Abstract
OBJECTIVE
To study how type 2 diabetes adversely affects brain volumes, changes in volume, and cognitive function.
RESEARCH DESIGN AND METHODS
Regional brain volumes and ischemic lesion volumes in 1,366 women, aged 72–89 years, were measured with structural brain magnetic resonance imaging (MRI). Repeat scans were collected an average of 4.7 years later in 698 women. Cross-sectional differences and changes with time between women with and without diabetes were compared. Relationships that cognitive function test scores had with these measures and diabetes were examined.
RESULTS
The 145 women with diabetes (10.6%) at the first MRI had smaller total brain volumes (0.6% less; P = 0.05) and smaller gray matter volumes (1.5% less; P = 0.01) but not white matter volumes, both overall and within major lobes. They also had larger ischemic lesion volumes (21.8% greater; P = 0.02), both overall and in gray matter (27.5% greater; P = 0.06), in white matter (18.8% greater; P = 0.02), and across major lobes. Overall, women with diabetes had slightly (nonsignificant) greater loss of total brain volumes (3.02 cc; P = 0.11) and significant increases in total ischemic lesion volumes (9.7% more; P = 0.05) with time relative to those without diabetes. Diabetes was associated with lower scores in global cognitive function and its subdomains. These relative deficits were only partially accounted for by brain volumes and risk factors for cognitive deficits.
CONCLUSIONS
Diabetes is associated with smaller brain volumes in gray but not white matter and increasing ischemic lesion volumes throughout the brain. These markers are associated with but do not fully account for diabetes-related deficits in cognitive function.
Many interrelated factors adversely affect the brain health of individuals with type 2 diabetes, including energy dysregulation, inflammation, reduced perfusion, and increased oxidative stress and protein deposition (1,2). In cross-sectional studies, type 2 diabetes is often associated with smaller brain volumes and, less consistently, with greater amounts of white matter hyperintensities and other markers of cerebrovascular disease (3,4). Two longitudinal magnetic resonance imaging (MRI) studies have documented increased rates of total brain atrophy, which appeared to be greater among individuals with lower cognitive function, but not increased accumulations of white matter hyperintensities (5,6). The relative increases in atrophy associated with diabetes remained after covariate adjustment for many risk factors for cognitive dysfunction, including age, blood pressures, education, lipid levels, and BMI.
This article is organized with three aims. In a large cohort of older women, we first describe the cross-sectional associations that diabetes had with brain tissue volumes and ischemic lesion loads. We do so for the whole brain and separately for white matter, gray matter, and major lobes. Second, among the women assessed longitudinally with MRI, we examine whether changes in brain tissue and ischemic lesion volumes varied according to diabetes status. Finally, we examine associations that brain volumes and ischemic lesion volumes had with global cognitive function and its subdomains and examine the degree to which they account for diabetes-related relative deficits. There has been one report that diabetes is associated with greater adverse effects on changes in brain structure among women than men (5), perhaps because diabetes often co-occurs with relatively more vascular risk factors among women with versus without diabetes than is the case among men (7). We analyze data from the first sufficiently large cohort of women to characterize the extent to which diabetes-related brain changes account for relative cognitive deficits.
RESEARCH DESIGN AND METHODS
This article draws data from the MRI component of the Women’s Health Initiative (WHI) Memory Study (WHIMS-MRI). Volunteers were recruited from 14 U.S. academic centers. These women had participated in the Women’s Health Initiative Memory Study (WHIMS), an ancillary study to the WHI (which consisted of parallel, placebo-controlled, randomized clinical trials of 0.625 mg/day conjugated equine estrogens with and without 2.5 mg/day medroxyprogesterone acetate in women with a uterus or posthysterectomy) (8). At enrollment into WHIMS, women were 65 to 80 years of age and free of dementia.
WHIMS-MRI was designed to contrast MRI outcomes among women who had been assigned to active versus placebo therapy (9–11). Exclusion criteria included presence of pacemakers, other implants, and foreign bodies, along with other contraindications to MRI. These women’s mean age at scanning, which occurred in 2005–2007, was 78.5 (SD 3.7) years. In 2008–2010, the women were invited to return for a second MRI. Once eligibility had been reconfirmed, scans were repeated according to an identical protocol. Written, informed consent was obtained for each MRI; the National Institutes of Health and the institutional review boards of participating institutions approved the protocols and consent forms.
Diabetes
At WHI enrollment, women self-reported a history of diabetes when not pregnant or of diabetes treatment. Fasting blood glucose was determined for a 5% sample. During WHI follow-up, women were periodically queried about diabetes treatment, and they brought their prescription medications to clinic visits to be recorded 1, 3, 6, and 9 years after enrollment (12). Women were classified as having diabetes on the basis of self-report of diabetes, self-report of diabetes treatment, or, for those with fasting glucose measurements, levels exceeding 126 mg/dL. One woman who reported diabetes onset before the age of 30 years and who was currently taking insulin was considered to have probable type 1 diabetes and therefore excluded from this analysis.
Risk factors for MRI outcomes
Risk factors for declines in structural brain MRI outcomes included in our analyses were those that may also be related to risk or co-occurrence of diabetes: age, education, race or ethnicity, BMI, waist girth, alcohol use, blood pressure, previous cardiovascular disease, previous stroke, and WHI treatment assignment. These were collected from self-reports and standardized assessments during WHI enrollment and annual follow-up visits at schedules that varied among measures. We used values from the most recent assessment before the MRI in our analyses.
MRI outcomes
Technicians trained on the study protocol collected the MRI scans. Regional brain volumes and ischemic lesion loads (i.e., volumes) were measured centrally at the WHIMS-MRI Quality Control Center (see appendix). Standardized and validated protocols were used for obtaining and processing MRI scans and for measuring volumes (10,11,13). Series were acquired with field of view of 22 cm and matrix size of 256 × 256. They included oblique axial spin density/T2-weighted spin echo (repetition time [TR] = 3,200 ms, echo time [TE] = 30/120 ms, slice thickness = 3 mm), fluid attenuation inversion recovery (FLAIR) T2-weighted spin echo (TR = 8,000 ms, inversion time [TI] = 2,000 ms, TE = 100 ms, slice thickness = 3 mm), and axial three-dimensional spoiled gradient recalled T1-weighted gradient echo (TR = 21 ms, TE = 8 ms, flip angle = 30°, slice thickness = 1.5 mm) images from the vertex to skull base parallel to the anterior commissure–posterior commissure plane. T1-weighted volumetric MRI scans were first preprocessed according to a standardized protocol for alignment, removal of extracranial material, and segmentation of brain into gray and white parenchyma and cerebrospinal fluid. Regional volumetric measurements were obtained with an automated computer-based template warping method that summed the number of respective voxels falling within each anatomical region of interest (ROI). Intracranial volume was estimated as the total cerebral hemispheric volumes plus cerebrospinal fluid within the ventricular and sulcal spaces. After additional preprocessing steps, including histogram standardization and coregistration, the ischemic lesion segmentation component of the algorithm was applied, based on local signal features extracted from coregistered multiparametric MRI sequences (i.e., T1, proton density, T2, and FLAIR). A support vector machine classifier was first trained on expert-defined small-vessel ischemic disease (SVID) lesions in 45 cases from the Action to Control Cardiovascular Risk in Diabetes—Memory in Diabetes (ACCORD-MIND) study (14) and then used to classify SVID in new scans. For algorithm training purposes, SVID was operationally defined as a nonmass lesion having FLAIR signal greater than that of normal gray matter in a vascular distribution. The computer-assisted methodology was validated against manual segmentation, (i.e., manual drawing of ROIs) by an experienced expert neuroradiologist (13) and has been used successfully in other cohorts (14–17).
All supratentorial brain tissue was classified as normal or abnormal (ischemic) gray or white matter and assigned to one of 92 anatomical ROIs of the cerebrum (13,14). These ROIs were organized in an anatomically hierarchical system that was collapsed into anatomical regions for this analysis—total brain, gray matter, white matter, and four lobes (frontal, occipital, parietal, and temporal). We analyzed the volumes and the ischemic lesion volumes within each of these lobes and also the volume of ventricular cerebrospinal fluid.
We originally reported outcomes from 1,403 baseline MRIs on the basis of an earlier protocol for measurement (10,11). Baseline and follow-up MRIs were subsequently reprocessed with a refined image analysis protocol. This report is based on 1,366 of the original baseline MRIs (97.4%) and 698 follow-up MRIs of women for whom type 2 diabetes status was recorded.
Tests of cognitive function and classification of cognitive impairment
Global cognitive function was assessed with the Modified MiniMental State (3MS) examination, administered annually by trained and certified technicians from WHIMS enrollment until the first MRI (8,18). Possible scores ranged from 0 to 100, with a higher score reflecting better cognitive functioning. A factor analysis of WHIMS 3MS scores yielded four major components: 1) verbal memory and verbal fluency, 2) language and executive function, 3) orientation, and 4) language and praxis (19). We used the 3MS score collected most recently before the first MRI.
Statistical methods
General linear models with covariate adjustment were fitted to assess differences in volumes between women with and without diabetes at WHIMS-MRI enrollment with a model that included main effects for diabetes status, covariates, an interaction term between diabetes status, and a variable for time that took on a value of 0 at the initial MRI scan and the time between scans for the second MRI. A compound symmetry model was used for intrasubject correlations. In this model, changes in volumes occurred as a linear function of time between scans, and the rates of these changes were allowed to vary between women with and without diabetes. The advantage of this model is that it used all available data to estimate mean differences in volumes at the time of the first MRI. A supporting analysis limited to only volumes collected on the first MRI yielded similar results and is not reported. Because the distribution of ischemic lesion volumes was right skewed, an offset logarithm transformation was used to provide a more symmetrical distribution for analysis by taking the logarithm of the sum of the measured volume plus 1. Changes in volumes between the first and second MRI were computed, and differences in mean changes were described with analyses of covariance. Changes in ischemic lesion volumes were also highly skewed. We used the log-transformed values described here and applied linear regression to characterize the effect of diabetes on the second MRI measure, after adjustment for the log-transformed value of the baseline MRI. All models included age, clinic site, time from WHI enrollment, and time between scans as covariates. In addition, because a difference in intracranial volume was detected between the cross-sectional diabetes groups, this measure was also included as a covariate in their analyses.
RESULTS
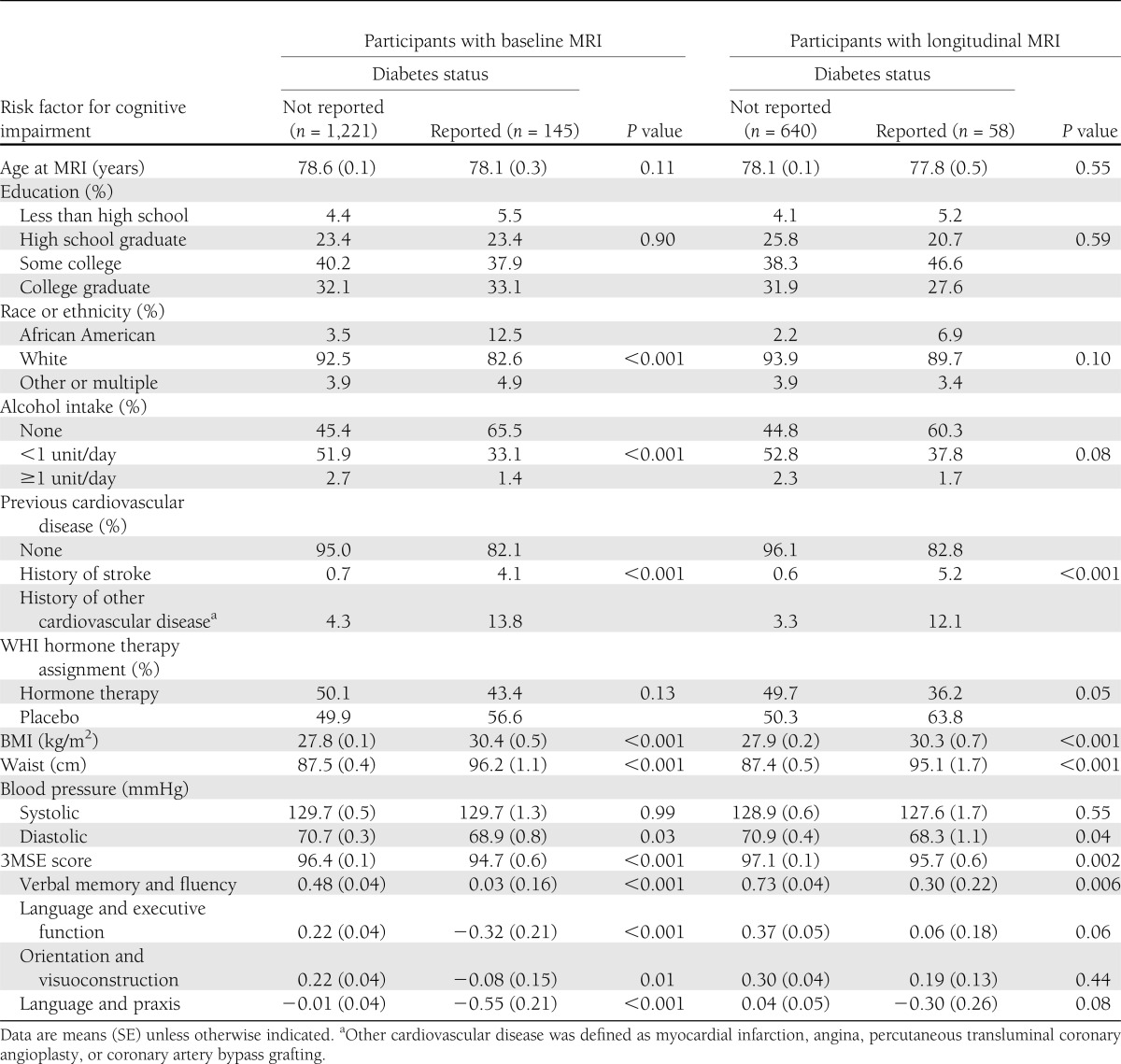
At the first examination, standardized MRI measures were obtained from 145 women with recorded diabetes and from 1,221 without; these women comprise the cross-sectional cohort. A second standardized scan was obtained for 58 of the 145 women from the cross-sectional cohort with diabetes (40.4%) and for 640 of the women with diabetes (52.4%); this subset of women comprises the longitudinal cohort. Women for whom a second scan was not obtained (i.e., who were in the cross-sectional cohort but not the longitudinal cohort) tended to be older (mean of 79.0 vs. 78.1 years; P < 0.001), were less likely to be white (89.3 vs. 93.6%; P = 0.002), and tended to have lower 3MS scores (mean of 95.4 vs. 97.0; P < 0.001), but did not differ significantly (P > 0.05) otherwise.
Within the cross-sectional cohort (Table 1), women with diabetes were more likely to be African American, report no alcohol consumption, and have cardiovascular disease. They also had higher mean BMI and waist circumference and lower mean diastolic blood pressure. These associations were also evident among the women in the longitudinal cohort, although statistical significance was attenuated with the smaller sample size. Mean global cognitive function, as measured by the 3MS, was significantly lower among women with diabetes, as were means for each of the four components of cognitive function identified through factor analysis.
Table 1.
Distribution of risk factors for atrophy and cerebrovascular disease by diabetes status among women enrolled in WHIMS-MRI at the most recent assessment before first MRI
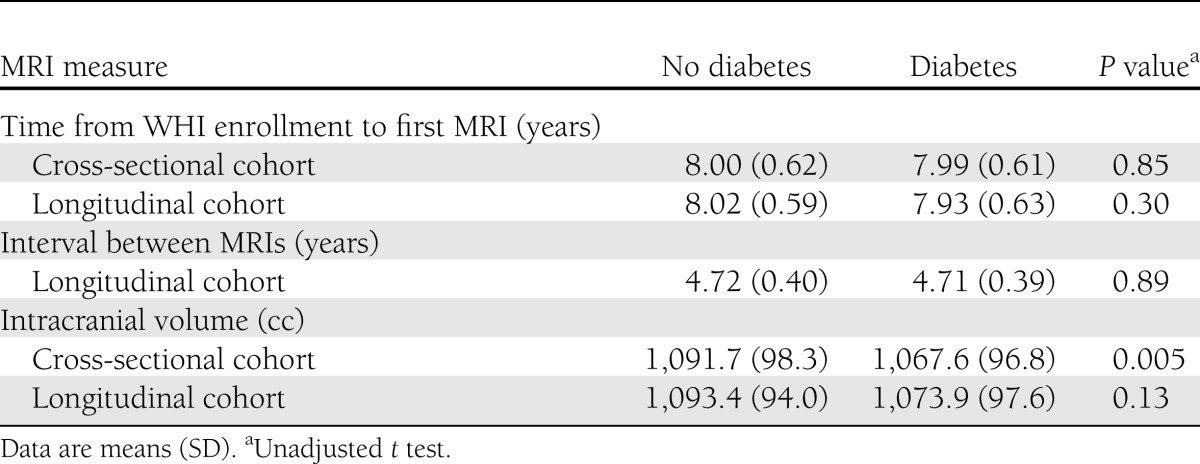
As seen in Table 2, the initial MRI scans were collected an average of 8.0 years after enrollment in the WHI for women with and without diabetes in the cross-sectional and longitudinal cohorts. The second MRI scans were obtained an average of 4.7 years after the first for women with and without diabetes in the longitudinal cohort. In the cross-sectional cohort, the mean intracranial volume of women with diabetes was about 24 cc lower than that in women without diabetes (P = 0.005).
Table 2.
Time frames and intracranial volumes for women without and with diabetes
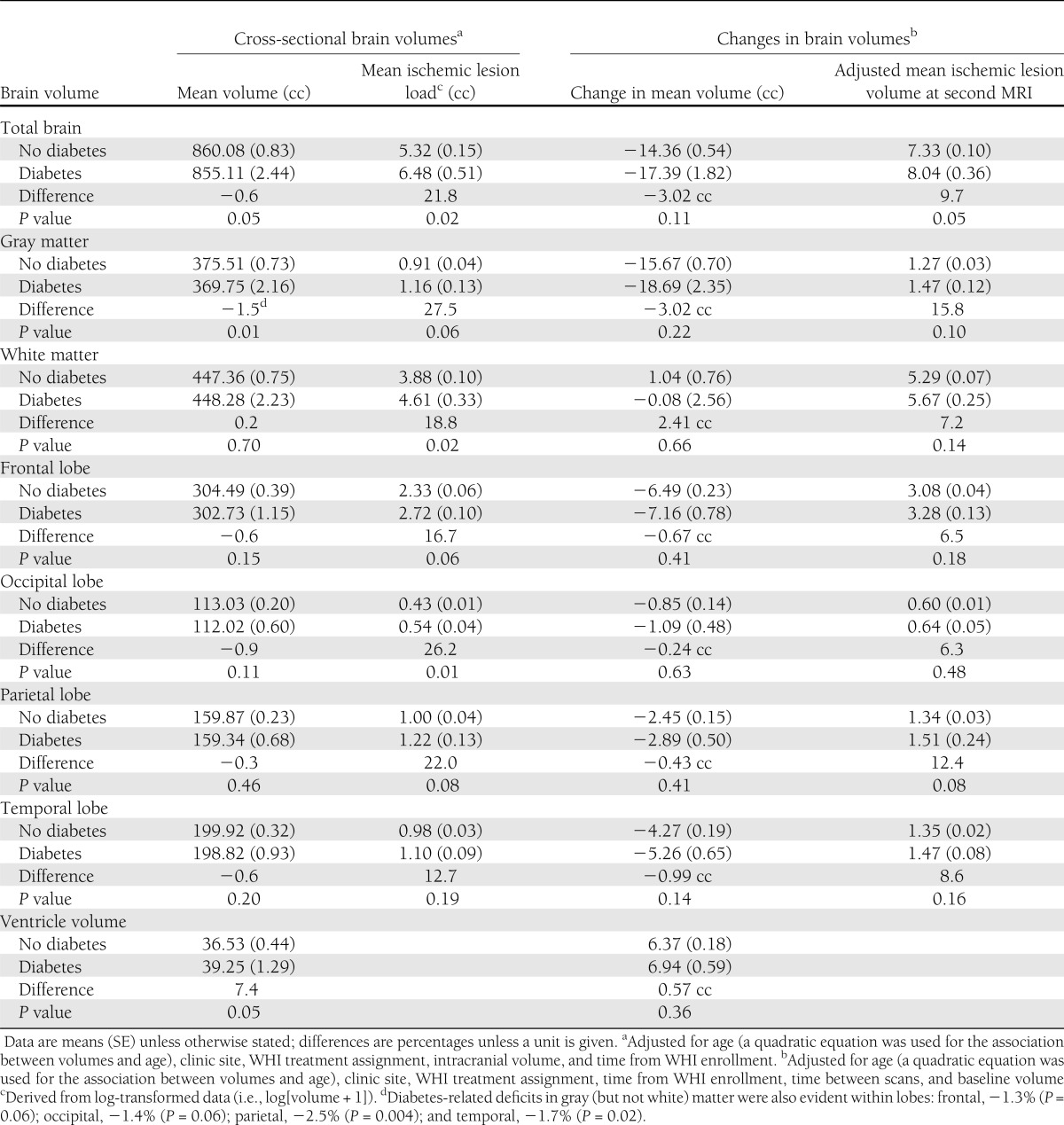
Table 3 provides mean volumes at the first MRI from general linear models with adjustment for age, clinic site, WHI treatment assignment, time from WHI enrollment, and intracranial volume. Volumes tended to be lower among women with diabetes for total brain (−0.6%; P = 0.05) and gray matter (−1.5%; P = 0.01). Among the four lobes we analyzed, differences in overall volumes between women with and without diabetes did not reach statistical significance, as seen in Table 3. As noted in the footnote to Table 3, we separately examined gray matter within each of the lobes and found evidence for diabetes-related deficits in gray matter within each: frontal, −1.3% (P = 0.06); occipital, −1.4% (P = 0.06); parietal, −2.5% (P = 0.004); and temporal, −1.7% (P = 0.02). Mean white matter volume was not related to diabetes overall or in any lobe. Mean ventricular volume was 7.4% larger among women with diabetes (P = 0.05).
Table 3.
Covariate-adjusted relationships of regional brain volumes and ischemic lesion volumes with diabetes from analyses of all women
Mean ischemic lesion loads were consistently greater among women with diabetes throughout all regions and in both gray and white matter. These differences reached P < 0.05 for the total brain (21.8%; P = 0.02), white matter (18.8%; P = 0.02), and occipital lobe (26.2%; P = 0.01). Additional covariate adjustment for all factors in Table 1 did not materially affect the magnitudes of these estimated differences, which also were consistent with findings when analyses were restricted to white women (data not shown).
After similar covariate adjustment, fitted mean (SE) changes in total brain volumes between MRIs were −14.36 cc (0.54) for women without diabetes and −17.39 cc (1.82) for women with diabetes (P = 0.11). Across regions, mean volumes tended to decrease more rapidly and ventricular volume to increase more rapidly among women with diabetes; however, no differences were statistically significant (P > 0.05). After adjustment for baseline levels, the covariate-adjusted total brain ischemic lesion loads at the second MRI were 7.33 cc (0.10) for women without diabetes compared with 8.04 cc (0.36) for women with diabetes (P = 0.05). This trend toward increased ischemic lesion volumes among women with diabetes was apparent in both white and gray matter and for several lobes but did not reach statistical significance for these measures (P > 0.05).
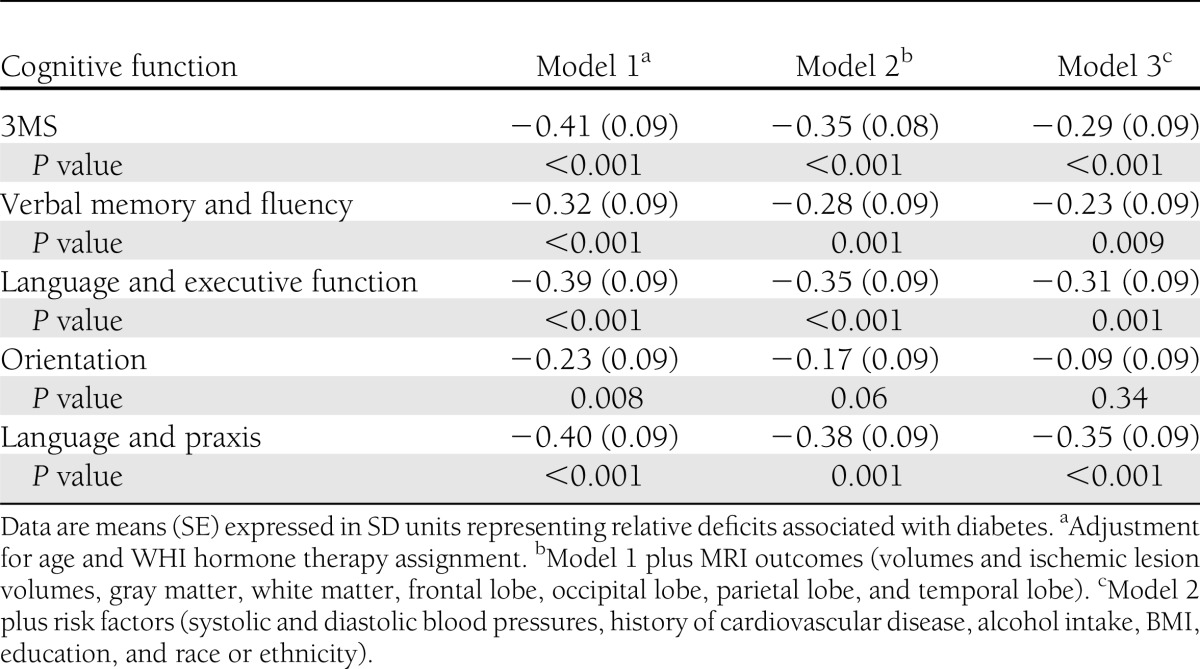
Poorer cognitive function, as evidenced by lower 3MS scores, was correlated with smaller brain volumes (r = −0.08; P < 0.001), larger ischemic lesion volumes (r = 0.06; P = 0.006), greater loss in brain volume (r = 0.10; P = 0.006), and greater increase in ischemic lesion volume (r = 0.15; P < 0.001) in models with covariate adjustment for intracranial volume. We fitted three models with varying levels of covariate adjustment to describe relationships that diabetes had with deficits in global and domain-specific cognitive function, expressing these in standard deviation units to facilitate comparisons (Table 4). After adjustment for age and WHI treatment assignment, the mean deficit for 3MS scores was 0.41 SD units (SE 0.09; (P < 0.001), and deficits were seen in each subdomain, ranging from 0.23 SD units (0.09) for orientation to 0.40 SD units (0.09) for language and praxis (all P < 0.001). Covariate adjustment for all MRI volumes and ischemic lesion volumes (total and regional) attenuated the mean diabetes-related deficits only slightly (model 2). Additional adjustment for all other risk factors for cognitive impairment in Table 1, further attenuated diabetes-related differences; however, these remained highly significant for all cognitive measures except orientation.
Table 4.
Deficits in mean 3MSE scores and principal factor scores associated with diabetes with varying levels of covariate adjustment
CONCLUSIONS
The analyses described here yielded three principal findings, which we will discuss in turn. First, in a large cohort of older women, diabetes was independently associated with significantly smaller volumes of gray matter but not white matter, significantly greater ventricular volumes, and significantly greater ischemic lesion loads throughout the brain. Second, diabetes was associated with trends toward greater progression of ischemic lesion loads and loss of total brain volumes throughout the brain but not loss of white matter during 4.7 years of average follow-up. Finally, although lower brain volumes and greater ischemic lesion volumes were all related to poorer cognitive function, these MRI measures did not fully account for the diabetes-related deficits in cognitive function. Significant diabetes-related deficits in cognitive deficits remained after adjustments for MRI measures and other factors in Table 1.
Associations of diabetes with brain volumes and ischemic lesion loads
There are numerous reports linking diabetes with lower brain volumes later in life. In a 2006 systematic review, van Harten et al. (3) found consistent associations across many brain regions, and more recent reports add support (6,20–24). Our finding that these smaller volumes are limited to gray matter agrees with two prior reports of marked diabetes-related decrements in gray but not white matter volumes (25,26). The impact of diabetes on ischemic lesion loads and white matter hyperintensities has been less consistently shown, perhaps because of differences in measurement protocols and definitions (3,27). Certainly many shared risk factors and metabolic pathways would be expected to link diabetes with increased levels of ischemic lesion loads, including impaired perfusion and increased inflammation (2,28). Adjustment for BMI, waist circumference, blood pressure, previous cardiovascular disease, education, race or ethnicity, and alcohol intake did not materially affect these relationships, suggesting that the impact of diabetes on brain volumes and ischemic lesion loads in our study was not channeled through these risk factors.
Associations of diabetes with changes in brain volumes and ischemic lesion loads
Comparisons in the rates of changes in brain volumes and ischemic lesion loads between individuals with and without diabetes have been reported from two previous cohorts. The Utrecht Diabetic Encephalopathy Study collected brain MRIs 4 years apart from 55 individuals with diabetes and 28 controls with a mean age of 65 years and found greater increases in ventricular volumes but no significant differences in the rates of total brain volumes or white matter hyperintensities (29). Subgroup analyses found that the increase in ventricular volume occurred in women but not men. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial collected brain MRIs 3 years apart from 89 individuals with diabetes and 438 controls with a mean age of 75 years and found increased rates of total brain atrophy but no increases in white matter hyperintensity volumes in association with diabetes (6). We found some evidence for increased rates of overall atrophy in our cohort across 4.7 years among women with diabetes, although the difference did not reach statistical significance; however, we did see stronger evidence of increased ischemic lesion loads. These differences remained after extensive covariate adjustment. Our cohort was slightly older than the previous studies, and our measurement protocol was different, which may have influenced our findings. It may also be the case that the effects of diabetes on brain structure are greater among women. Another possibility is that diabetes-related increases in ischemic lesion volumes occur later in life, which is consistent with Sonnen et al. (30), who found diabetes-related markers of cerebrovascular disease to be more pronounced among individuals in later stages of cognitive decline (i.e., with dementia).
Relationships of MRI measures with global cognitive function and its subdomains
Diabetes has been repeatedly documented to be associated with relative deficits in global and domain-specific cognitive function, similar to those that we report (2,31). Adverse cross-sectional and longitudinal MRI findings are also related to poorer cognitive function individuals with and without diabetes (29,32,33). Including MRI outcomes as covariates attenuated the estimated diabetes-related deficits only slightly, which is consistent with other reports (31). Inclusion of demographic markers and risk factors such as cardiovascular disease, alcohol use, waist girth, BMI, and blood pressures similarly did not fully account for these deficits. Although other candidates for mediation, such as apolipoprotein E status and proinflammatory cytokines, were not available, our findings suggest that factors not tightly linked to MRI volumes may be in play. One possibility is dysregulation of glucose metabolism. Insulin plays a central role in maintaining normal cognitive and brain function in older adults, and insulin dysregulation has been implicated in the pathophysiology of mild cognitive impairment, Alzheimer disease, and vascular dementia (34,35). Chronic insulin resistance and impaired glucose tolerance have been reported in mild cognitive impairment and early Alzheimer disease. Peripheral hyperinsulinemia and low brain insulin concentrations may reduce β-amyloid clearance and also promote inflammatory response. Recent observations have suggested that lower brain concentrations of insulin and reduced insulin receptors may be associated with increased incidence of Alzheimer disease; however, variable results on the effects of oral hypoglycemic agents on β-amyloid production have been reported in animal studies, and findings from recent human clinical trials have not been encouraging (36,37–39). Aggressive pharmacological management of diabetes may have mixed effects on brain structure, marginally reducing atrophy but increasing ischemic lesion volumes (14). Insulin given intranasally has shown significant promise in early mild cognitive impairment and Alzheimer disease clinical trials (40).
Limitations
Our sample is drawn from former volunteers in a trial of postmenopausal hormone therapy and may not represent general populations. Follow-up MRIs were obtained for about half of the original cohort, and women who did return differed from those who did not in several characteristics. Diabetes status for some women was based on self-report, and reliable data on duration of diabetes were not available. Our MRI protocol is different from some other studies that we cite, but it included well-validated quantitative measures of brain tissue and ischemic lesion volumes. The covariates available to us did not include some related to potential mechanisms (i.e., cholesterol levels, inflammatory markers, and insulin and glucose levels). We did not attempt to control for medication use (e.g., aspirin or statins), which likely varied with time. It is also possible that observed relationships may have been attenuated by measurement error.
Summary
Many processes adversely influence brain health and ultimately increase the risks of cognitive impairment with diabetes. Cognitive deficits emerge early in diabetes, and perhaps in prediabetes, and are maintained and may increase with time (31). In this large and diverse cohort of women, gray matter was decreased and accumulation of ischemic lesions was accelerated. Large scale measures of brain structure and changes in brain structure were modestly correlated with cognitive function but only partially explained diabetes-related deficits.
Acknowledgments
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health, U.S. Department of Health and Human Services. Contracts HHSN-268200464221C and N01-WH-4-4221 provided additional support. S.M.R. is supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health. The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals, Inc, St. Davids, PA. No other potential conflicts of interest relevant to this article were reported.
M.A.E. performed analyses and wrote the manuscript. R.N.B. oversaw data collection and coauthored the manuscript. J.S.G., J.G.R., M.S.S., and S.L. coauthored sections of the manuscript. P.E.H. performed analyses and coauthored sections of the manuscript. R.C., L.H.C., K.Y., K.M., and R.R. collaborated on the drafting of the manuscript, reviewed the work, and provided critical input. S.M.R. participated fully in the development and writing of this manuscript. M.A.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the American Diabetes Association Scientific Sessions, Philadelphia, Pennsylvania, 8–12 June 2012.
Appendix
WHIMS-MRI Clinical Centers
Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, Mimi Goodwin, Richard DeNise, Michael Lipton, James Hannigan, Anthony Carpini, David Noble, Wilton Guzman; Medical College of Wisconsin, Milwaukee: Jane Morley Kotchen, Joseph Goveas, Diana Kerwin, John Ulmer, Steve Censky, Troy Flinton, Tracy Matusewic, Robert Prost; Stanford Center for Research in Disease Prevention, Stanford University, CA: Marcia L. Stefanick, Sue Swope, Anne Marie Sawyer-Glover, Susan Hartley; The Ohio State University, Columbus: Rebecca Jackson, Rose Hallarn, Bonnie Kennedy, Jill Bolognone, Lindsay Casimir, Amanda Kochis; University of California at Davis, Sacramento: John Robbins, Sophia Zaragoza, Cameron Carter, John Ryan, Denise Macias, Jerry Sonico; University of California at Los Angeles: Lauren Nathan, Barbara Voigt, Pablo Villablanca, Glen Nyborg, Sergio Godinez, Adele Perrymann; University of Florida, Gainesville/Jacksonville: Marian Limacher, Sheila Anderson, Mary Ellen Toombs, Jeffrey Bennett, Kevin Jones, Sandy Brum, Shane Chatfield, Kevin Vantrees; University of Iowa, Davenport: Jennifer Robinson, Candy Wilson, Kevin Koch, Suzette Hart, Jennifer Carroll, Mary Cherrico; University of Massachusetts, Worcester: Judith Ockene, Linda Churchill, Douglas Fellows, Anthony Serio, Sharon Jackson, Deidre Spavich; University of Minnesota, Minneapolis: Karen Margolis, Cindy Bjerk, Chip Truwitt, Margaret Peitso, Alexa Camcrena, Richard Grim, Julie Levin, Mary Perron; University of Nevada, Reno: Robert Brunner, Ross Golding, Leslie Pansky, Sandie Arguello, Jane Hammons, Nikki Peterson; University of North Carolina, Chapel Hill: Carol Murphy, Maggie Morgan, Mauricio Castillo, Thomas Beckman, Benjamin Huang; University of Pittsburgh, PA: Lewis Kuller, Pat McHugh, Carolyn Meltzer, Denise Davis, Joyce Davis, Piera Kost, Kim Lucas, Tom Potter, Lee Tarr.
WHIMS-MRI Clinical Coordinating Center
Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker, Mark Espeland, Laura Coker, Jeff Williamson, Debbie Felton, LeeAnn Gleiser, Steve Rapp, Claudine Legault, Maggie Dailey, Ramon Casanova, Julia Robertson, Patricia Hogan, Sarah Gaussoin, Pam Nance, Cheryl Summerville, Ricardo Peral, Josh Tan.
WHIMS-MRI Quality Control Center
University of Pennsylvania, Philadelphia: Nick Bryan, Christos Davatzikos, Lisa Desiderio.
U.S. National Institutes of Health
National Institute on Aging, Bethesda, MD: Neil Buckholtz, Susan Molchan, Susan Resnick; National Heart, Lung, and Blood Institute, Bethesda, MD, Jacques Rossouw, Linda Pottern.
Footnotes
A slide set summarizing this article is available online.
A list of participants in the WHIMS-MRI Study Group can be found in the Appendix.
References
- 1.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev 2007;56:384–402. [DOI] [PubMed]
- 2.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008;29:494–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 2006;29:2539–2548 [DOI] [PubMed] [Google Scholar]
- 4.Tiehuis AM, van der Graaf Y, Visseren FL, et al. SMART Study Group Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 2008;39:1600–1603 [DOI] [PubMed] [Google Scholar]
- 5.de Bresser J, Tiehuis AM, van den Berg E, et al. Utrecht Diabetic Encephalopathy Study Group Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Elderen SGC, de Roos A, de Craen AJM, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 2010;75:997–1002 [DOI] [PubMed] [Google Scholar]
- 7.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002;162:1737–1745 [DOI] [PubMed] [Google Scholar]
- 8.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 1998;19:604–621 [DOI] [PubMed] [Google Scholar]
- 9.Jaramillo SA, Felton D, Andrews LA, et al. Women’s Health Initiative Memory Study Research Group Enrollment in a brain magnetic resonance study: results from the Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI). Acad Radiol 2007;14:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coker LH, Hogan PE, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology 2009;72:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resnick SR, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 2009;72:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis KL, Lihong Qi, Brzyski R, et al. Women Health Initiative Investigators Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol 2008;15:300–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND investigators Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977DOI: 10.1016/S1474-4422(11)70188-0 10.1016/S1474-4422(11)70188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 2009;72:1906–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging 2002;21:1421–1439 [DOI] [PubMed] [Google Scholar]
- 17.Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Grond J. Automatic segmentation of different-sized white matter lesions by voxel probability estimation. Med Image Anal 2004;8:205–215 [DOI] [PubMed] [Google Scholar]
- 18.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318 [PubMed] [Google Scholar]
- 19.Rapp SR, Espeland MA, Hogan P, Jones BN, Dugan E, WHIMS investigators Baseline experience with Modified Mini Mental State Exam: The Women’s Health Initiative Memory Study (WHIMS). Aging Ment Health 2003;7:217–223 [DOI] [PubMed] [Google Scholar]
- 20.Manschot SM, Brands AMA, van der Grond J, et al. Utrecht Diabetic Encephalopathy Study Group Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006;55:1106–1113 [DOI] [PubMed] [Google Scholar]
- 21.Korf ESC, van Straaten ECW, de Leeuw FE, et al. LADIS Study Group Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet Med 2007;24:166–171 [DOI] [PubMed] [Google Scholar]
- 22.Last D, Alsop DC, Abduljalil AM, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 2007;30:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007;50:711–719 [DOI] [PubMed] [Google Scholar]
- 24.Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res 2009;1280:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jongen C, van der Grond J, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JP, Utrecht Diabetic Encephalopathy Study Group Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia 2007;50:1509–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Haroon E, Darwin C, et al. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J Magn Reson Imaging 2008;27:14–19 [DOI] [PubMed] [Google Scholar]
- 27.Jongen C, Biessels GJ. Structural brain imaging in diabetes: a methodological perspective. Eur J Pharmacol 2008;585:208–218 [DOI] [PubMed] [Google Scholar]
- 28.Williamson JD, Miller ME, Bryan RN, et al. ACCORD Study Group The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol 2007;99(12A):112i–122i [DOI] [PubMed] [Google Scholar]
- 29.Reijmer YD, van den Berg E, de Bresser J, et al. Utrecht Diabetic Encephalopathy Study Group Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev 2011;27:195–202 [DOI] [PubMed] [Google Scholar]
- 30.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol 2009;66:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espeland MA, Miller ME, Goveas JS, et al. Cognitive function and fine motor speed in older women with diabetes mellitus: results from the Women's Health Initiative Study of Cognitive Aging. J Womens Health (Larchmt) 2011;20:1435–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdelho A, Madureira S, Moleiro C, et al. LADIS Study White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology 2010;75:160–167 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol 2005;58:610–616 [DOI] [PubMed] [Google Scholar]
- 34.Craft S. Alzheimer disease: Insulin resistance and AD—extending the translational path. Nat Rev Neurol 2012;19:360–362a [DOI] [PubMed] [Google Scholar]
- 35.Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci 2012;67:754–759 [DOI] [PMC free article] [PubMed]
- 36.Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA 2009;106:3907–39612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbatecola AM, Lattanzio F, Molinari AM, et al. Rosiglitazone and cognitive stability in older individuals with type 2 diabetes and mild cognitive impairment. Diabetes Care 2010;33:1706–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold M, Alderton C, Zvartau-Hind M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 2010;30:131–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington C, Sawchak S, Chiang C, et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res 2011;8:592–606 [DOI] [PubMed] [Google Scholar]
- 40.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012;69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]