Abstract
OBJECTIVE
We aimed to examine insulin clearance, a compensatory mechanism to changes in insulin sensitivity, across sex, race/ethnicity populations, and varying states of glucose tolerance.
RESEARCH DESIGN AND METHODS
We measured insulin sensitivity index (SI), acute insulin response (AIR), and metabolic clearance rate of insulin (MCRI) by the frequently sampled intravenous glucose tolerance test in 1,295 participants in the Insulin Resistance Atherosclerosis Study.
RESULTS
MCRI was positively related to SI and negatively to AIR and adiposity across sex, race/ethnicity populations, and varying states of glucose tolerance, adiposity, and family history of diabetes. Differences in MCRI by race/ethnicity (lower in African Americans and Hispanics compared with non-Hispanic whites) and glucose tolerance were largely explained by differences in adiposity, SI, and AIR.
CONCLUSIONS
Insulin sensitivity, insulin secretion, and adiposity are correlates of insulin clearance and appear to explain differences in insulin clearance by race/ethnicity and glucose tolerance status.
Reduced insulin clearance has been demonstrated in experimental models of insulin resistance (1) and conditions associated with insulin resistance (2–5). Insulin clearance partially explains the variability of fasting insulin independently of the effect of insulin resistance, insulin secretion, adiposity, and plasma glucose (6). In response to their higher insulin resistance, minority populations have lower insulin clearance than non-Hispanic whites (4,5,7). In these studies, however, results were not adjusted for insulin resistance. Therefore, we aimed to examine insulin clearance across sex, race/ethnicity populations, and varying states of glucose tolerance in the Insulin Resistance Atherosclerosis Study (IRAS) (8).
RESEARCH DESIGN AND METHODS
The design and methods of the IRAS have previously been described in detail (8). The present report includes information on 1,295 participants, none of whom were treated with glucose-lowering medications.
Insulin sensitivity, insulin secretion, and insulin clearance were measured by the frequently sampled intravenous glucose tolerance test. Insulin sensitivity, expressed as the insulin sensitivity index (SI), was calculated using mathematical modeling methods (MINMOD version 3.0 [1994]; Harms Software, Los Angeles, CA). Acute insulin secretion (AIR) was calculated as the mean of 2- and 4-min insulin concentrations after glucose administration. MCRI was calculated as the ratio of the insulin dose over the incremental area under the curve of insulin from 20 min to infinity (9).
Statistical analyses were performed using the SAS statistical software (version 9.2; SAS Institute, Cary, NC). Means ± SE or proportions (95% CI) were calculated by one-way ANCOVA or logistic regression to account for the effect of covariates (age, sex, race/ethnicity, and clinic). Pearson correlation analysis was used to examine the relationship of MCRI with SI, AIR, fasting insulin, and waist circumference. Independent relationships of relevant variables with MCRI were established using the GENMOD procedure to account for the effect of categorical and standardized continuous variables. Log-transformed values of age, insulin, SI, AIR, and MCRI were used to meet statistical assumptions.
RESULTS
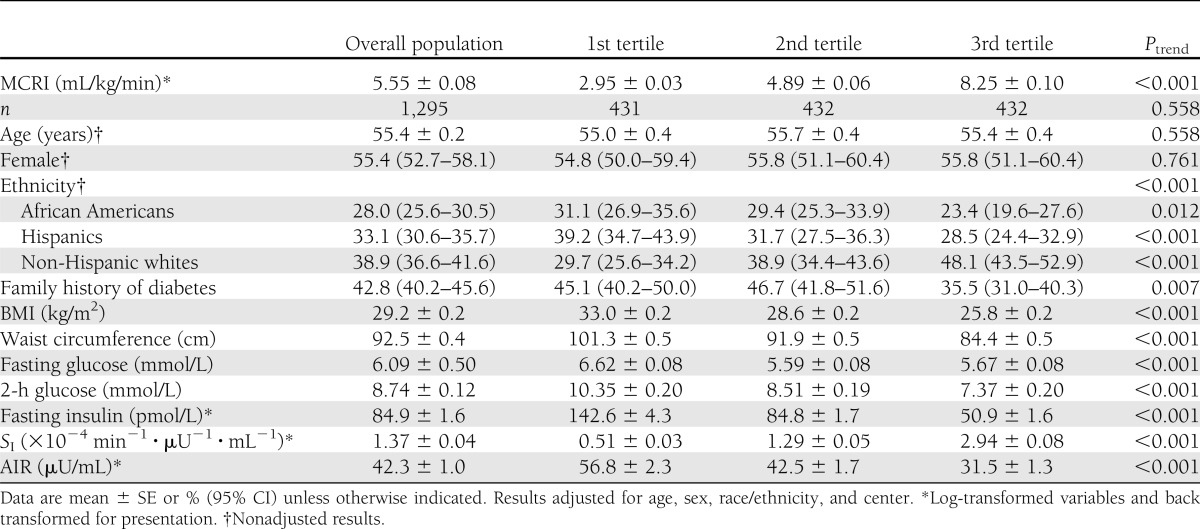
MCRI was not related to age and sex (Table 1). Minority populations and family history of diabetes were associated with lower MCRI. MCRI was directly related to SI and inversely to adiposity, plasma glucose, fasting insulin, and AIR. Compared with impaired glucose tolerance, MCRI was higher in normal glucose tolerance (5.64 ± 0.10 vs. 4.53 ± 0.12 mL/kg/min, P < 0.001) and lower in diabetes (3.94 ± 0.11 mL/kg/min, P < 0.001).
Table 1.
Characteristics in the overall population by tertiles of MCRI
MCRI correlated directly with SI in both nondiabetic (r = 0.77) and diabetic (r = 0.58) individuals and inversely with fasting insulin (r = −0.69 and −0.64, respectively), waist circumference (r = −0.56 and −0.46), AIR (r = −0.47 and −0.61) (Supplementary Fig. 1).
The relation of race/ethnicity, family history of diabetes, and glucose tolerance to MCRI was no longer statistically significant after adjustment for BMI, waist circumference, SI, and AIR (Supplementary Table 1). BMI (β −7.2 ± 1.7, P < 0.001), waist circumference (β −3.9 ± 1.8, P < 0.05), SI (β 27.4 ± 1.1, P < 0.001), and AIR (β −12.5 ± 0.9, P < 0.001) were independently related to MCRI. The relation of BMI, SI, and AIR to MCRI was consistent across sex, race/ethnicity populations, and varying states of glucose tolerance, adiposity, and family history of diabetes (Supplementary Table 2).
CONCLUSIONS
Our study has several novel findings: 1) insulin clearance is not associated with age or sex; 2) insulin sensitivity, insulin secretion, and adiposity are independently related to insulin clearance across sex, race/ethnic populations, varying states of glucose tolerance and adiposity, and family history of diabetes; and 3) insulin sensitivity, insulin secretion, and adiposity appear to explain differences in insulin clearance by race/ethnicity and glucose tolerance status.
In the fasting state, the liver clears ~40–60% of the insulin concentration in the portal blood (10). Results from a study of intentional weight gain in men with normal weight (change in BMI from 21.8 to 23.8 kg/m2 in 15 weeks) suggest that reduced insulin clearance may be the most important compensatory mechanism for explaining the increase in basal and stimulated insulin concentrations (11). Insulin clearance has also been described as the first compensatory mechanism to experimental fat-induced insulin resistance (1), even though insulin clearance is not altered by acute hyperglycemia (12). The reduction in insulin clearance enhances glucose uptake and suppress lipolysis by increasing insulin levels. Consequently, it has been hypothesized that insulin clearance is reduced in insulin-resistant states to lessen the demands on the β-cell (1).
In an animal model of alloxan-induced selective decrease in β-cell mass, insulin secretion decreases in proportion to β-cell mass (13). Insulin secretion after meal ingestion is impaired along with worsening of hepatic insulin clearance. Human studies have also shown that skeletal muscle contributes to peripheral insulin clearance (14,15). Physiological hyperinsulinemia recruits skeletal muscle capillaries, but insulin clearance is reduced because of the saturation of the trans endothelial insulin transport (a rate-limiting process for insulin action) (14). Obesity has been shown to impair microvascular recruitment (15). The cross-sectional nature of our study precludes us from making causal inferences. However, our results suggest that there is a complex relationship between insulin secretion and insulin clearance independently of the effect of obesity and insulin sensitivity.
Acknowledgments
This study was supported by grants from the National Heart, Lung, and Blood Institute (HL-47887, HL-47889, HL-47890, HL-47892, HL-47902, and DK-079888) and the General Clinical Research Centers Program (NCRR GCRC, M01 RR431, and M01 RR01346).
No potential conflicts of interest relevant to this article were reported.
C.L. contributed to the study concept and design, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. A.J.G.H. and L.E.W. contributed to discussion and reviewed and edited the manuscript. M.J.R. and D.S. researched data and contributed to discussion. M.O.G. contributed to discussion and reviewed and edited the manuscript. S.M.H. researched data, contributed to the study concept and design, contributed to discussion, and reviewed and edited the manuscript. C.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0101/-/DC1.
References
- 1.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes 2000;49:2116–2125 [DOI] [PubMed] [Google Scholar]
- 2.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 3.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 1983;32:438–446 [DOI] [PubMed] [Google Scholar]
- 4.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 1994;11:755–762 [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Stern MP, Watanabe RM, Bergman RN. Relationship of insulin clearance and secretion to insulin sensitivity in non-diabetic Mexican Americans. Eur J Clin Invest 1992;22:147–153 [DOI] [PubMed] [Google Scholar]
- 6.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab 2011;301:E402–E408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liew CF, Seah ES, Yeo KP, Lee KO, Wise SD. Lean, nondiabetic Asian Indians have decreased insulin sensitivity and insulin clearance, and raised leptin compared to Caucasians and Chinese subjects. Int J Obes Relat Metab Disord 2003;27:784–789 [DOI] [PubMed] [Google Scholar]
- 8.Wagenknecht LE, Mayer EJ, Rewers M, et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 9.Polonsky KS, Pugh W, Jaspan JB, et al. C-peptide and insulin secretion. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest 1984;74:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding PE, Bloom G, Field JB. Effect of infusion of insulin into portal vein on hepatic extraction of insulin in anesthetized dogs. Am J Physiol 1975;228:1580–1588 [DOI] [PubMed] [Google Scholar]
- 11.Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metab 2008;294:E568–E575 [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol 1983;244:E517–E527 [DOI] [PubMed] [Google Scholar]
- 13.Kjems LL, Kirby BM, Welsh EM, et al. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes 2001;50:2001–2012 [DOI] [PubMed] [Google Scholar]
- 14.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 2007;56:2958–2963 [DOI] [PubMed] [Google Scholar]
- 15.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006;55:1436–1442 [DOI] [PubMed] [Google Scholar]