Abstract
OBJECTIVE
To test whether early, insulin-mediated microvascular recruitment in skeletal muscle predicts steady-state glucose metabolism in the setting of physiological elevation of free fatty acid concentrations.
RESEARCH DESIGN AND METHODS
We measured insulin’s microvascular and metabolic effects in 14 healthy young adults during a 2-h euglycemic insulin clamp. Plasma free fatty acid concentrations were raised (Intralipid and heparin infusion) for 3 h before the clamp and maintained at postprandial concentrations during the clamp. Microvascular blood volume (MBV) was measured by contrast-enhanced ultrasound (CEU) continuously from baseline through the first 30 min of the insulin clamp. Muscle glucose and insulin uptake were measured by the forearm balance method.
RESULTS
The glucose infusion rate (GIR) necessary to maintain euglycemia during the clamp varied by fivefold across subjects (2.5–12.5 mg/min/kg). The early MBV responses to insulin, as indicated by CEU video intensity, ranged widely, from a 39% decline to a 69% increase. During the clamp, steady state forearm muscle glucose uptake and GIR each correlated significantly with the change in forearm MBV (P < 0.01). To explore the basis for the wide range of vascular and metabolic insulin sensitivity observed, we also measured Vo2max in a subset of eight subjects. Fitness (Vo2max) correlated significantly with the GIR, the forearm glucose uptake, and the percentage change in MBV during the insulin clamp (P < 0.05 for each).
CONCLUSIONS
Early microvascular responses to insulin strongly associate with steady state skeletal muscle insulin-mediated glucose uptake. Physical fitness predicts both metabolic and vascular insulin responsiveness.
Insulin recruits underperfused capillaries to increase skeletal muscle microvascular blood volume (MBV), as measured by contrast-enhanced ultrasound (CEU), within 20 min in both rats (1) and humans (2,3). This effect occurs with physiological insulin concentrations (2,4) and precedes both changes in total limb blood flow (1,5,6) and insulin’s metabolic action (1). In rodents, microvascular recruitment enhances the rate at which insulin is delivered to muscle interstitium (7), thereby facilitating insulin’s metabolic action, and exercise training has been shown to enhance insulin-induced microvascular recruitment and muscle glucose disposal in rodents (8).
Raising plasma concentrations of free fatty acids (FFAs) induces insulin resistance within 2–4 h, can induce inflammation in muscle (9) and in circulating leukocytes (10), and produces endothelial dysfunction (10,11). Clinical studies have shown a marked impairment in insulin’s ability to recruit both muscle and skin microvasculature in chronically insulin-resistant obese subjects (12–14). FFA-induced insulin resistance impairs insulin-mediated microvascular recruitment in skin with elevation of FFA to physiological levels (∼1 mmol/L) (15) and in muscle microvasculature with higher FFA levels ∼3 mmol/L (16).
Both acute exercise and training can affect the metabolic response to raising plasma FFA. Raising plasma FFA acutely through lipid and heparin infusion has less effect on insulin sensitivity in individuals who exercised intensively the preceding day (17). Exercise training also prevents FFA-induced hepatic and peripheral insulin resistance (18). It is not known whether training affects insulin-induced microvascular recruitment or the ability of FFA to inhibit recruitment in humans.
Recently, we reported that human skeletal muscle insulin uptake (product of forearm blood flow and arteriovenous concentration) could be quantified and that it occurred through a saturable transport process at physiological concentrations of insulin (2). Whether FFA elevation would, by blocking insulin-induced increases in MBV, also limit muscle insulin uptake is not known.
In this study, CEU was used to measure muscle microvascular perfusion and paired arterial and venous sampling to measure muscle insulin and glucose uptake in response to a physiologic insulin infusion in 14 healthy volunteers whose plasma FFA levels were maintained in a range encountered in human insulin-resistant states (∼1.0 mmol/L). To examine whether fitness was predictive of these responses, a subset of 8 volunteers underwent maximal exercise testing to quantify the relationship between Vo2max and muscle metabolic and microvascular insulin sensitivity.
RESEARCH DESIGN AND METHODS
Studies were performed in 14 lean (BMI 22 ± 1 kg/m2), healthy volunteers (7 male and 7 female), aged 18–35 years without a personal or family history of hypertension or diabetes and receiving no medications. The studies were not scheduled to correspond to a particular phase of the menstrual cycle in the 7 females because insulin clamp–measured insulin sensitivity in lean, healthy young women is not significantly affected by cycle phase (19). The study protocol was approved by the University of Virginia institutional review board, and each subject gave written consent. All studies were performed in the General Clinical Research Center.
All subjects had normal results of physical examination, liver function tests, fasting glucose, and lipid profile. Eight of the 14 subjects performed a treadmill exercise test after an overnight fast, with the standard Bruce protocol used to determine Vo2max. Each participant began walking at an initial velocity of 60 m/min, with velocity increasing by 10 m/min every 3 min (the duration of each stage) until volitional exhaustion. Metabolic measures were obtained through standard open-circuit spirometry (Viasys Vmax 229; CareFusion, Yorba Linda, CA). Vo2 peak was determined as the highest 1-min oxygen consumption value obtained.
For the insulin clamp, each of the 14 subjects was admitted to the General Clinical Research Center the evening before the study. After an overnight 12-h fast, volunteers were studied while supine according to the following protocol: A brachial arterial catheter and a retrograde median antecubital venous catheter were placed for blood sampling. In the contralateral arm, a venous catheter was placed for the infusion of lipid, glucose, insulin and Definity microbubbles. An infusion of 20% lipid emulsion (Abbott Laboratories, Chicago, IL) was initiated at a rate of 45 mL/h for 1 h and then at 30 mL/h for 4 h. A second venous catheter was placed in the same arm for an infusion of heparin at a rate of 0.2 units/kg/min. Both these infusions continued for 5 h. After 150 min of lipid infusion, paired arterial and venous samples were taken every 10 min three times for measurement of plasma glucose, insulin, FFAs, and lactate. Forearm blood flow was measured after each set of arterial and venous samples by Doppler ultrasound. At 175 min of lipid infusion, CEU measurements of forearm muscle MBV were initiated and continued for 40 min as described below.
Euglycemic insulin clamp
At time 180 min, a primed 3 mU/min/kg insulin infusion was started in the arm contralateral to the arterial catheter. This infusion was decreased by 0.2 mU/min/kg each min during the next 10 min and then maintained at a rate of 1 mU/kg/min for the next 110 min. Arterial plasma glucose was maintained at basal levels with a variable rate 20% glucose infusion (euglycemic clamp) (20). Whole-body glucose disposal at steady state (80–120 min of the clamp) was estimated from the glucose infusion rate (GIR) required to keep arterial glucose constant. Forearm glucose and FFA balances (net uptake or release) were determined from the arteriovenous concentration difference obtained every 10 min from 150 to 300 min of lipid infusion. To avoid interference with the CEU images, no arterial or venous samples were collected from 180 to 210 min of lipid infusion.
MBV was measured with a SONOS 7500 ultrasound system (Philips Medical Systems, Bothell, WA) with harmonic imaging during the continuous infusion of perfluorocarbon gas–filled lipid microbubbles (Definity; Lantheus Medical Imaging Co., Billerica, MA), as described previously (2). CEU images were downloaded to an off-line image analysis system (Q-Laboratory; Philips Medical Systems, Andover, MA). Background-subtracted acoustic intensity was measured from a region of interest around the deep forearm flexor muscles, as described previously (12,21). Changes in MBV with time during insulin exposure were calculated from the acoustic intensity expressed as mean decibels.
Brachial artery blood flow was measured at baseline and every 20 min from 40 to 120 min of the insulin clamp with the SONOS 7500 ultrasound system with a linear-array transducer and a transmit frequency of 12 MHz. Two-dimensional imaging of the brachial artery was performed in the long axis approximately 10 cm proximal to the antecubital fossa. Images were triggered to the R wave of the cardiac cycle, and the brachial artery diameter was measured with online video calipers. At the same location, the time average mean blood velocity was measured with pulsed-wave Doppler ultrasound. Brachial artery mean blood flow was calculated according to the following equation: Q = v ⋅ π(d/2)2, where Q is brachial blood flow, v is mean brachial artery blood flow velocity, and d is brachial artery diameter.
Insulin was measured with a solid-phase two-site chemiluminescent assay (Diagnostic Products Corporation, Los Angeles, CA). The FFA level was measured with a colorimetric assay (Waco Diagnostics, Richmond, VA). Glucose and lactate were measured in duplicate with a YSI 2300 analyzer (Yellow Springs Instruments, Yellow Springs, OH). Baseline coagulation parameters, liver function tests, and fasting lipid profile were performed by standard assays in the University of Virginia Clinical Chemistries Laboratory.
Forearm balances for glucose, FFAs, and insulin were calculated as follows: balance = ([A] – [V]) ⋅ F, where [A] and [V] are arterial and venous concentrations and F is forearm blood flow in milliliters per minute per 100 mL forearm volume. A positive balance corresponded to a net uptake, whereas a negative balance signaled a net release of substrate. For calculation of glucose balance, blood flow was used; for FFA and insulin, we used forearm plasma flow, derived as blood flow ⋅ (1 – Hematocrit). The clearance of insulin was calculated as the product of the extraction fraction of insulin, derived as ([A] – [V])/[A], and forearm plasma flow per 100 mL forearm volume.
Statistical analysis
Data are presented as means ± SE. Comparisons were made by paired Student t test for the following: between mean baseline (−30 to −10 min) and mean steady state (80 to 120 min) values for forearm glucose uptake (FGU), forearm insulin uptake (FIU), FFA balance, insulin clearance and total forearm blood flow; between baseline and 25 min for CEU acoustic intensity; between 0 and 30 min for arterial FFA concentration; and between the highest and lowest tertile of percentage MBV change. Pearson product-moment correlation coefficient was computed to determine the relationship between specific variables. For all analyses, P < 0.05 was considered statistically significant. Statistics were calculated with Sigmastat 3.2 (Systat Co., Richmond, CA).
RESULTS
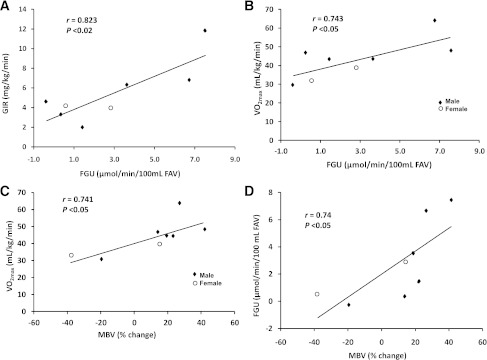
Table 1 gives the clinical characteristics of all 14 subjects studied broken down into the two groups who either did not (group 1) or did (group 2) have Vo2max measured. All were normotensive, were nonobese, and had normal values for serum lipids. Before beginning the insulin clamp, the 3-h Intralipid and heparin infusion had raised the arterial plasma FFA concentration to 2.0 ± 0.2 mmol/L. Plasma glucose averaged 5.1 ± 0.1 mmol/L, and forearm blood flow was 6.5 ± 0.4 mL/min/100 mL. The basal forearm glucose and FFA balances averaged 0.65 ± 0.1 and −0.1 ± 0.2 μmol/min/100 mL, respectively. The basal arterial insulin concentration was 37 ± 5 pmol/L, which significantly (P < 0.001) exceeded that in the forearm venous blood (32 ± 4 pmol/L), resulting in a significant FIU of 17.8 ± 3.0 fmol/min/100 mL. Basal forearm insulin clearance averaged 0.49 ± 0.07 mL/min/100 mL.
Table 1.
The measured phenotypic characteristics of all subjects studied
The intravenous insulin infusion raised arterial insulin concentrations from 36 to 251 ± 11 pmol/L during the 120 min of hyperinsulinemia. Arterial glucose averaged 5.1 ± 0.8 mmol/L during the baseline period and was maintained within 5% of baseline throughout. Arterial plasma FFA concentrations had declined sharply by 30 min of insulin infusion (P < 0.001) and plateaued at 1.1 mmol/L by 80 min. Forearm blood flow was unchanged from basal during the insulin infusion (6.5 ± 0.4 vs. 6.7 ± 0.5 mL/min/100 mL).
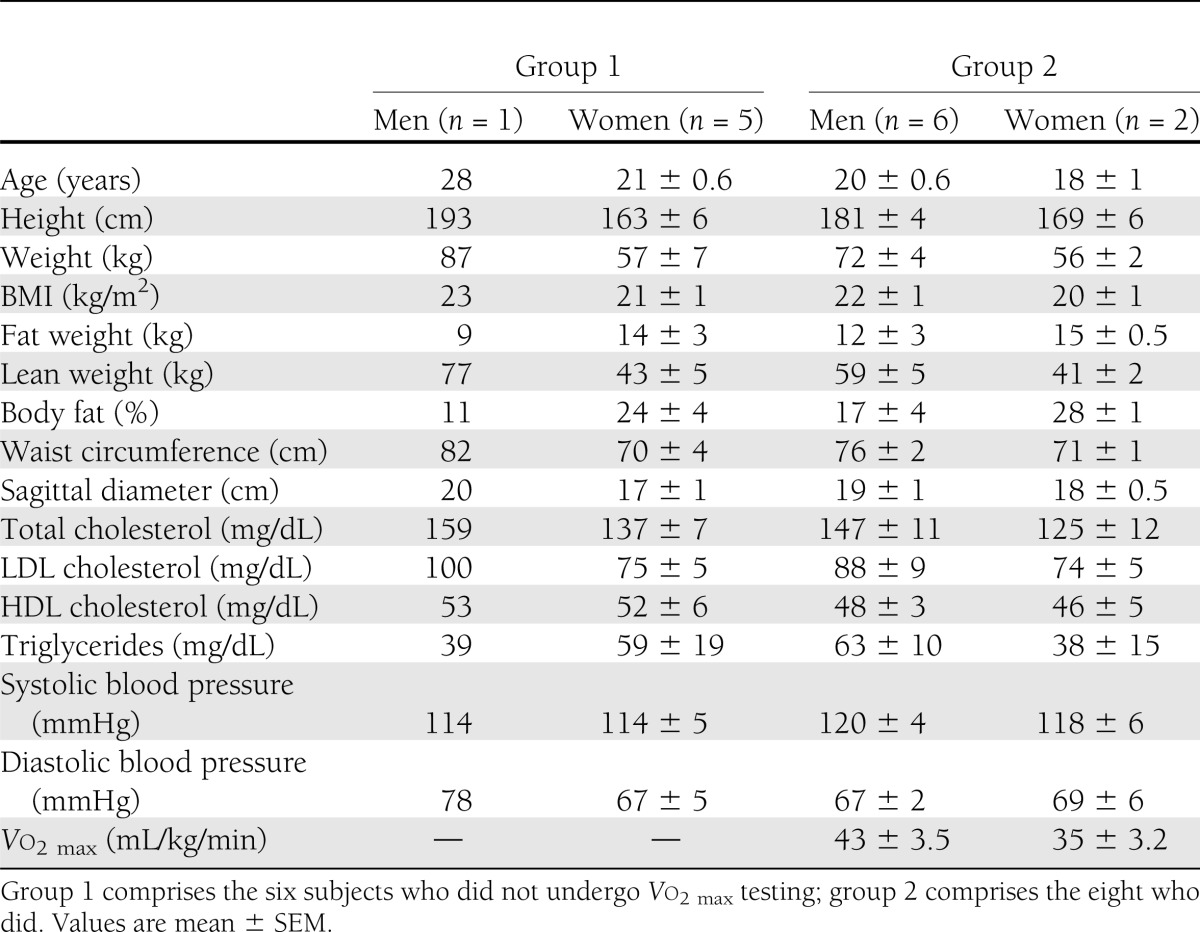
FGU during the last 40 min of the insulin infusion averaged approximately sixfold the basal value (0.65 ± 0.1 vs. 3.8 ± 0.8 μmol/min/100mL; P < 0.01) and ranged across subjects from 0.2 to 9.9 μmol/min/100 mL. FFA balance was unchanged (−0.1 ± 0.2 vs. 0.1 ± 0.3 μmol/min/100 mL). The whole-body GIR during the last 40 min of the insulin clamp ranged from 11 to 68 μmol/kg/min (average 31.3 ± 4.6 μmol/kg/min). There was the expected strong correlation (r = 0.876; P < 0.001) between FGU and the GIR (Fig. 1A), each measured during the last 40 min of the clamp, underscoring the role of skeletal muscle in body glucose disposal under hyperinsulinemic conditions. FIU also rose significantly during hyperinsulinemia (18 ± 3 to 80 ± 12 fmol/min/100mL; P < 0.01), whereas forearm clearance of insulin trended downward (0.49 ± 0.09 vs. 0.33 ± 0.05 ml/min/100 mL; P = 0.14).
Figure 1.
A: The correlation between the GIR and FGU, each measured during the last 40 min of the euglycemic insulin clamp. B: The correlation between FGU measured during the last 40 min of the insulin clamp and the percentage change of MBV measured during the first 30 min of insulin infusion. C: The correlation of GIR measured during the last 40 min of the insulin clamp with the percentage change in MBV during the initial 30 min of insulin infusion. FAV, forearm volume.
On average, there was no change in the MBV observed between baseline and 30 min of insulin infusion (5.4 ± 1.0 vs. 5.8 ± 1.1 acoustic intensity units), consistent with FFA elevation blocking the vascular effect of insulin to increase MBV. We did, however, observe that the microvascular responses varied considerably across individuals, from a 39% decline to a 69% increase in MBV. Furthermore, there was a strong correlation between the increase in FGU and the percentage change in MBV (r = 0.80; P < 0.01), consistent with a positive relationship between perfusion volume and the metabolic effect of insulin (Fig. 1B). Likewise, there was a significant correlation between the GIR and percentage change in MBV (Fig. 1C). There was no significant correlation between forearm blood flow and GIR (r = 0.16; P = NS). Likewise, we found no correlation between percentage change in MBV and the plasma FFA concentration measured during the first hour of the insulin clamp (r = 0.09; P = NS).
This study was not powered to address whether there was an effect of sex on this response. We did, however, observe a significant correlation between percentage change in MBV and FGU in both women (r = 0.77; P < 0.05) and men (r = 0.88; P < 0.01) and a correlation between percentage change in MBV and whole-body GIR that was not significant in women (r = 0.60; P = NS) although it was nearly significant in men (r = 0.74; P = 0.06). This suggests that the relationship between MBV and muscle glucose uptake holds for both sexes.
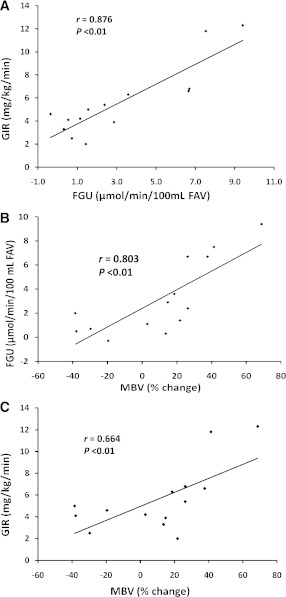
Comparing the five subjects in the highest tertile with the five in the lowest tertile of MBV percentage change, we found that FGU was markedly higher (6.5 ± 1.2 vs. 0.7 ± 0.3 μmol/min/100 mL; P < 0.01) in the group that had the more responsive microvasculature (Fig. 2). FIU averaged nearly threefold greater in that group(92 ± 29 vs. 32 ± 7 fmol/min/100 mL; P = 0.08), but this difference was of borderline significance (Fig. 2).
Figure 2.
A: The mean ± SEM of FGU observed in the five individuals who either had no increase in MBV or had an actual decline (lowest tertile) versus that in the five individuals who had the greatest percent increase in MBV (highest tertile). B: The mean ± SEM changes in FIU between baseline and the last 40 min of the insulin clamp in the same two groups. The P values were determined by unpaired t tests. FAV, forearm volume.
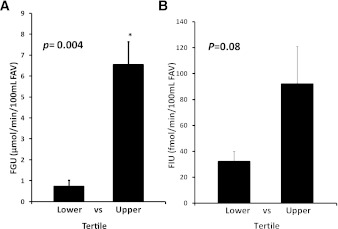
Of the 14 subjects, 8 agreed to have Vo2 max measured on a separate day from the clamp study. The mean Vo2max was 43 ± 4 mL/min/kg and ranged from 29 to 63 mL/min/kg. Compared with the other 6 subjects there were no differences in these 8 in BMI, age, fasting insulin or glucose, or the plasma concentrations of FFA (1.05 ± 0.16 vs. 1.14 ± 0.13 mmol/L), insulin (250 ± 15 vs. 252 ± 18 pmol/L), or glucose (4.9 ± 0.1 vs. 5.1 ± 0.1 mmol/L) during the last 40 min of the clamp. We observed that in this subgroup the ranges of responses to insulin of GIR (2.0–12 mg/min/kg), FGU (−0.1 to +8.0 mmol/min/100 mL), and MBV percentage change (−40 to +40) were comparable to those of the group as a whole (Fig. 1A–C ). In this subgroup there was again the expected correlation (r = 0.823; P < 0.02) between FGU and GIR (Fig. 3A). In these subjects there were significant correlations between Vo2max and FGU (Fig. 3B) and between Vo2max and percentage change in MBV (Fig. 3C). Finally, in this subgroup we again found a significant correlation (r = 0.743; P < 0.05) between the percentage change in MBV and FGU (Fig. 3D). In contrast, there was no correlation between changes in blood flow (either absolute or percentage change from basal) and GIR or Vo2max, suggesting that under these experimental conditions regulation of MBV is more closely linked than is total blood flow to insulin’s metabolic effect.
Figure 3.
A: The correlation between whole-body GIR and FGU, each measured during the last 40 min of the insulin clamp, in eight individuals in whom fitness was assessed by Vo2max. B: The correlation between the Vo2max and FGU in the same individuals. C: The correlation between Vo2max and the percentage change in MBV seen during the first 30 min of insulin infusion in the same individuals. D: The correlation between FGU measured during the last 40 min of the insulin clamp and the percentage change of MBV measured during the first 30 min of the insulin infusion in the same individuals. FAV, forearm volume.
CONCLUSIONS
Previously, we reported that euglycemic hyperinsulinemia significantly enhanced forearm MBV in healthy humans (3) and that metabolic insulin resistance, such as occurs with obesity (12) and with lipid infusion (16), blunts insulin’s action to increase MBV. In those studies we did not directly measure muscle glucose uptake, however, and MBV was measured at baseline and after 2 h of hyperinsulinemia. Because insulin’s microvascular action in muscle occurs within 15–30 min (2) of infusion and because we (22) and others (23,24) have hypothesized that insulin’s access to muscle interstitium is rate limiting for insulin’s metabolic action in muscle, we wanted to compare early insulin-induced changes in MBV with subsequent muscle glucose metabolism and to do so in the setting of physiological FFA elevation to levels observed in the postprandial state in insulin-resistant individuals. In this study, changes in MBV were measured during the first 30 min of hyperinsulinemia and forearm glucose metabolism between 80 and 120 min. In the current study, by maintaining postprandial plasma FFA concentrations (∼1.1 mmol/L), we found that both muscle MBV and FGU varied over a wide range in healthy young adults. Most intriguingly, there was a strong correlation between insulin’s early microvascular action and subsequent metabolic action in muscle, underscoring the physiological importance of microvascular insulin sensitivity to muscle glucose metabolism. Beyond that, we noted that the level of fitness appeared to impact both microvascular and metabolic responses to insulin during the lipid infusion. This is of particular interest in light of recent reports that both an acute bout of endurance exercise (17) and overall fitness (18,25) interfere with the ability of lipid infusions to diminish insulin sensitivity. This suggests that muscle microvasculature, like muscle itself, responds to exercise and training to preserve insulin responsiveness.
FFAs are thought to induce muscle insulin resistance at least in part through the activation of an inflammatory response (9), which itself may result from increased oxidative stress (26). In humans, acutely raising plasma FFA level (as was done here) has been observed to enhance nuclear factor-κB activity in circulating mononuclear cells and plasma concentrations of macrophage migration inhibition factor, consistent with an acute inflammatory response. This was accompanied by a decrease in brachial artery flow-mediated dilation consistent with an impact of raised FFA level, with or without inflammation, on endothelial function (10). Exercise has repeatedly been shown to increase production of reactive oxygen species; however this reactive oxygen species production appears to play a synergistic role in activating and regulating antioxidant pathways (27), including manganese superoxide dismutase (28), glutathione peroxidase (29), and heme oxygenase-1 (30). This tightly regulated bidirectional redox signaling appears to occur in part through the NF-κB and mitogen-activated protein kinase (27) signal transduction pathways. The observation that fitness mitigates the inhibitory effect of FFAs on muscle’s microvascular response to insulin suggests that the muscle vasculature of fit volunteers has developed a capacity to protect against oxidative stress induced by FFA infusion. Insulin’s action to enhance microvascular perfusion is dependent on nitric oxide production. FFAs have been shown in rats to impair endothelial cell nitric oxide production acting through the inhibitor of κB kinase β pathway (31) and to impair insulin-induced nitric oxide production and leg blood flow changes in humans (32). We have shown that insulin’s effect to increase MBV is blocked by inhibition of nitric oxide synthase. The greater response of MBV to insulin in fit individuals seen here suggests that fitness may abrogate the effect of FFAs to inhibit vascular nitric oxide production.
In the current study, we observed a significant uptake of insulin by forearm muscle under both basal and hyperinsulinemic conditions. The basal FIU and clearance of insulin observed here were not different than we reported previously in healthy controls not receiving lipid (2). Likewise, insulin uptake by muscle during the clamp was comparable to that which we reported earlier (2). We noted however that there was a wide range of FIU among subjects. As was seen with glucose, there appeared to be greater uptake among persons who responded to insulin by increasing MBV (Fig. 1). Among the 8 subjects who had Vo2max measured, the mean rate of FIU during the last 40 min of the insulin clamp ranged from 22 to 112 fmol/min/100 mL. We divided these 8 subjects into two groups, four with high Vo2max and four with low Vo2max (average 50 ± 4 vs. 36 ± 3 mL/min/kg) and compared FIU rates. FIU during the clamp was nearly twofold greater in the 4 physically fit individuals (82 ± 16 vs. 46 ± 9; P = 0.06). This suggests that enhanced insulin delivery in physically fit individuals may contribute to the increased skeletal muscle insulin sensitivity seen with increasing fitness.
As noted in results, FGU was much greater in subjects with good microvascular responses to insulin, as reflected by increases in MBV. In four subjects the MBV actually declined below basal level during insulin infusion. We had previously observed this behavior during Intralipid infusion in rat studies and found that the decline could be prevented by coinfusion of BQ123, an endothelin A receptor blocker (33). This led us to suggest it may be due to selective inhibition by FFAs of endothelial nitric oxide synthase activation with preservation of insulin’s action to increase endothelin 1 production in the microvasculature, as has been observed in the Zucker (fa/fa) rat (34) and in several in vitro studies (35,36). A similar decrease in microvascular perfusion was reported for human cardiac muscle in response to meal ingestion in diabetic patients but was not seen in healthy volunteers (37).
A limitation of the current study is that we do not have measures of MBV before beginning the lipid infusion. This is due to the limitation of the amount of Definity that can be infused in humans during a single study. In rats, Intralipid with heparin infusion alone did not increase MBV (33). Another limitation relates to whether fitness per se or some other lifestyle difference associated with fitness explains the correlation between Vo2max and insulin-induced changes in MBV.
In summary, we have observed that during mild, physiological increases in plasma FFA concentrations, both metabolic and vascular insulin sensitivities vary widely in otherwise healthy humans. Early microvasculature recruitment correlates strongly with subsequent muscle glucose uptake. This is consistent with a role for insulin’s microvascular action in modulating insulin’s metabolic action in muscle. Impaired microvascular responses may also diminish muscle insulin uptake, perhaps accounting in part for the muscle insulin resistance seen. Finally, physical fitness appears to blunt the inhibitory effect of raising plasma FFA on insulin-induced muscle microvascular recruitment and glucose.
Acknowledgments
This work was supported by National Institutes of Health grants DK-073759 (E.J.B.) and RR-00847 (University of Virginia General Clinical Research Center).
No potential conflicts of interest relevant to this article were reported.
E.M.E. and L.A.J. participated equally in the design and conduct of the studies and data analysis. E.J.B. participated in the design, conduct, data analysis, and manuscript preparation and provided grant support for the study. E.J.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
References
- 1.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–1423 [DOI] [PubMed] [Google Scholar]
- 2.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 2007;56:2958–2963 [DOI] [PubMed] [Google Scholar]
- 3.Coggins MP, Lindner J, Rattigan S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001;50:2682–2690 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Vincent MA, Richards SM, et al. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 2004;53:447–453 [DOI] [PubMed] [Google Scholar]
- 5.Yki-Järvinen H, Utriainen T. Insulin-induced vasodilatation: physiology or pharmacology? Diabetologia 1998;41:369–379 [DOI] [PubMed] [Google Scholar]
- 6.Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol 1996;271:E1067–E1072 [DOI] [PubMed] [Google Scholar]
- 7.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 2007;56:2194–2200 [DOI] [PubMed] [Google Scholar]
- 8.Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes 2001;50:2659–2665 [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 2001;108:437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003;52:2882–2887 [DOI] [PubMed] [Google Scholar]
- 11.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 1997;100:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006;55:1436–1442 [DOI] [PubMed] [Google Scholar]
- 13.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 2009;32:1672–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jongh RT, Ijzerman RG, Serné EH, et al. Visceral and truncal subcutaneous adipose tissue are associated with impaired capillary recruitment in healthy individuals. J Clin Endocrinol Metab 2006;91:5100–5106 [DOI] [PubMed] [Google Scholar]
- 15.de Jongh RT, Serné EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 2004;53:2873–2882 [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 2009;94:3543–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 2007;117:1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haus JM, Solomon TPJ, Marchetti CM, Edmison JM, González F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 2010;95:323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yki-Järvinen H. Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab 1984;59:350–353 [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 21.Vincent MA, Clerk LH, Lindner JR, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006;290:E1191–E1197 [DOI] [PubMed] [Google Scholar]
- 22.Barrett EJ, Eggleston EM, Inyard AC, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009;52:752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 1989;84:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prager R, Wallace P, Olefsky JM. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest 1986;78:472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon TPJ, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab 2009;297:E552–E559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic Biol Med 2008;44:142–152 [DOI] [PubMed] [Google Scholar]
- 28.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 2000;105:1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol 1994;267:R439–R445 [DOI] [PubMed] [Google Scholar]
- 30.Niess AM, Sommer M, Schneider M, et al. Physical exercise-induced expression of inducible nitric oxide synthase and heme oxygenase-1 in human leukocytes: effects of RRR-alpha-tocopherol supplementation. Antioxid Redox Signal 2000;2:113–126 [DOI] [PubMed] [Google Scholar]
- 31.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 2005;25:989–994 [DOI] [PubMed] [Google Scholar]
- 32.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 2000;49:1231–1238 [DOI] [PubMed] [Google Scholar]
- 33.Inyard AC, Chong DG, Klibanov AL, Barrett EJ. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes 2009;58:2457–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 1999;104:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 2002;56:464–471 [DOI] [PubMed] [Google Scholar]
- 36.Bakker W, Sipkema P, Stehouwer CD, et al. Protein kinase C theta activation induces insulin-mediated constriction of muscle resistance arteries. Diabetes 2008;57:706–713 [DOI] [PubMed] [Google Scholar]
- 37.Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation 2005;112:179–184 [DOI] [PubMed] [Google Scholar]