Abstract
OBJECTIVE
Adverse effects of hypercaloric, high-fructose diets on insulin sensitivity and lipids in human subjects have been shown repeatedly. The implications of fructose in amounts close to usual daily consumption, however, have not been well studied. This study assessed the effect of moderate amounts of fructose and sucrose compared with glucose on glucose and lipid metabolism.
RESEARCH DESIGN AND METHODS
Nine healthy, normal-weight male volunteers (aged 21–25 years) were studied in this double-blind, randomized, cross-over trial. All subjects consumed four different sweetened beverages (600 mL/day) for 3 weeks each: medium fructose (MF) at 40 g/day, and high fructose (HF), high glucose (HG), and high sucrose (HS) each at 80 g/day. Euglycemic-hyperinsulinemic clamps with [6,6]-2H2 glucose labeling were used to measure endogenous glucose production. Lipid profile, glucose, and insulin were measured in fasting samples.
RESULTS
Hepatic suppression of glucose production during the clamp was significantly lower after HF (59.4 ± 11.0%) than HG (70.3 ± 10.5%, P < 0.05), whereas fasting glucose, insulin, and C-peptide did not differ between the interventions. Compared with HG, LDL cholesterol and total cholesterol were significantly higher after MF, HF, and HS, and free fatty acids were significantly increased after MF, but not after the two other interventions (P < 0.05). Subjects’ energy intake during the interventions did not differ significantly from baseline intake.
CONCLUSIONS
This study clearly shows that moderate amounts of fructose and sucrose significantly alter hepatic insulin sensitivity and lipid metabolism compared with similar amounts of glucose.
In the U.S., the consumption of fructose increased by more than 25% between 1970 and 1997 as the total sugar intake of the population rose (1). During the same period, the prevalence of obesity rose dramatically, paralleling the increase in fructose consumption and the introduction of high-fructose corn syrup (2). Whether there is a causal relationship between those developments, however, remains unclear. Total fructose consumption from natural and added sources, estimated from food disappearance data, was estimated to be 97 g/person/day in 1997 in the U.S. (1) and 83 g/person/day in 1998 in Switzerland (3).
Epidemiologic and intervention studies of fructose and other caloric sweeteners have shown detrimental effects on health. In a cross-sectional study in U.S. adults, for example, the consumption of caloric sweeteners was associated with increased dyslipidemia (4) and in the Health Professionals Follow-up Study, high intakes of sugar-sweetened beverages (SSB) increased the risk for type 2 diabetes (5). Intervention trials have provided evidence that high- to very high–fructose doses led to increases in de novo lipogenesis, blood triglycerides, and hepatic insulin resistance (6–8).
Not all of these studies found consistent effects for all parameters, however. In the study by Lê et al. (6), where 1.5 g fructose/kg body weight were consumed during a 4-week period, fasting lipids and glucose were affected, but insulin resistance, as determined by a euglycemic-hyperinsulinemic clamp, did not change. However, their study only tested fructose, without comparison with other sugars. Furthermore, relatively high amounts of fructose were consumed in most of these studies, reaching up to 25% of total energy intake. In a recent intervention study in healthy Swiss men, we found adverse effects of low to moderate amounts of fructose—but also glucose and sucrose—on fasting glucose and inflammatory markers, whereas only beverages containing fructose seemed to negatively affect LDL particle size. Even though fasting glucose was altered, none of the interventions showed any effect on glucose tolerance or on indices of insulin sensitivity calculated during an oral glucose tolerance test (9).
The aim of the current study was therefore to assess the effect of moderate amounts of fructose and sucrose, compared with the same amounts of glucose, specifically on hepatic insulin sensitivity but also on lipid profiles of healthy human subjects using euglycemic-hyperinsulinemic clamps with [6,6]-2H2 labeled glucose.
RESEARCH DESIGN AND METHODS
Study design
The study consisted of four different interventions in random sequence. Each intervention lasted 3 weeks and was directly followed by an examination in our clinic. Thereafter, a washout of a minimum of 4 weeks was implemented before the next intervention began. The first subject started the study in February 2009, and the last subject completed the study in March 2011. During each intervention, subjects were supplied with SSB containing different sugars in different concentrations: medium fructose (MF), 40 g/day; high fructose (HF), 80 g/day; high glucose (HG), 80 g/day; and high sucrose (HS), 80 g/day. The drinks were provided in containers of 200 mL each, with blinded content, and the subjects had to consume three drinks (total, 600 mL) daily. The sugar concentrations of the drinks were 66.5 g/L for the medium concentrations and 133.5 g/L for the high concentrations. Subjects were advised to consume the drinks together with the three main meals. To assess compliance, subjects were asked to return beverages not consumed on the day of visit to the metabolic ward.
The drinks were produced by the Nestlé Product Technology Center in Konolfingen, Switzerland, under good manufacturing practice conditions and according to our instructions. Before their use in the study, the drinks underwent quality control at the Product Technology Center. During the study, sugar content of the drinks was monitored and found to be stable.
The order of the four different interventions was randomly assigned to the subjects (physical randomization), and the study was carried out in a double-blind manner with intention-to-treat analysis of the data. The random allocation of the order of interventions was implemented by a coworker not otherwise involved in the study. Participants, the nurse taking the anthropometric measurements, and the laboratory technicians were blinded to the order of interventions.
Subjects
Nine healthy, normal-weight male volunteers (BMI between 20 and 24 kg/m2, age between 21 and 25 years) living in the region of Zurich, Switzerland, were included in this study. Subjects were recruited through advertisements at the universities in Zurich by an author (I.A.). Written informed consent was obtained from all subjects before entering the study. The study was approved by the ethics committee of the University Hospital Zurich.
Sample size calculation was based on an estimated difference in hepatic suppression between two interventions of 10%, with a standard deviation of 6% (α = 0.016 after Bonferroni correction for three comparisons), and it was determined a sample size of nine volunteers would be sufficient. Volunteers were eligible for the study if they were male, had a normal BMI (19–25 kg/m2), were healthy, and aged 20 to 50 years. Volunteers taking regular medication or consuming SSB with a total carbohydrate content exceeding 60 g/day were not included in the study.
Protocol
One day before each examination, subjects were asked not to engage in strenuous physical activity. On the examination day, they were asked to present at the Clinical Trials unit of the University Hospital Zurich at 7.30 a.m. after a 12-h overnight fast. Upon arrival, weight was determined to the nearest 100 g using a digital balance (WB 100 P, Tanita, Hoofddorp, the Netherlands), and height was measured to the nearest 0.5 cm using a wall-mounted stadiometer at the first examination. BMI was calculated as weight (kg)/height (m)2. Waist and hip circumference were determined using a nonstretchable measuring tape. Percentage of body fat was measured by bioelectrical impedance (AKERN BIA 101, AKERN, Pontassieve, Italy) with the subject supine.
Blood pressure was measured using an automated device (Omron M6, upper arm blood pressure monitor) after a 15-min rest while supine. After this, with the subjects resting quietly in a bed, an indwelling catheter was inserted into the vein of the right arm for blood sampling. Another indwelling catheter was inserted into an antecubital vein of the left arm for the infusion of glucose, insulin, and the tracer ([6,6]-2H2 glucose). In the fasted state, blood samples were collected for the measurement of glucose, insulin, C-peptide, lipid profile, and leptin. After blood sampling, a primed continuous infusion of [6,6]-2H2 glucose was administered during 5 h to determine endogenous glucose production (bolus of 2 mg/kg over 10 min, followed by a continuous rate of 0.02 mg/kg/min). After 180 min of tracer equilibration, a hyperinsulinemic-euglycemic clamp was started for the following 120 min. Insulin was infused continuously (bolus of 60 mU/m2/min for 3 min, followed by continuous rate of 15 mU/m2/min). A relatively low insulin infusion rate with incomplete suppression of hepatic glucose production was chosen to reveal differences in insulin sensitivity in our generally insulin-sensitive study group and based on our previous experiences (10).
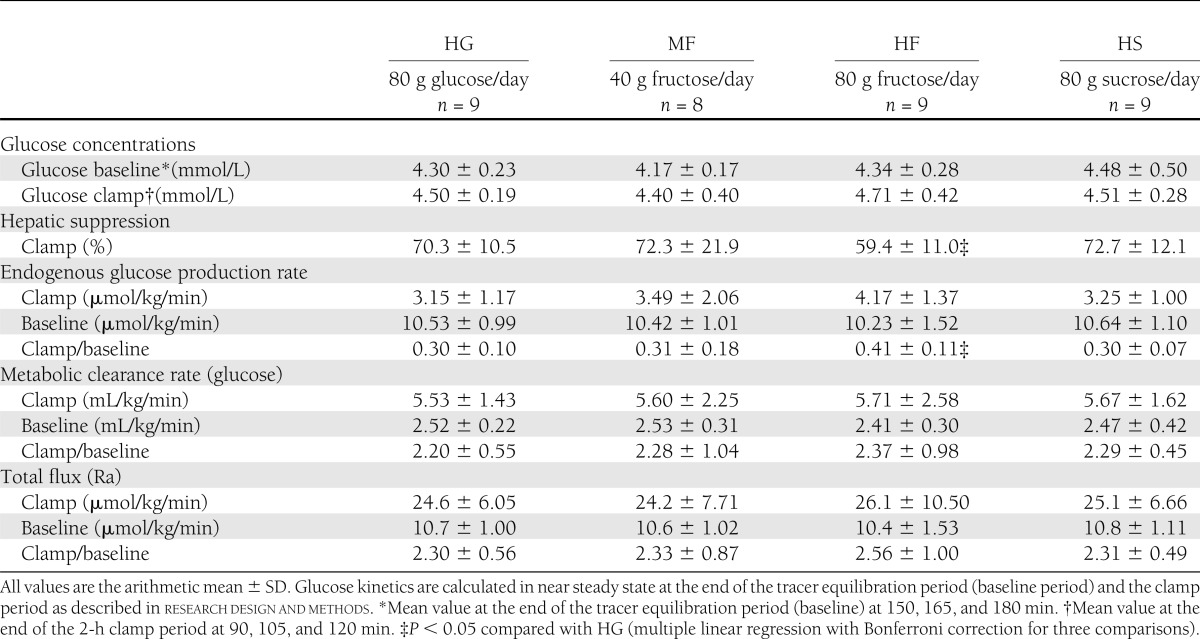
Blood samples were collected every 5 min during the clamp to monitor blood glucose concentrations, and glucose (20% w/v) was infused at variable rates to keep the blood glucose euglycemic (∼4.5 mmol/L) (Table 2). The 20% glucose infusion contained 1.2% [6,6]-2H2 glucose to maintain a constant plasma D2-glucose tracer–to–tracee ratio (TTR) during the clamp. Blood was drawn at 150, 165, and 180 min of the 3-h tracer equilibration (baseline) period and at 60, 90, 105, and 120 min of the 2-h clamp period for the determination of tracer concentrations (TTR of [6,6]-2H2 glucose). Glucose kinetics were calculated as described previously (11) at near-steady state at the end of the tracer equilibration baseline period (150–180 min) and during the last 30 min of the clamp (mean values from time points given above). Total glucose flux (Ra), endogenous glucose production rate (EGP), glucose metabolic clearance rate (Rd/glucose concentration, i.e., insulin mediated glucose disposal, a standard parameter of whole-body insulin sensitivity), and percentage of hepatic suppression of glucose production (a parameter of hepatic insulin sensitivity) were calculated as follows:
Table 2.
Glucose metabolism during the clamp in all subjects after each of the four 3-week interventions
Ra = F/TTR, with F being the rate of tracer infusion;
EGP = Ra – glucose infusion rate;
glucose metabolic clearance rate = Rd/glucose concentration = Ra/glucose concentration; and
hepatic suppression = 100% × [(EGPbasal− EGPclamp)/EGPbasal].
In the week before each examination and before the start of the first intervention, all subjects filled in a 3-day (2 weekdays and 1 weekend day) weighed food record (12). During those 3 days, all foods and drinks consumed had to be weighed on a digital kitchen scale whenever possible, and if not possible, amounts had to be documented in standard kitchen measures to allow quantitative estimation of dietary intake. Subjects were asked not to change their usual eating habits during the days of recording.
The individual 3-day food records of each subject were carefully checked on the day of the examination to ensure completeness and comprehensibility. Data were then entered into the Swiss version of the EBISpro nutrition software (J. Erhardt, University of Hohenheim, Germany) to convert the amount of food eaten into individual nutrients. The 3-day energy and nutrient intakes were averaged to obtain a mean daily energy and nutrient intake for each subject.
Free fructose and free glucose refer to fructose and glucose that is contained in the food as monosaccharide, whereas total fructose and total glucose refer to the monosaccharides and also the part derived from the disaccharide sucrose (50% fructose and 50% glucose).
The primary outcome measure of this trial was the change in insulin sensitivity, determined as the hepatic glucose suppression during the euglycemic-hyperinsulinemic clamp after fructose and sucrose interventions compared with glucose. Secondary outcome measures were changes in fasting concentrations of lipids, glucose, insulin, and C-peptide, as well as changes in anthropometric measures.
Laboratory analysis
Blood glucose was directly measured from whole blood samples (fasting and during the clamp) using an automated enzymatic method (YSI 2300; YSI Life Sciences, Yellow Springs, OH). The remaining blood samples were centrifuged, and the serum and plasma were directly processed for the lipid profile or stored at −20°C for further analysis. Triglycerides, cholesterol, and free fatty acids were measured in fresh serum on Roche MODULAR by enzymatic reactions (triglyceride GPO-PAP and cholesterol CHOP-PAP; Roche Diagnostics, Mannheim, Germany), on Roche INTEGRA by a homogenous enzymatic color reaction (HDL cholesterol plus third generation; Roche Diagnostics), and on Konelab (Free Fatty Acids; Thermo Scientific, Dreieich, Germany). From frozen serum, C-peptide was measured using RIA (IRMA-C-PEP; CIS Bio International, Bagnols-sur-Cèze Cedex, France) and leptin using ELISA (EZHL-80 SK; Linco Research, St. Charles, MO). Plasma [6,6]-2H2 glucose enrichment (TTR) was measured by gas chromatography-mass spectrometry (Hewlett-Packard Instruments, Palo Alto, CA) as described elsewhere (13).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (IBM SPSS, Chicago, IL). All variables were checked for normal distribution before data analysis. Data are expressed as arithmetic mean ± SD for normally distributed variables and as geometric mean ± SD for non-normally distributed data. Non-normally distributed data were log-transformed, and further analysis was done using the transformed data. According to the intention-to-treat design of the study, all subjects (completers and noncompleters) were included in the final analysis. The effect of the interventions and the order of the interventions on anthropometric and metabolic parameters was examined using multiple linear regression, as described elsewhere (14), always controlling for between-patient differences. Post hoc Bonferroni correction was applied to account for multiple comparisons. In the main analysis, the three other interventions were compared with the glucose intervention, and thus, a correction factor of three (three interventions) was used. For the dietary intake, all four interventions were compared with baseline, and thus, a correction factor of four was used. A value of P < 0.05 after correction was considered significant.
RESULTS
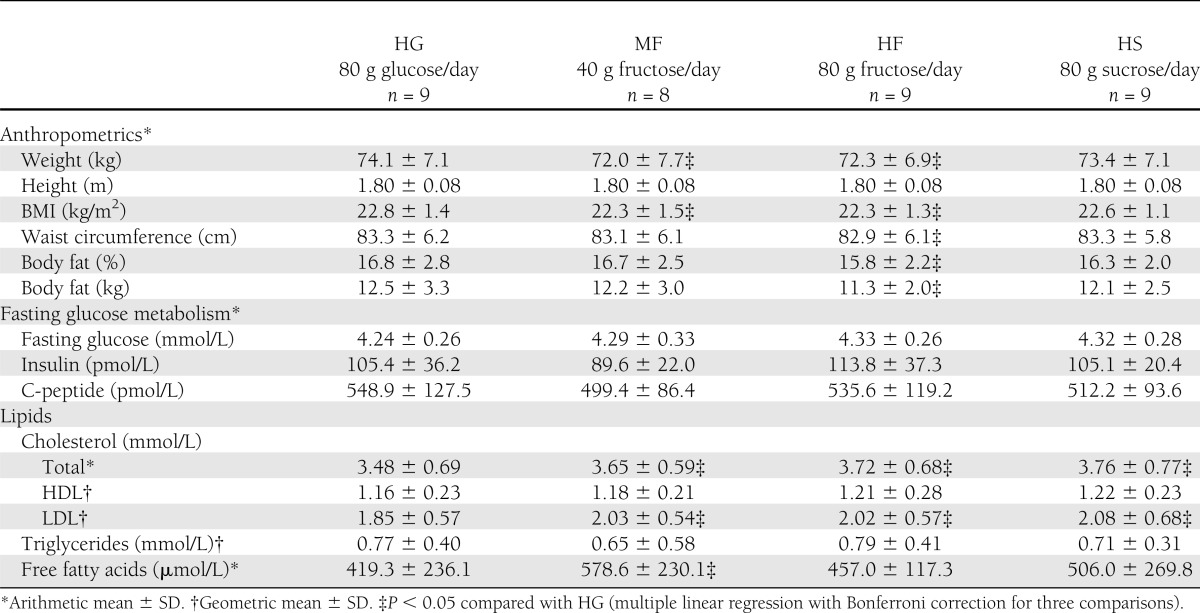
Nine subjects participated in the study. Except for one, who was not able to finish the last intervention (MF) because he moved abroad, all subjects completed all four interventions. At baseline, the subjects were a mean age of 22.8 ± 1.7 years. Their anthropometric characteristics after each of the interventions are reported in Table 1. Compared with the HG intervention, body weight, BMI, body fat, and waist circumference were slightly but significantly lower after the HF intervention (P < 0.05, general linear model with Bonferroni correction for three comparisons). Body weight and BMI were also significantly lower after the MF intervention compared with HG (P < 0.01).
Table 1.
Anthropometric characteristics as well as fasting glucose, insulin, C-peptide, and lipid concentrations of all subjects after each of the four 3-week interventions
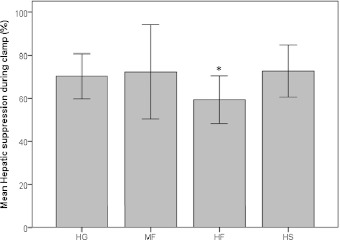
The results of the euglycemic-hyperinsulinemic clamps after each intervention are reported in Table 2. The hepatic suppression of glucose production during the clamp was significantly lower after the HF intervention compared with HG (P = 0.015), as also shown by the higher ratio of endogenous production during the clamp at baseline (P = 0.009), whereas there was no difference among HG and MF or HS (compare Fig. 1). This shows a significant decrease in hepatic insulin sensitivity after relatively small amounts of daily fructose consumption. In contrast, no significant differences among diets were seen in glucose metabolic clearance rate (i.e., insulin-mediated glucose clearance), which is a parameter of whole-body insulin sensitivity. Mean glucose levels during the baseline measurements and the clamp were kept in the same range.
Figure 1.
Hepatic suppression of glucose production (%) after 3 weeks’ consumption of different SSBs (HG: 80 g glucose/day, MF: 40 g fructose/day, HF: 80 g fructose/day, HS: 80 g sucrose/day). *P < 0.05 compared with HG. Values are means ± 1 SD.
Also reported in Table 1 are the fasting metabolic characteristics (glucose, insulin, C-peptide, lipids) of the subjects after each intervention. Fasting levels of glucose, insulin, and C-peptide did not differ significantly between HG and any of the other interventions. Compared with the HG intervention, LDL cholesterol and total cholesterol were significantly higher after the MF, HF, and HS interventions (P < 0.05). Furthermore, the free fatty acid concentration was increased after MF compared with HG (P = 0.033), with a trend toward higher values after HF and HS, albeit not significant. No differences were seen among the interventions for HDL cholesterol or triglycerides.
Compared with HG (2.02 ± 2.28 ng/mL) leptin concentrations were significantly lower after MF (1.26 ± 1.22, P = 0.012) and HF (1.37 ± 2.54 , P = 0.012), whereas the difference compared to HS (1.71 ± 2.99) was not significant.
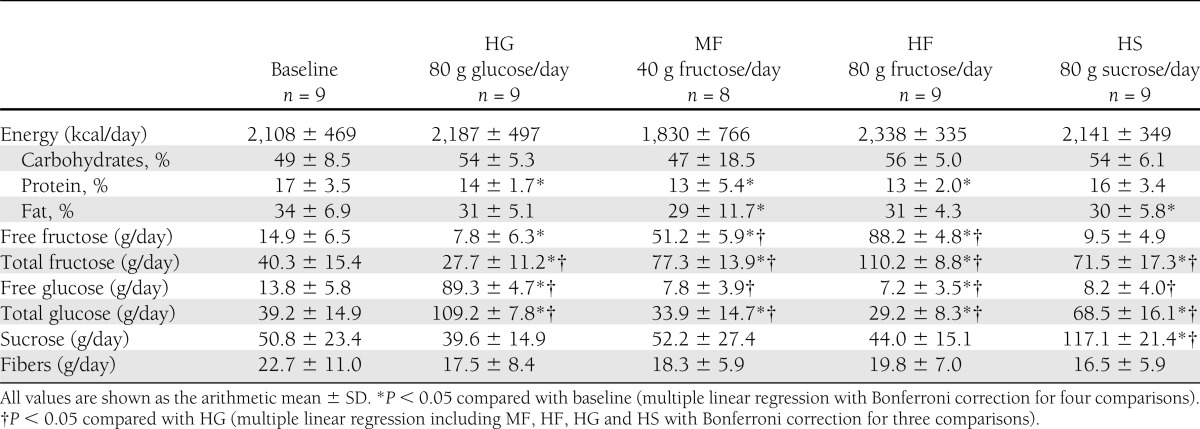
Dietary intake was assessed at baseline and after each of the four interventions. The consumption of energy, macronutrients, fiber, and the different sugars are reported in Table 3. Energy intake and the percentage of energy from fat, carbohydrates, and protein did not differ significantly between HG and the other interventions, and neither did fiber intake. However, protein intake was significantly lower in all interventions, except for HS, compared with baseline, whereas fat intake was significantly lower in the MF and the HS interventions, again compared with baseline. Carbohydrate intake was higher in the HF, HG, and HS interventions compared with baseline, but the differences were not significant. The consumption of the individual sugars varied according to the interventions.
Table 3.
Dietary intake of all subjects at baseline and after each of the four 3-week interventions
CONCLUSIONS
This study has investigated the effect of SSBs containing fructose or sucrose compared with those containing glucose and has resulted in two important findings: 1) Compared with HG, suppression of endogenous glucose production is reduced after the HF diet during the euglycemic-hyperinsulinemic clamp, indicating reduced hepatic insulin sensitivity after the HF diet, and 2) after all diets containing fructose (MF, HF, and HS), total and LDL cholesterol were elevated compared with HG.
Glucose and fructose have a similar caloric content, but intermediary fructose metabolism has unique features. After a dietary fructose load, fructose is rapidly cleared from the plasma and efficiently metabolized in the liver in an insulin-independent manner (2). While glucose metabolism via hexokinase and glycolysis is tightly regulated by the energy status of the cell and insulin levels, fructose metabolism via fructokinase bypasses these regulatory steps. Hence, rapid breakdown of fructose into trioses leads to high fluxes through the downstream steps of the glycolytic pathway, generating, for example, precursors and substrates for de novo lipogenesis. Fructose ingestion also affects lipid metabolism via enhanced and extended activity of regulator proteins (e.g., sterol regulatory element-binding protein-1c for de novo lipogenesis) (1,8,15,16).
Several studies have shown that supplementation with high amounts of fructose, associated with excess energy intake, induces features similar to those encountered in the metabolic syndrome. The most striking effect is an increase in fasting and postprandial triglycerides, which can be explained by a stimulation of hepatic de novo lipogenesis (8,17), a stimulation of VLDL-triglyceride secretion, and a decreased VLDL-triglyceride clearance (18,19). In addition, several studies have reported a mildly impaired hepatic insulin sensitivity, as indicated by an increase in fasting hepatic glucose production or by a blunted suppression of glucose production during hyperinsulinemia or a deposition of ectopic fat in liver cells (7,17,20).
In the current study, we observed a significant decrease in hepatic insulin sensitivity even with relatively small amounts of daily fructose consumption. This was documented by using an insulin clamp at low insulin infusion rates, which incompletely suppressed hepatic glucose production. In addition, care was taken to have a long tracer infusion time before measurement to avoid erroneous results linked to incomplete tracer equilibration, which may explain why similar results were not observed with former experiments (6). This clearly indicates that hepatic insulin sensitivity is exquisitely sensitive to fructose intake. The mechanisms underlying these effects remain unknown but may involve a stimulation of gluconeogenesis and increased glycogen stores, or may be related to hepatic lipotoxicity.
In contrast to this impaired hepatic insulin sensitivity, whole-body (presumably essentially muscle) insulin sensitivity was not significantly altered by fructose-containing drinks. This is consistent with other studies that used higher amounts of fructose (7,17) but may appear at odds with the observation that high fructose intakes can impair glucose tolerance. This strongly suggests that this impaired glucose tolerance is explained by impaired suppression of hepatic glucose output rather than by muscle insulin resistance, at least with short-term high-fructose diets. It remains possible, however, that fructose administration over longer periods of time may also alter muscle insulin sensitivity, possibly through a progressive deposition of ectopic fat in skeletal muscle, as shown by Lê et al. (7).
In contrast to other studies (6,21), we did not observe a significant increase in plasma triglyceride concentrations. This is most likely related to the relatively low amount of fructose administered in the current study. A meta-analysis (22) showed fasting triglyceride concentrations increase with daily fructose intake above 100 g/day (i.e., somewhat higher than used in the present experiments). However, interestingly, we found an increase in total and LDL-cholesterol concentrations after the HF, MF, and HS diets compared with HG.
Similarly, the study by Bantle et al. (21) revealed differences in total and LDL-cholesterol between fructose and glucose diets after a study duration of 4 weeks, but they were no longer significant after 6 weeks, which was the study end point. At this point, the only parameter that did differ between the glucose and fructose diets was triglyceride concentration in men (21). However, despite similar amounts of fructose and glucose, the study population in their trial was not necessarily comparable to the one in the current study. All subjects in our study were aged between 21 and 25 years, whereas in the Bantle et al. study, half of the subjects were aged older than 40 years. Further, the mean BMI of our healthy volunteers was 22.3 kg/m2 at baseline but was 24.7 and 25.8 kg/m2 in the subjects aged younger and older than 40 years of age, respectively, in the Bantle et al. (21) study.
Following along the same line, another recent study investigating the effect of different sugars on lipid metabolism reported results comparable to ours, despite methodologic differences (25% of energy requirements given as SSB, duration 2 weeks). This group observed increased concentrations of LDL cholesterol and also of 24-h triglyceride area under the curve (a parameter we did not assess) after fructose and high-fructose corn syrup, but not after glucose consumption, whereas fasting triglycerides were similar after all interventions (23).
A special feature of the current study is that it provided a direct comparison of the effects of fructose-containing drinks to glucose alone. Only few studies have performed such a direct comparison. Stanhope et al. (8) found an increase in fasting glucose and a decrease in insulin sensitivity after a 10-week intervention with fructose-containing beverages but not after glucose-containing beverages. However, the energy provided by the fructose and glucose beverages in their study accounted for 25% of total energy intake. In our study, diminished hepatic insulin sensitivity could be seen despite a considerably lower amount of sugar given (15% of baseline energy intake) and a much shorter study duration. This indicates that already relatively low amounts of fructose over a short period of time may negatively affect glucose metabolism, even in healthy lean subjects. In disagreement with our results, recent studies by Silbernagel et al. (24) and Ngo Sock et al. (20) found similar effects of high-glucose and high-fructose diets with regard to insulin sensitivity determined by an oral glucose tolerance test (24) or to intrahepatic fat content (20); however, there was a significant increase in fasting triglyceride concentrations with fructose only.
Compared with the glucose intervention, we found significantly lower weight and BMI after the MF and HF intervention and significantly lower body fat and waist circumference after HF only. Even though the differences were relatively small, the finding was consistent over the different anthropometric measurements and could not be explained by higher energy intake during the HG intervention or by reduced physical activity. One previous study comparing weight and fat changes after HF and HG diets found that, even though overall weight gain was similar, fructose induced more gain in intra-abdominal adipose tissue, whereas glucose led to increased subcutaneous adipose tissue (8). The duration of the interventions in that study, however, was 10 weeks, and 25% of energy requirements were provided by sugar. We did not distinguish between intra-abdominal and subcutaneous adipose tissue in our study and can therefore not be sure what the changes we have observed were attributable to. However, previous studies have shown that leptin is mainly secreted in subcutaneous adipose tissue (25,26). Thus, the increased secretion of leptin after HG compared with HF and MF we have observed also points toward an increase in subcutaneous adipose tissue after this intervention. The small sample size in our study and the relatively short study duration may have blunted other changes seen in previous studies.
A limitation of the current study may be the relatively short duration of the interventions and the moderate amount of sugars given. However, our aim was to study the effect of the different sugars in amounts that are likely to be consumed in normal life. That we did see certain effects already at this level and after 3 weeks seems to justify our decision. Another limitation is the lack of baseline measurements before each of the interventions. Still, based on the complexity of the method used and the already high subject burden, we decided against them. However, to control for possible baseline differences, we used a randomized crossover design and controlled for the order of interventions in the statistical analysis.
In conclusion, this study shows that, with regard to glucose metabolism and, specifically, hepatic insulin sensitivity, fructose, even in moderate amounts, seems to be more harmful than the same amount of glucose. Furthermore, all fructose-containing drinks (including sucrose) showed significant effects on the lipid profile compared with glucose. However, anthropometric measurements pointed toward higher adiposity after the glucose intervention, even though differences were small. Thus, even when consumed in moderate amounts and over a limited period of time, SSB, especially those containing fructose, can result in alterations of hepatic glucose metabolism and lipid profile in healthy young men, which may possibly be associated with increased cardiometabolic risk. Further research will be needed to better understand the underlying mechanisms, specifically with regard to the lipid metabolism and also to understand other influencing factors such as age, sex, or genetic predisposition.
Acknowledgments
The study was funded by the Swiss National Science Foundation (Grant 32003B-119706 to K.B.) and the Vontobel Foundation (G.A.S.). Neither of the funding organizations was involved in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
I.A., M.H., and P.A.G. conducted the research, analyzed data or performed statistical analysis, and wrote, read, and edited the manuscript. L.S. conducted the research, analyzed data or performed statistical analysis, and read and edited the manuscript. S.B.M. conducted the research and analyzed data or performed statistical analysis. L.T. and G.A.S. wrote, read, and edited the manuscript. K.B. designed and conducted the research; analyzed data or performed statistical analysis; wrote, read, and edited the manuscript; and had primary responsibility for final content of the manuscript. I.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was presented as a poster at the 19th European Congress on Obesity (ECO2012), Lyon, France, 9–12 May 2012.
The authors thank Valeria Meyer, University Hospital Zurich, Switzerland, the study nurse assisting in the entire project, and Cornelia Zwimpfer, University Hospital Zurich, Switzerland, and Valentine Rey, University of Lausanne, Switzerland, for expert laboratory and technical work. The authors also thank all of the subjects participating in this study and the Nestlé Product and Technology Centre in Konolfingen, Switzerland, for providing the study drinks.
Footnotes
Clinical trial reg. no. NCT01021969, clinicaltrials.gov.
See accompanying commentary, p. 11.
References
- 1.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 2002;76:911–922 [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–543 [DOI] [PubMed] [Google Scholar]
- 3.Eidgenössische Ernährungskommission Kohlenhydrate in der Ernährung. Keller U, Ed. Zurich, Bundesamt für Gesundheit, 2009 [Google Scholar]
- 4.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010;303:1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–1327 [DOI] [PMC free article] [PubMed]
- 6.Lê KA, Faeh D, Stettler R, et al. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 2006;84:1374–1379 [DOI] [PubMed] [Google Scholar]
- 7.Lê KA, Ith M, Kreis R, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89:1760–1765 [DOI] [PubMed]
- 8.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334. [DOI] [PMC free article] [PubMed]
- 9.Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–485 [DOI] [PubMed] [Google Scholar]
- 10.Berneis K, Ninnis R, Häussinger D, Keller U. Effects of hyper- and hypoosmolality on whole body protein and glucose kinetics in humans. Am J Physiol 1999;276:E188–E195 [DOI] [PubMed] [Google Scholar]
- 11.Vella A, Rizza RA. Application of isotopic techniques using constant specific activity or enrichment to the study of carbohydrate metabolism. Diabetes 2009;58:2168–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson RS. Principles of Nutritional Assessment. 2nd ed. New York, Oxford University Press, 2005 [Google Scholar]
- 13.Schneiter P, Gillet M, Chioléro R, Jéquier E, Tappy L. Hepatic nonoxidative disposal of an oral glucose meal in patients with liver cirrhosis. Metabolism 1999;48:1260–1266 [DOI] [PubMed] [Google Scholar]
- 14.Senn S. Crossover Trials in Clinical Research. 2nd ed. Chichester, U.K., Wiley Publishing, 2002 [Google Scholar]
- 15.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis and metabolic syndrome. Am J Physiol Endocrinol Metab 2010;299:E685–E694 [DOI] [PubMed]
- 16.Stanhope KL, Havel PJ. Fructose consumption: recent results and their potential implications. Ann N Y Acad Sci 2010;1190:15–24 [DOI] [PMC free article] [PubMed]
- 17.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54:1907–1913 [DOI] [PubMed] [Google Scholar]
- 18.Parks EJ. Dietary carbohydrate’s effects on lipogenesis and the relationship of lipogenesis to blood insulin and glucose concentrations. Br J Nutr 2002;87(Suppl. 2):S247–S253 [DOI] [PubMed] [Google Scholar]
- 19.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 2007;85:1511–1520 [DOI] [PubMed] [Google Scholar]
- 20.Ngo Sock ET, Lê KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr 2010;103:939–943 [DOI] [PubMed] [Google Scholar]
- 21.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr 2000;72:1128–1134 [DOI] [PubMed] [Google Scholar]
- 22.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 2008;88:1419–1437 [DOI] [PubMed]
- 23.Stanhope KL, Bremer AA, Medici V, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011;96:E1596–E1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silbernagel G, Machann J, Unmuth S, et al. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr 2011;106:79–86 [DOI] [PubMed]
- 25.Russell CD, Petersen RN, Rao SP, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol 1998;275:E507–E515 [DOI] [PubMed] [Google Scholar]
- 26.Van Harmelen V, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998;47:913–917 [DOI] [PubMed] [Google Scholar]