Abstract
OBJECTIVE
To examine whether diabetes genetic risk testing and counseling can improve diabetes prevention behaviors.
RESEARCH DESIGN AND METHODS
We conducted a randomized trial of diabetes genetic risk counseling among overweight patients at increased phenotypic risk for type 2 diabetes. Participants were randomly allocated to genetic testing versus no testing. Genetic risk was calculated by summing 36 single nucleotide polymorphisms associated with type 2 diabetes. Participants in the top and bottom score quartiles received individual genetic counseling before being enrolled with untested control participants in a 12-week, validated, diabetes prevention program. Middle-risk quartile participants were not studied further. We examined the effect of this genetic counseling intervention on patient self-reported attitudes, program attendance, and weight loss, separately comparing higher-risk and lower-risk result recipients with control participants.
RESULTS
The 108 participants enrolled in the diabetes prevention program included 42 participants at higher diabetes genetic risk, 32 at lower diabetes genetic risk, and 34 untested control subjects. Mean age was 57.9 ± 10.6 years, 61% were men, and average BMI was 34.8 kg/m2, with no differences among randomization groups. Participants attended 6.8 ± 4.3 group sessions and lost 8.5 ± 10.1 pounds, with 33 of 108 (30.6%) losing ≥5% body weight. There were few statistically significant differences in self-reported motivation, program attendance, or mean weight loss when higher-risk recipients and lower-risk recipients were compared with control subjects (P > 0.05 for all but one comparison).
CONCLUSIONS
Diabetes genetic risk counseling with currently available variants does not significantly alter self-reported motivation or prevention program adherence for overweight individuals at risk for diabetes.
Nearly 80 million Americans are currently at increased risk for diabetes, with worldwide diabetes prevalence projected to reach 440 million by 2030 (1,2). Robust clinical trial evidence has demonstrated that lifestyle changes leading to increased exercise and weight loss can substantially reduce the risk for diabetes (3–5). Despite the relative cost-effectiveness of nonpharmacologic approaches to diabetes prevention (6,7), efforts to translate the intensive behavioral change interventions of clinical trials into the community setting have had only modest success (8–11).
New approaches to conveying personal risk, such as individualized diabetes genetic risk testing, may enable more effective diabetes prevention (12). One promise of genome-based “personalized medicine” has been the potential to motivate individuals to make lifestyle changes that ameliorate their disease risk (13,14). Type 2 diabetes is an ideal clinical paradigm for testing this assumption given the high prevalence of an easily identified predisease phenotype, the strong evidence linking behavior change to risk reduction, suboptimal translation of proven behavioral change interventions into clinical practice, and recent rapid progress in diabetes genetic epidemiology.
Patients and providers have both indicated that learning about higher genetic risk results would likely motivate individuals to change their behavior to prevent diabetes (15,16). This prediction has not yet been convincingly demonstrated in controlled trials (17). In addition, genetic testing that reveals a decreased genetic risk could provide false reassurance to individuals with a high phenotypic risk (18). This concern has also not yet been rigorously examined. Therefore, we conducted a randomized, controlled trial to test the hypothesis that diabetes genetic risk testing and counseling can motivate the behavior changes necessary to prevent diabetes. Given the potential for false reassurance with lower-risk results, we separately investigated the effect of disclosing increased or decreased diabetes genetic risk compared with untested control participants.
RESEARCH DESIGN AND METHODS
Study design
The Genetic Counseling/Lifestyle Change for Diabetes Prevention (GC/LC) Study was a prospective, three-arm parallel group, randomized, controlled clinical trial conducted among individuals at increased phenotypic risk for type 2 diabetes that tested two hypotheses: 1) receiving a higher diabetes genetic risk result would improve motivation and participation in a 12-session weekly diabetes prevention program compared with untested control subjects; and 2) receiving a lower diabetes genetic risk result would decrease motivation and participation compared with untested control subjects. This study was investigator-initiated, funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and conducted at Massachusetts General Hospital in Boston.
Details of the study design have been published previously (19). Briefly, eligible individuals were randomly allocated using random numbers in concealed envelopes in a four-to-one ratio to diabetes genetic risk testing versus no testing. Investigators implemented the random allocation, enrollment, and assignment. Participants with top and bottom quartile of diabetes genetic risk were enrolled with untested control subjects into a 12-week group-based Lifestyle Balance diabetes prevention program modeled after the Diabetes Prevention Program protocol and previously validated in patients with metabolic syndrome (9). Participants determined to have average diabetes genetic risk received their results but were not studied further. This study was approved by the Partners Human Research Committee Institutional Review Board. All participants provided written informed consent before enrollment.
Study participants
Participants were recruited from primary care practices within our institution, with the permission of their primary care physicians, between January 2010 and March 2011. Patients were eligible to participate in the study if they were aged 21 years or older, overweight (defined as BMI ≥29.1 kg/m2 in men, ≥27.2 kg/m2 in women), met one other criterion for metabolic syndrome without an existing diagnosis of type 2 diabetes (20), and were physically able and willing to participate in a 12-week group session program designed to achieve weight loss through dietary change and increased physical activity.
Blood samples for individuals randomized to genetic testing were drawn for diabetes genetic risk assessment and genotyped at the Broad Institute (Cambridge, MA) using the Sequenom MassARRAY iPLEXGold platform (Sequenom, Inc., San Diego, CA). A summary genetic risk score was calculated from 36 successfully genotyped risk alleles previously associated with type 2 diabetes (21).
Calculation of relative and absolute diabetes genetic risk
Individualized genetic risk assessment was performed by multiplying the relative genetic risk, as determined using diabetes incidence data from the Framingham Offspring Study (FOS) (22), by the absolute phenotypic risk of the study population. In FOS, 17.9% patients in the top quartile of genetic risk score distribution (>38) developed diabetes (46% relative increase compared with middle two average quartiles), whereas 9.9% in the bottom quartile (scores <34) developed diabetes (18% relative decrease compared with “average” risk). We estimated the absolute diabetes incidence for participants meeting our study eligibility criteria as ∼11% over 3 years using previously published data from within our hospital network (23). Multiplying relative genetic risk by this absolute phenotypic-based risk resulted in posttest absolute 3-year risk estimates of 17% (higher genetic risk recipients), 11% (untested control subjects), and 9% (lower genetic risk recipients) for type 2 diabetes.
Intervention implementation
Participants with results showing higher and lower diabetes genetic risk each received a 15-min structured, individual genetic counseling session conducted by a certified genetic counselor. Details of the session have published previously (24). Each participant received a detailed diabetes genetic risk report listing results for each successfully tested single nucleotide polymorphisms and the individual's overall diabetes genetic risk category (Supplementary Data). The one-on-one genetic counseling sessions explained the genetic test results, discussed the relative contributions of genetic versus lifestyle factors to the development of diabetes, and placed the recipient’s genetic risk results within the context of his or her overall risk for diabetes. A primary goal of the counseling session was to use the genetic test result as an opportunity to encourage overall diabetes risk reduction through behavioral changes.
Counseled intervention participants and untested control subjects participated in a 12-week diabetes prevention program conducted by an experienced dietitian from our institution’s Diabetes Center who was masked to the genetic results. The diabetes prevention groups combined intervention and control participants to eliminate any group-based intervention effects. Participants were asked to refrain from discussing their genetic testing status and results. Masking was well preserved, with the correct prediction of participant testing status by the dietitian after completion of the 12-week program no better than chance (33.2%).
Outcome measures
We posited a causal pathway for diabetes prevention by which changes in patient motivation would lead to changes in the health-related behaviors that in turn would induce the physiologic changes necessary to prevent diabetes. To capture changes in motivation, we assessed self-reported confidence and motivation to make diabetes-related lifestyle changes (exercise, weight loss, and adoption of a low-fat diet) and stage of change for achieving these behaviors (25–27). To assess behavioral changes, we measured number of sessions attended for the 12-week Lifestyle Balance program because prior research has demonstrated a positive dose–response relationship between attendance and diabetes prevention (28). Finally, we also assessed weight change from baseline to program completion.
Statistical analyses
For our primary analyses, we examined changes in self-reported responses from baseline to study end, separately comparing higher-risk and lower-risk recipients with untested control participants. The study was designed to have sufficient power for assessing 1) the difference between comparison arms from baseline to study completion in self-reported stages of change, and 2) differences in program attendance. We estimated that with 30 intervention participants in each arm and 30 control participants, we had 97% power to detect a 90% increase in higher-risk intervention patients versus a 50% increase in control participants regarding stage of change at 0.05 two-sided significance and 78% power to show a 21% decrease in motivation comparing lower-risk intervention patients with control participants. For attendance, we estimated that the study sample size would provide 96% power to detect a 20% difference in number of diabetes prevention sessions attended (i.e., difference of 1.7 visits assuming that control participants attended 8.5 of 12 visits).
For stages of change, we dichotomized the results into percentage of participants who improved or increased versus all others. Changes from baseline in 10-point scales for motivation and confidence were analyzed as continuous data using t tests and dichotomized data (increase vs. no increase) using χ2 tests. Program attendance was analyzed as a continuous variable (using t tests and Wilcoxon rank sum to compare means and medians) and also dichotomized as proportion of participants attending at least seven sessions (a threshold consistent with the Centers for Disease Control and Prevention’s attendance criteria for diabetes prevention recognition programs) (29) as the goal for group-based diabetes prevention programs. All analyses were intention-to-treat.
In a planned secondary analysis, we also compared the relative effect and durability of the genetic counseling intervention between higher- versus lower-risk intervention recipients after completion of the 12-week program.
RESULTS
Study participants
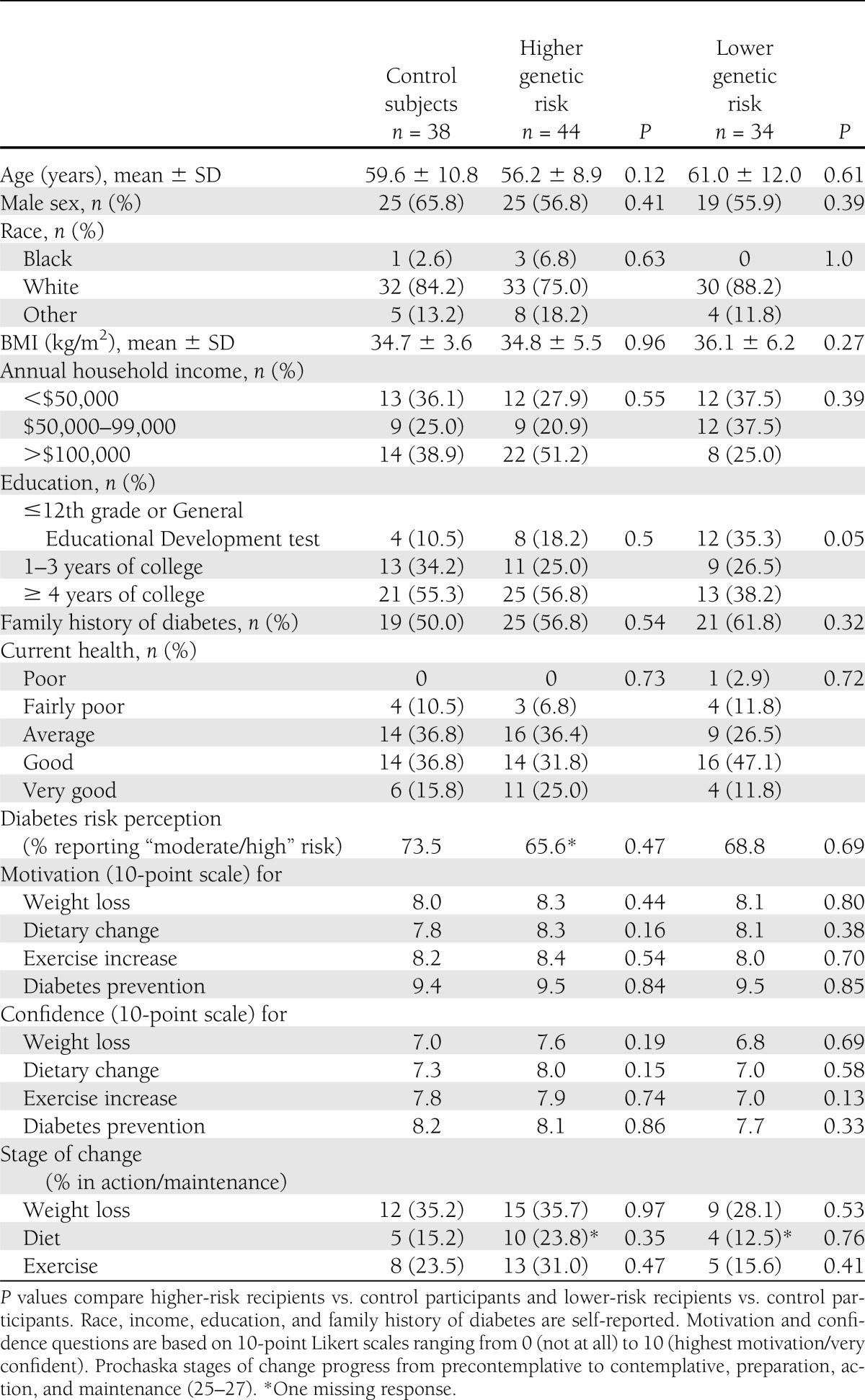
We contacted 687 potentially eligible participants by telephone. After excluding ineligible 83 individuals, 177 of 604 participants (29.3%) consented to participate. Study allocation arms were well balanced (Table 1). All participants reported high motivation (9.4 on a scale of 0–10) to prevent diabetes, although motivation and confidence for making specific changes involving weight loss, diet, and increasing exercise were lower, ranging from 6.8 to 8.4.
Table 1.
Baseline characteristics of the 116 participants by intervention allocation
Changes in self-reported attitudes
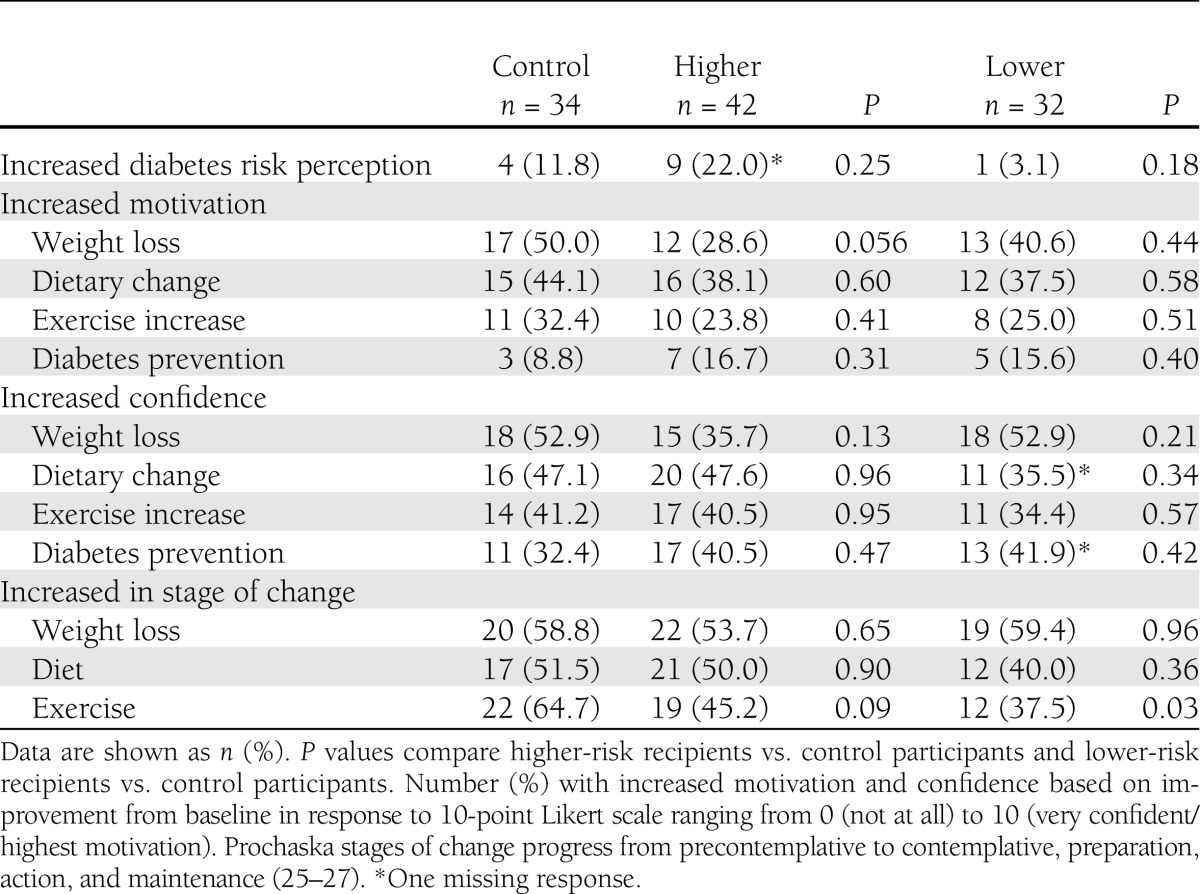
Enrollment in the 12-week diabetes prevention program led to small, generally favorable changes in risk perception, motivation (with the exception of exercise), and confidence that were not statistically different comparing higher- or lower-risk result recipients with control participants (Table 2). There was some evidence that lower-risk result recipients had less intent to exercise, with 37.5% of these participants increasing their stage of change for exercise compared with 64.7% of control participants (P = 0.03).
Table 2.
Changes from baseline in self-reported measures of risk perception, motivation, confidence, and stage of change for diabetes prevention behaviors, comparing recipients of higher and lower genetic risk results with untested control subjects
Differences in program attendance and weight loss
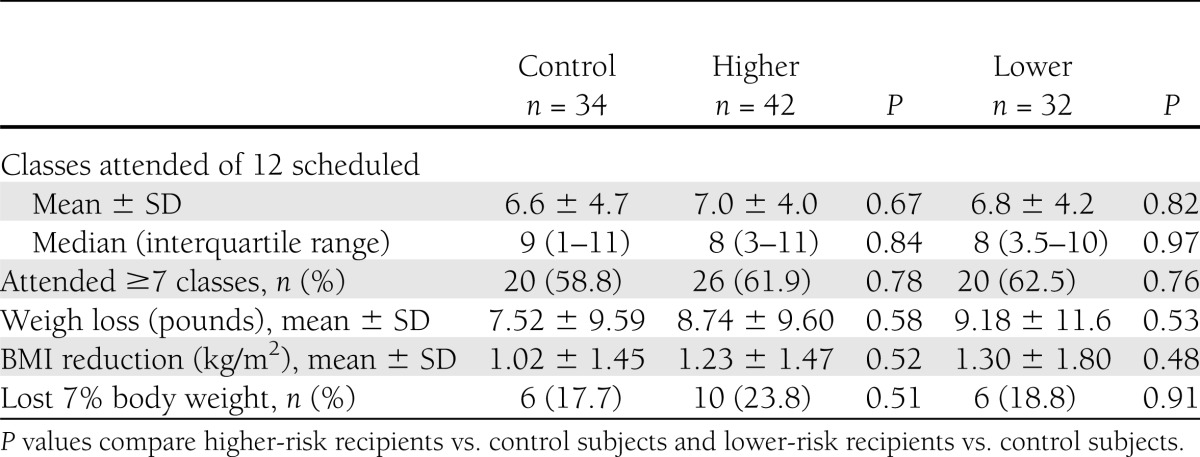
Study participants attended a mean of 6.8 ± 4.3 of 12 diabetes prevention group sessions. The 12-week Lifestyle Change program had a beneficial overall effect, with enrollees losing a mean of 8.5 ± 10.1 pounds (P < 0.001) and 33 of 108 participants (30.6%) losing ≥5% body weight. However, despite clear room for improvement in program attendance and goal weight achievement, receipt of personal genetic risk information and counseling had no statistically significant effect on measured behaviors compared with untested control participants (Table 3 and Fig. 1). Recipients of higher-risk results attended 0.4 more sessions (95% CI −1.6 to 2.5; P = 0.67) and lost more weight (BMI difference −0.2 kg/m2 [−0.5 to 0.9]; P = 0.52) compared with control participants. Lower-risk recipients attended 0.3 fewer sessions (−1.9 to 2.4; P = 0.82) and also lost more weight (BMI difference −0.3 kg/m2 [−0.5 to 1.1]; P = 0.48) compared with control participants.
Table 3.
Differences in program attendance and weight loss, comparing recipients of higher and lower genetic risk results with untested control subjects
Figure 1.
Proportion of participants attending each week of the 12-week Diabetes Prevention Program.
Secondary analysis of higher- versus lower-risk intervention arms
After completing the 12-week prevention program, 74 intervention participants (96% ) recalled their diabetes genetic risk status (e.g., “higher” or “lower”), although only 2 (3%) could accurately recall their numeric genetic risk score. In an exploratory analysis, we found that higher-risk result recipients more often reported that the initial genetic counseling intervention had made them “much more/somewhat more” motivated to participate in the 12-week program (78.6% vs. 43.8% for lower-risk participants, P = 0.003) and to make lifestyle changes to prevent diabetes (85.7% vs. 56.3% for lower-risk participants, P = 0.008). A significant proportion of lower-risk result recipients reported that they had not thought about their genetic risk results in the prior 3 months (43.8% vs. 16.7% of higher result recipients, P = 0.02). Despite these self-reported differences, program attendance and weight loss were not statistically different when the two intervention arms were compared (P > 0.05).
CONCLUSIONS
Among overweight primary care patients at increased phenotypic risk for type 2 diabetes, receiving a higher genetic risk result and counseling did not significantly improve motivation to adopt diabetes prevention behaviors or significantly increase program attendance or weight loss compared with untested control patients. Conversely, receiving a lower genetic risk result did not appear to significantly detract from motivation or attendance.
The GC/LC Study is one of the first rigorous, controlled trials to directly address the effect of diabetes genetic risk information on patient behavior. Our study has several important strengths. We designed our genetic counseling intervention to be intensive yet relatively brief to maximize the translatability to the real-world clinical setting. Delivered by an experienced genetic counselor, the counseling intervention was designed to educate recipients about the relative contributions of both genetic and behavioral risk and to emphasize that changing their behavior could reduce their overall diabetes risk. This approach received positive preliminary feedback by study participants for its effect on perceived control and general satisfaction with the genetic counseling process (24). Crucially, all study participants were then enrolled in an evidence-based and validated diabetes prevention program designed to provide them with the tools and skills necessary to achieve the required behavior changes for diabetes prevention. We believe that using the genetic test disclosure as a “teachable moment” to engage patients in risk-reducing behavior change represents a powerful model for how genetic testing for common chronic diseases can be implemented into primary care practice. By coupling information (genetic test results and counseling) to a mechanism for participants to act on the information (diabetes prevention program), the GC/LC Study created an ideal context for genetic testing to succeed as a motivator.
Our study was also designed to directly address the potential for false reassurance from receiving results showing a lower genetic risk, particularly among patients with increased risk based on family history or validated phenotypic measures. We did not uncover a strong negative influence of receiving a “lower” risk result, with the possible exception of one exercise measure. From our exit surveys, it appears that many lower-risk recipients underemphasized their genetic test results. We suspect that most of the genetically tested patients with average results would have had a similar response, indicating that diabetes genetic risk testing and counseling, even if it ultimately provides greater predictive power, will likely have little benefit but will do little harm for most tested patients who do not have higher results.
Despite these study strengths, several limitations must be considered. Perhaps foremost of these is the limited predictive value of current diabetes genetic risk testing, which required us to focus on the highest-risk patients to maximize the resulting contrast between tested and untested participants. Even in this group, with an estimated 3-year risk for diabetes of 11%, the recalculated risk based on relative genetic risk results resulted in only modest changes. Thus, we do not know whether greater genetic predictive ability in the future will have a bigger impact on changing behavior. However, given that 96% of our intervention patients remembered their qualitative genetic risk (i.e., “higher” or “lower”) but not their quantitative risk score (provided as a hand-out during the counseling session), we suspect that marginal improvements in risk prediction will not lead to substantially greater impact on patient behavior.
Another limitation of current genetic knowledge is that results do not alter the actual behavioral intervention; thus, although the risk information is personalized, the intervention itself is not. The Diabetes Prevention Program recently showed that an intensive lifestyle intervention benefits participants regardless of overall genetic risk (30), but future research focused on unique gene-environment interactions may help to further tailor interventions (e.g., some patients may benefit preferentially from caloric restriction or certain dietary plans, others from aerobic exercise or resistance training) (31) and therefore lead to truly personalized behavioral treatments. Finally, although we cannot exclude small differences in self-reported measures that did not reach statistical significance, we have good confidence that any difference in self-reported measures did not translate into significant changes in attendance behavior.
Our results must be considered within the context of the study design. We randomized in two stages to provide a clinically relevant test of genetic testing versus no testing on preventive behavior, while also efficiently focusing on the genetic risk extremes among tested participants. For the second-stage allocation of intervention participants, we relied on the concept of Mendelian randomization (e.g., the random allocation of parental alleles during gametogenesis) to identify top and bottom quartiles of genetic risk score distribution (32). A strategy of using the lowest quartile of score as “normal” would have increased the effect size in the highest quartile and would have eliminated the problem of presenting “lower” genetic risk results to otherwise phenotypically high-risk individuals. However, it seemed unethical to portray low-score outliers as normal given the population distribution in which the vast majority of individuals fall within a relatively narrow middle range. In addition, creating more extreme cut points, such as the top decile of risk, would have resulted in higher relative risk differences at the expense of identifying an increasingly smaller number of participants, which would have diminished the clinical relevance of our results.
Focusing as we did on phenotypically high-risk participants might have limited the effect of the genetic risk results because these participants might already have been maximally motivated. The paradox of this limitation is that less motivated individuals are also less likely to be interested in genetic testing. Given the modest success rate among control participants in our study and among other community-based programs described in the literature (8–11), new tools to achieve enduring behavior change are clearly needed. One challenge for the future is to identify the subset of patients for whom genetic test results represent the tipping point from inaction to action.
Our findings build on an emerging literature in translational genomics and health outcomes (33–35) and have implications for current direct-to-consumer home genetic testing (36). Such tests may benefit self-selected individuals but may also have negative consequences in patient-borne costs and the potential for triggering expensive diagnostic cascades (37). Without further evidence of efficacy from controlled clinical trials, such testing cannot yet be recommended in routine clinical care for diabetes prevention. Other important applications of personalized genetic testing deserve further study, including clinically applied pharmacogenomic profiling (38) and evaluation of phenotypically lower-risk younger patients who have yet to manifest diabetes-related phenotypic traits.
In summary, a diabetes genetic risk assessment and counseling intervention for overweight individuals based on 36 single nucleotide polymorphisms neither improved nor substantially detracted from an evidence-based behavioral intervention to prevent diabetes.
Acknowledgments
This study received funding support from National Institute of Diabetes and Digestive and Kidney Diseases Grants R21-DK84527 and K24-DK080140. No potential conflicts of interest relevant to this article were reported.
R.W.G. obtained funding, oversaw the clinical trial, researched data, and wrote the manuscript. K.E.O., J.L.W., and K.G.S. contributed to the conduct of the clinical trial, researched data, and reviewed and edited the manuscript. J.L.V. researched data and reviewed and edited the manuscript. L.M.D., L.G.B., R.C.G., C.G., E.R.P., J.C.F., and J.B.M. contributed to the conduct of the clinical trial and reviewed and edited the manuscript. R.W.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The results of this study, titled “Can Genetic Testing Motivate Behavior Change and Weight Loss?” were presented at the “New Frontiers in Weight Management” symposium at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors acknowledge the Diabetes Prevention Support Center of the University of Pittsburgh for providing the written materials for the Group Lifestyle Balance program.
Footnotes
Clinical trial reg. no. NCT01034319, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0884/-/DC1.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 5.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 6.Jacobs-van der Bruggen MA, van Baal PH, Hoogenveen RT, et al. Cost-effectiveness of lifestyle modification in diabetic patients. Diabetes Care 2009;32:1453–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman WH, Hoerger TJ, Brandle M, et al. Diabetes Prevention Program Research Group The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the diabetes prevention program into the community. The DEPLOY pilot study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care 2008;31:684–689 [DOI] [PubMed] [Google Scholar]
- 10.Absetz P, Oldenburg B, Hankonen N, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL Lifestyle Implementation Trial. Diabetes Care 2009;32:1418–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med 2009;37:505–511 [DOI] [PubMed] [Google Scholar]
- 12.McBride CM, Bowen D, Brody LC, et al. Future health applications of genomics: priorities for communication, behavioral, and social sciences research. Am J Prev Med 2010;38:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010;363:301–304 [DOI] [PubMed] [Google Scholar]
- 14.McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health 2010;31:89–103 [DOI] [PubMed] [Google Scholar]
- 15.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia 2009;52:2299–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markowitz SM, Park ER, Delahanty LM, O’Brien KE, Grant RW. Perceived impact of diabetes genetic risk testing among patients at high phenotypic risk for type 2 diabetes. Diabetes Care 2011;34:568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev 2010:CD007275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans JP, Meslin EM, Marteau TM, Caulfield T. Genomics. Deflating the genomic bubble. Science 2011;331:861–862 [DOI] [PubMed] [Google Scholar]
- 19.Grant RW, Meigs JB, Florez JC, et al. Design of a randomized trial of diabetes genetic risk testing to motivate behavior change: the Genetic Counseling/Lifestyle Change (GC/LC) Study for Diabetes Prevention. Clin Trials 2011;8:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 21.de Miguel-Yanes JM, Shrader P, Pencina MJ, et al. MAGIC Investigators. DIAGRAM+ Investigators Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care 2011;34:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008;359:2208–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hivert MF, Grant RW, Shrader P, Meigs JB. Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BMC Health Serv Res 2009;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waxler JL, O'Brien KE, Delahanty LM, et al. Genetic counseling as a tool for type 2 diabetes prevention: a new genetic counseling framework for common polygenetic disorders. J Genet Couns 2012 Feb 3 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 25.Greene GW, Rossi SR, Reed GR, Willey C, Prochaska JO. Stages of change for reducing dietary fat to 30% of energy or less. J Am Diet Assoc 1994;94:1105–1110; quiz 1111–1112 [DOI] [PubMed] [Google Scholar]
- 26.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992;63:60–66 [DOI] [PubMed] [Google Scholar]
- 27.O’Connell D, Velicer WF. A decisional balance measure and the stages of change model for weight loss. Int J Addict 1988;23:729–750 [DOI] [PubMed] [Google Scholar]
- 28.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 29.Williamson DF, Marrero DG. Scaling up type 2 diabetes prevention programs for high risk persons: progress and challenges in the United States. In Diabetes Prevention in Practice. Schwarz P, Reddy P, Greaves C, Dunbar JA, Schwarz J, Eds. Dresden, TUMAINI Institute for Prevention Management, 2010, p. 69–82 [Google Scholar]
- 30.Hivert MF, Jablonski KA, Perreault L, et al. DIAGRAM Consortium. Diabetes Prevention Program Research Group Updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the Diabetes Prevention Program. Diabetes 2011;60:1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosado EL, Bressan J, Martins MF, Cecon PR, Martínez JA. Polymorphism in the PPARgamma2 and beta2-adrenergic genes and diet lipid effects on body composition, energy expenditure and eating behavior of obese women. Appetite 2007;49:635–643 [DOI] [PubMed] [Google Scholar]
- 32.Thanassoulis G, O’Donnell CJ. Mendelian randomization: nature’s randomized trial in the post-genome era. JAMA 2009;301:2386–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins F. Has the revolution arrived? Nature 2010;464:674–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green RC, Roberts JS, Cupples LA, et al. REVEAL Study Group Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med 2009;361:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA 2008;299:1320–1334 [DOI] [PubMed] [Google Scholar]
- 36.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med 2011;364:524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verrilli DW, Welch HG. The impact of diagnostic testing on therapeutic interventions. JAMA 1996;275:1189–1191 [PubMed] [Google Scholar]
- 38.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011;364:1144–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]