis B vaccine is considered very safe. Public health costs for outbreak investigations and benefits from herd immunity were not included in the model. The model assumed that vaccination would be provided during regularly scheduled health care visits (which could underestimate costs) and that all people vaccinated would receive the complete series. People with diagnosed diabetes <60 years of age were estimated to be twice as likely to be infected with HBV compared with people without diabetes (22), and hepatitis B–associated morbidity is potentially high. Although not reflected in our calculations, people with diabetes who develop HBV infection might be more likely than otherwise healthy adults to develop serious sequelae of infection; acute HBV infection leads to the development of chronic infection (which can lead to cirrhosis, liver failure, and liver cancer) in ∼5% of otherwise healthy adults (23), but development of chronic infection might be more frequent among older adults (24). Mortality after acute infection is also increased; hepatitis B–associated deaths among people with diabetes in recent outbreaks in long-term care facilities reached as high as 75% (25), whereas 1–3% of otherwise healthy adults in nonoutbreak settings (CDC, unpublished data) die as a result of their acute infection. In addition, a recent population-based study found that people with both diabetes (defined as fasting glucose ≥126 mg/dL or history of oral hypoglycemic or insulin use, or both) and chronic HBV infection had ∼30 times the risk of death as people without diabetes with chronic infection (26).
Given the higher incidence of hepatitis B among people with diagnosed diabetes and the cost-effectiveness ratio of $75,094 per QALY gained of hepatitis B vaccination for people with diagnosed diabetes 20–59 years of age, on 25 October 2011, the Advisory Committee on Immunization Practices recommended hepatitis B vaccination for these individuals as a primary preventive measure. For older adults with diagnosed diabetes, the frequency of current or anticipated future need for assisted monitoring of blood glucose and other diabetes procedures should be incorporated into the clinical decision-making process for recommending hepatitis B vaccination, particularly early in the course of diabetes.
Acknowledgments
This study was supported by the CDC (contract 200-2009-30991 TO3).
No potential conflicts of interest relevant to this article were reported.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC or the Agency for Toxic Substances and Disease Registry.
T.J.H. directed the study, wrote the manuscript, and researched data. S.S. wrote the manuscript and researched data. J.S.W. modified the cost-effectiveness model and analyzed data. C.L.B. researched and analyzed data. F.Z. created the cost-effectiveness model. K.B. contributed to the discussion and researched data. T.V.M. secured funding for the study, wrote the manuscript, and researched data. T.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0759/-/DC1.
References
- 1.Centers for Disease Control and Prevention (CDC). Hepatitis B vaccination—United States, 1982-2002. MMWR Morb Mortal Wkly Rep 2002;51:549–552, 563 [PubMed]
- 2.Centers for Disease Control and Prevention (CDC). Viral hepatitis statistics and surveillance, United States 2010. Available at http://www.cdc.gov/hepatitis/Statistics/2010Surveillance/PDFs/2010HepSurveillanceRpt.pdf Accessed 6 August 2012
- 3.Daniels D, Grytdal S, Wasley A, Centers for Disease Control and Prevention (CDC) Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ 2009;58:1–27 [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011;60:1709–1711 [PubMed] [Google Scholar]
- 5.Murphy T. Hepatitis B vaccination for adults with diabetes: summary of considerations. Advisory Committee on Immunization Practices (ACIP). 25 October 2011 [PubMed]
- 6.Schillie S, Smith E, Reilly M, Murphy T. Odds of acute hepatitis B among persons with diabetes at eight emerging infections program sites. Advisory Committee on Immunization Practices. 25 October 2011.
- 7.Thompson ND, Perz JF. Eliminating the blood: ongoing outbreaks of hepatitis B virus infection and the need for innovative glucose monitoring technologies. J Diabetes Sci Tech 2009;3:283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Euler GL, McPhee SJ, et al. Economic analysis of promotion of hepatitis B vaccinations among Vietnamese-American children and adolescents in Houston and Dallas. Pediatrics 2003;111:1289–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA 1995;274:1201–1208 [PubMed] [Google Scholar]
- 10.Kim SY, Billah K, Lieu TA, Weinstein MC. Cost effectiveness of hepatitis B vaccination at HIV counseling and testing sites. Am J Prev Med 2006;30:498–506 [DOI] [PubMed] [Google Scholar]
- 11.Jiles R. Epidemiology of Acute Hepatitis B. Atlanta, GA, Division of Viral Hepatitis, Centers for Disease Control and Prevention, 2010 [Google Scholar]
- 12.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. CDC vaccine price list: adult vaccine price list [Internet], 2011. Available at http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm Accessed 9 May 2011
- 14.Miriti MK, Billah K, Weinbaum C, et al. Economic benefits of hepatitis B vaccination at sexually transmitted disease clinics in the U.S. Public Health Rep 2008;123:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freed GL, Clark SJ, Cowan AE, Coleman MS. Primary care physician perspectives on providing adult vaccines. Vaccine 2011;29:1850–1854 [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996 [Google Scholar]
- 17.Tambour M, Zethraeus N. Bootstrap confidence intervals for cost-effectiveness ratios: some simulation results. Health Econ 1998;7:143–147 [DOI] [PubMed] [Google Scholar]
- 18.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010;33:1872–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007;44:1280–1288 [DOI] [PubMed] [Google Scholar]
- 20.Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007;25:8326–8337 [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie I, Khoury AE, Cui Y, Dasbach E, Grabenstein JD, Goetghebeur M. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine 2009;27:4891–4904 [DOI] [PubMed] [Google Scholar]
- 22.Reilly ML, Poissant T, Vonderwahl CW, Gerard K, Murphy TV. Incidence of acute hepatitis B among adults with and without diabetes, 2009-2010. Abstract presented at the 49th Annual Meeting of Infectious Diseases Society of America, 20–23 October 2011, Boston, Massachusetts. [Google Scholar]
- 23.Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis 1995;20:992–1000 [DOI] [PubMed] [Google Scholar]
- 24.Kondo Y, Tsukada K, Takeuchi T, et al. High carrier rate after hepatitis B virus infection in the elderly. Hepatology 1993;18:768–774 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Notes from the field: deaths from acute hepatitis B virus infection associated with assisted blood glucose monitoring in an assisted-living facility—North Carolina, August-October 2010. MMWR Morb Mortal Wkly Rep 2011;60:182. [PubMed] [Google Scholar]
- 26.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut 2010;59:1410–1415 [DOI] [PubMed] [Google Scholar]