Classification of diabetes
As stated by Zimmet et al. (1) in the initial chapter of a classical textbook on diabetes, the major requirement for orderly epidemiologic, genetic, and clinical research on diabetes and glucose intolerance, and indeed for their clinical management, is an appropriate classification. Furthermore, a hallmark in the process of understanding the etiology of a disease and studying its natural history is the ability to identify and differentiate its various forms and place them into a rational etio-pathologic framework. In 1984, Ahrén and Corrigan (2) reported the existence of a subgroup of diabetic patients observed in Tanzania where the need for insulin replacement therapy fluctuates with time, waxes and wanes, and transient ketoacidosis develops. This subtype of diabetes is not unusual in African Americans and sub-Saharan Africans and currently recognized under the term of ketosis-prone atypical diabetes (KPD) (review in ref. 3).
Classifying KPD in relation with the other more frequent forms of diabetes is not easy. At the onset, KPD often appears as type 1 diabetes with acute hyperglycemia and ketosis or ketoacidosis and the obvious need for insulin therapy but the signs of autoimmunity against islet β-cells are absent. In contrast, during near-normoglycemic remission, patients with KPD usually display multisite insulin resistance similar to that seen in type 2 diabetes (4,5). In 2008, Sobngwi et al. (6) hypothesized that KPD is a subtype of type 2 diabetes with acute onset at diagnosis as the result of an environmental factor such as a human herpes virus infection that would severely impair glucose-stimulated insulin secretion and favor ketogenesis.
In this issue of Diabetes Care, Choukem et al. (7) present original data on the islet dysfunction present in KPD during remission.
Islet dysfunction in ketosis-prone atypical diabetes
Previous studies have shown severe insulin secretory deficiency during the acute ketotic phase of KPD while during remission the insulin response to intravenous glucose clearly improves but remains lower than in healthy control subjects (4,8). In the study reported here by Choukem et al. (7), the insulin secretory defect during KPD in remission was confirmed, and careful analysis of the data suggested that KPD is associated with both decreased β-cell sensitivity to glucose and reduced β-cell mass. These findings are similar to those reported in classic cases of type 2 diabetes and support the concept that KPD is a subtype of type 2 diabetes (6). More interesting is the finding that KPD is associated with basal hyperglucagonemia and reduced glucagon suppression in response to oral or intravenous glucose, but not in response to intravenous insulin. Thus it appears that during near-normoglycemic remission the islet α-cell in KPD is sensitive to exogenous insulin but does not fully respond to glucose-induced endogenous insulin release. These observations should be discussed in the frame of the recently developed concept that diabetes should be seen as a paracrinopathy of the islets of Langerhans, in which hyperglucagonemia would result from intraislet insulin paracrine deficiency (9,10), while glucagon should be reconsidered as a key factor in the pathophysiology of diabetes (11) as proposed decades ago (12,13).
Revisiting the microanatomy of the islets of Langerhans
In his thesis defended in Berlin on 18 February 1869 and devoted to the microscopic anatomy of the pancreatic gland, Paul Langerhans reported the presence of some cell groups that were not in connection with the ducts and quite genuinely wrote, “I admit frankly that I am not able to explain the nature and function of these cells...” (10). And so, the islets of Langerhans were born.
Today these islets appear as remarkable micro-organs of extraordinary complexity. The pancreatic islet is composed of five endocrine cell types: the β-cells that produce insulin, the α-cells that produce glucagon, the δ-cells that produce somatostatin, the pancreatic polypeptide cells that produce the pancreatic polypeptide, and the most recently identified ε-cells that produce ghrelin. The interplay between insulin and glucagon in physiology and pathophysiology should be considered in view of the possible connections between the α- and β-cells inside the islets of Langerhans, knowing that insulin strongly inhibits glucagon secretion and, conversely, that glucagon stimulates insulin secretion.
For obvious reasons, most studies on the islet as a micro-organ have been performed in rodents. In rodents, most β-cells are located in the center of the islet while the other endocrine cells, and particularly the α-cells, are located at the periphery. In these species, the microcirculation appears remarkable in it microanatomy and microphysiology (14).
The arterial blood enters the core of the islet where, potentially, the β-cells can enrich it with the highest concentration of insulin in the body as it flows to the mantle and reaches the α-cells. With such a setting, a clear possibility exists for β-cell products such as zinc, γ-aminobutyric acic and, of course, insulin itself to modulate α-cell function. These observations have led to the theory that the hyperglucagonemia reported in all forms of experimental or clinical diabetes is the result of decreased inhibition of glucagon secretion by insulin or other β-cell products. Support of this theory has been brought by the observation that a 60% reduction of the β-cell mass in minipigs results in a decrease in the amplitude of the insulin pulses in the portal blood associated with a significant increase of the intraportal glucagon pulses (15). In this model, mathematical analysis has suggested that, in normal animals, intraislet pulsatile insulin directly suppresses glucagon secretion, but that this association is lost after selective partial reduction in β-cell mass thus supporting the concept that diabetes should be considered as a paracrinopathy of the islets of Langerhans (9,10).
β-Cells embracing α-cells in human islets
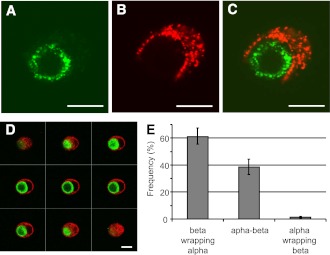
It can be argued that the microanatomy of the islets in man differs from that reported in rodents and that the core-to-mantle microcirculation model may not be applicable in man. The most detailed analysis of the microanatomy of the human islets of Langerhans has been reported by Bosco et al. (16).The β-cells form a lower percentage of the endocrine population than in rodent islets, but, as in rodent islets, they occupy a core position surrounded by mantles of the α-cells in pseudo-lobular divisions with blood vessels at their periphery. Three-dimensional analysis revealed that islet cells are in fact organized into trilaminar epithelial plates, which are folded with different degrees of complexity and bordered by vessels on both sides. In epithelial plates, most β-cells are located in a central position but frequently show cytoplasmic extensions between outlying α-cells. The intimate relationship between human β- and α-cells is well illustrated in Fig. 1, which is reproduced from Bosco et al. (16). When the association between α- and β-cells is assessed in cultured isolated human islet cells, many α-cells are “wrapped” by β-cells and almost never the contrary. This strongly suggests that in some way human α-cells are “under the control” of β-cells. As discussed by the authors, this observation reveals a unique plasticity of the β-cells, which are able to spread around the α-cells, suggesting that this characteristic is intrinsic to β-cells and not dictated by some islet coercions, such as extracellular matrix or islet vasculature. The molecular mechanism of this extraordinary “embracing” of α-cells by β-cells remains to be elucidated, but the phenomenon in itself must be considered when discussing the paracrinology of the islets and the attractive suggestion that diabetes must be seen as a paracrinopathy in which glucagon cannot be ignored (9–11).
Figure 1.
Human islet cells were isolated and cultured for 24 h. After a double immunofluorescence for insulin (red) and glucagon (green), islet cells were analyzed by confocal microscopy. A–C: Images showing a cell pair composed of one α-cell surrounded by one β-cell, the merged image is shown in panel C. The cell pair shown here is representative of cell pairs observed in different human cell preparations from at least 10 different pancreata. D: One series of consecutive merged images of a cell pair composed of one α-cell (green) surrounded by a β-cell (red). Scale bars, 10 μm. All heterologous contacts between α- and β-cells were coded according to their type of association: a β-cell wrapping an α-cell (β wrapping α), neutral apposition between α- and β- cells (α-β), and an α-cell wrapping a β-cell (α wrapping β). Results are shown as relative frequencies, and columns are means ± SEM of five islet cell preparations from five different pancreata. From all heterogenous contacts between α- and β-cells, the percentage of α-cells whose profile was round and the perimeter almost completely wrapped by a β-cell, as in panel D, was 38 ± 8 (means ± SEM of three experiments) (16). Reprinted with permission from the American Diabetes Association.
Back to ketosis-prone atypical diabetes
The article by Choukem et al. (7) does not close the debate about the place in the classification of diabetes and the pathogeny of KPD. It has the merit to show that, as in most if not all other forms of diabetes, focusing on insulin secretion or action only does not give the full picture. Here, also, glucagon is involved and, as stated by Unger and Cherrington (11), a “pathophysiologic and therapeutic makeover” has become necessary. Further studies are obviously needed to better understand the role of glucagon in diabetes, but ignoring it today is not an option.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
The author thanks Dr. Domenico Bosco for reviewing the article and granting permission to reproduce the figure.
Footnotes
See Choukem et al., p. 118
References
- 1.Zimmet P, Cowie C, Ekoe J-M, Shaw JE. Classification of diabetes and other categories of glucose intolerance. International Textbook of Diabetes Mellitus. DeFronzo RA, Ferrannini E, Keen H, Zimmet P, Eds. Chichester, John Wiley and Sons, 2004, p. 3–14 [Google Scholar]
- 2.Ahrén B, Corrigan CB. Intermittent need for insulin in a subgroup of diabetic patients in Tanzania. Diabet Med 1985;2:262–264 [PubMed] [Google Scholar]
- 3.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 2008;29:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes 2004;53:645–653 [DOI] [PubMed] [Google Scholar]
- 5.Choukem SP, Sobngwi E, Fetita LS, et al. Multitissue insulin resistance despite near-normoglycemic remission in Africans with ketosis-prone diabetes. Diabetes Care 2008;31:2332–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobngwi E, Choukem SP, Agbalika F, et al. Ketosis-prone type 2 diabetes mellitus and human herpes virus 8 infection in sub-Saharan Africans. JAMA 2008;299:2770–2776 [DOI] [PubMed] [Google Scholar]
- 7.Choukem S-P, Sobngwi E, Boudou P, et al. β- and α-cell dysfunctions in Africans with ketosis-prone atypical diabetes during near-normoglycemic remission. Diabetes Care 2013;36:118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes 1995;44:790–795 [DOI] [PubMed] [Google Scholar]
- 9.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 2010;107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefèbvre P. Diabetes as a paracrinopathy of the islets of Langerhans. European Endocrinology 2011;7:79–82 [Google Scholar]
- 11.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unger RH. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes 1976;25:136–151 [DOI] [PubMed] [Google Scholar]
- 13.Lefèbvre PJ, Luyckx AS. Glucagon and diabetes: a reappraisal. Diabetologia 1979;16:347–354 [DOI] [PubMed] [Google Scholar]
- 14.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 1982;31:883–889 [DOI] [PubMed] [Google Scholar]
- 15.Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC. Postprandial suppression of glucagon secretion depends upon intact insulin pulsatile secretion: further evidence for the intra-islet insulin hypothesis. Diabetes 2006;55:1051–1056 [DOI] [PubMed] [Google Scholar]
- 16.Bosco D, Armanet M, Morel P, et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes 2010;59:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]